Abstract
Patients with multiple sclerosis (MS) experience varying rates of brain volume (BV) loss ranging from 0.5 to 1.5 % per year. In addition, 66 % of patients with MS experience cognitive impairment, resulting in impact on daily activities. A systematic literature review (2003–2013) was conducted to identify all studies reporting a relationship between whole BV measures and selected patient outcomes measuring cognition, including the Symbol Digit Modalities Test (SDMT), Paced Auditory Serial Addition Test (PASAT) and MS Functional Composite (MSFC) scores. We identified 18 studies reporting associations between whole BV and cognitive outcomes. Six studies (33 %) examined the association between BV and SDMT; all six studies reported that BV loss (BVL) was significantly associated with a decline in SDMT scores (all p < 0.05). Among 14 studies (78 %) that examined the association between BV and PASAT scores, 12 (86 %) found a significant relationship between BVL and lower PASAT scores (all p < 0.05). Of the seven studies (39 %) that looked at BV and MSFC, six studies (86 %) found BVL significantly associated with lower MSFC scores (all p < 0.05). Our study demonstrated that BVL is associated with declines in cognition in MS patients across several cognition measures. The results of this study suggest that BV is a critical component of disease activity and progression in MS and has implications for treatment decisions to minimize BVL and preserve cognitive functioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease of the central nervous system (CNS) [1]. Eighty-five percent of patients are initially diagnosed with relapsing–remitting MS (RRMS) characterized by episodes of disability and recovery; after 20–25 years, about 90 % of untreated RRMS patients transition to secondary-progressive MS (SPMS) characterized by increasing disability without recovery [2, 3]. The gradual decline of both physical and neurological function associated with MS disease progression is attributed to brain atrophy caused by axonal and neuronal loss [4, 5]. Multiple sclerosis patients experience varying rates of brain volume loss (BVL) with whole brain atrophy ranging from 0.5 to 1.5 % per year among RRMS patients [6]. With the introduction of sequential volumetric magnetic resonance imaging (MRI) applied to MS patients, measurement of BVL over time and assessment of therapeutic effects on slowing brain atrophy have become possible.
In addition to brain atrophy, approximately 66 % of patients with MS experience a form of cognitive impairment, often resulting in a significant impact on daily activities [7]. The most common cognitive impairments experienced by MS patients are cognitive inefficiency and memory decline followed by verbal abilities and attention span. Studies have indicated that cognitive impairment is progressive and cognitive decline is associated with disease duration [8–10]. The MS Functional Composite (MSFC) score and its components, Paced Auditory Serial Addition Test (PASAT), Nine Hole Peg Test (9HPT), Timed 25 Foot Walk Test (T25FWT), and Symbol Digit Modalities Test (SDMT) are used to measure both cognitive and physical disability among MS patients. As the only cognitive component of MSFC, PASAT is the most frequently administered test for measuring information processing speed (IPS). Low test scores for PASAT, SDMT, and MSFC indicate worse performance. From these measures, cognitive impairment has been shown to be correlated with MRI measures, including both lesion and whole brain volume (BV) [11].
A number of studies examined the associations between BV and various patient outcomes. This study aimed to systematically catalogue the full set of published associations reported in 2003–2013, and to review published evidence of associations between BV and patient outcomes relating to cognitive impairment. In particular, this study evaluated the correlations between BV and selected patient outcomes measuring cognition in MS patients, including SDMT, PASAT, and MSFC, as well as the reported correlations between BV and Expanded Disability Status Scale (EDSS) and lesion volume (LV) and selected patient outcomes.
Materials and methods
Study selection criteria
We included longitudinal and cross-sectional studies that reported the relationship between whole BV measurements in both male and female MS patients and selected patient outcomes measuring cognition, including SDMT, PASAT, and MSFC scores.
Search strategy
We searched Medline, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), the NHS Economic Evaluation Database (NHS EED), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) for studies published in English between January 2003 and September 2013. We hand searched references of included reviews published in 2013 and reviewed the US clinical trial registry to identify additional studies that were not indexed in the electronic databases.
Study selection process and data extraction
Two independent reviewers applied the inclusion criteria and assessed the quality of the data collected using a standardized methodology. Each reviewer evaluated the data from the eligible studies and electronically entered the information into an Excel database developed specifically for the review with prepared fields. Disagreements between reviewers were resolved by consensus or by consultation with a third researcher, referring to the original sources.
We collected information on the study design, population, comparison or treatment groups, sample size, duration of follow-up, whole BV algorithm, average baseline characteristics of patients, MS type, MS disease duration, reported BV and associations. For BV measures, we extracted information for whole BV measures, including percent BV change (PBVC), brain parenchymal fraction (BPF), brain parenchymal volume (BPAV), normalized BV (NBV), relative BV (RBV), cerebral parenchymal volume (CPV), cerebral volume fraction (CVF), and whole BV (WBV). In addition, T1-hypointense LV (T1LV) and T2-hyperintense LV (T2LV) data were captured. We recorded physical function measured by EDSS and patient outcomes (i.e., PASAT, SDMT, and MSFC).
Data management and reporting
We used Endnote version X5 to store the bibliographic citations from the electronic search. For data entry and descriptive analyses, we used Microsoft Excel 2010. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) in reporting the systematic review [12].
Results
Search
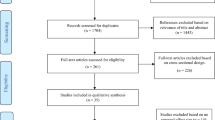
The literature search identified 2837 publications to be screened based on the predetermined screening criteria (Fig. 1). Of the 599 publications eligible for full-text screening, 581 were excluded based on publication type (n = 13), study design (n = 28), population (n = 178), and outcomes (n = 362). A total of 18 studies meeting the study inclusion criteria were included in the qualitative synthesis [13–30].
Overall study descriptions
The 18 studies included 13 cross-sectional studies and 5 longitudinal studies with study duration ranging from 12 to 96 months (Table 1). The study populations varied greatly across the 18 studies. Relapsing–remitting MS was the most common disease course with 12 (67 %) studies including patients with RRMS. In addition, nine (50 %) studies included patients with SPMS and five (28 %) studies included patients with primary-progressive MS (PPMS) (Table 2). Among the 17 studies reporting gender, 13 studies had over 50 % female patients. Among the 17 studies reporting age, the mean/median age of MS patients ranged from 34.2 to 55.7 years. In addition, the patient populations varied greatly with respect to disease duration; of the 17 studies reporting disease duration, the mean/median ranged from 1.4 to 19.9 years. The study with mean disease duration of 19.9 years was among a population of SPMS patients while the shortest disease duration (1.4 years) was among a population of RRMS patients (Table 1) [20, 23].
The structural image evaluation using normalization of atrophy cross-sectional (SIENAx) algorithm was the most frequently used algorithm for cross-sectional studies [13–15, 17, 19, 20]. Some studies reported SIENA as well as other algorithms such as Java Image, Statistical Parametric Mapping (SPM), MeVisLab Brain Volumetry, and in-house software programs to measure BV. BPF was the most frequently reported measure among the included studies (Table 2).
BV and SDMT
Six cross-sectional studies examined the association between BV and SDMT [13–17, 30]. Four (67 %) studies reported unadjusted correlations (Table 3) and two (33 %) studies adjusted for age and premorbid conditions in linear regression models. The six studies varied based on patient characteristics with the mean/median age of patients ranging from 34.2 to 47.0 years. In addition, the studies varied by MS disease course; one (17 %) study included only RRMS patients, four (67 %) studies included both RRMS and SPMS patients, and one (17 %) study included RRMS, SPMS, and PPMS patients.
The four studies reporting correlations found positive correlations ranging from 0.40 to 0.54 (all p < 0.05) indicating that BVL is associated with lower SDMT scores (Table 3). One study using a linear regression model controlling for age and premorbid intelligence showed that in a sample of RRMS and SPMS patients, a partial correlation of 0.62 (p < 0.001) was found between BPF and SDMT scores [16]. Another study, in a sample of RRMS and SPMS patients, a partial correlation of 0.58 (p < 0.01) was reported for BV and SDMT scores after adjusting for age and years of education [14].
BV and PASAT
Fourteen studies, including 3 longitudinal and 11 cross-sectional studies, examined the associations of BV and PASAT [13, 16–24, 26, 28–30]. Patient characteristics varied across the studies with mean age ranging from 36.9 to 55.7 years. The disease composition of the included study populations varied by studies; five (36 %) studies included only RRMS patients, three (21 %) studies included only SPMS patients, two (14 %) studies included only PPMS patients, two (14 %) included both RRMS and SPMS patients, one (7 %) included RRMS, SPMS, and PPMS patients, and one (7 %) included patients with remitting MS.
Of these 14 studies, 12 (86 %) reported correlations (Table 4) [13, 17–19, 21–24, 26, 28–30] (Table 4). Seven (58 %) studies reported statistically significant positive correlations ranging from 0.29 to 0.40 (all p < 0.05), demonstrating that BVL was associated with a decrease in PASAT scores [19, 22–24, 26, 28, 30].
In addition, three studies demonstrated statistically significant associations between BV and PASAT scores in linear regression models. One study among SPMS patients demonstrated in a multivariable regression model controlling for age, gender, disease duration, and duration of SPMS that NBV was a significant predictor of MSFC (p < 0.0001) [20]. Another study using a linear regression model controlling for age and premorbid intelligence demonstrated that BPF predicted PASAT scores (R p = 0.34, p = 0.01) [16]. In addition to correlating BPF and PASAT, one study also analyzed the relationship while controlling for age, disease duration, and EDSS and found that BPF was a significant predictor of PASAT (R p = 0.60, p < 0.0001) [24].
BV and MSFC
Nine studies (five longitudinal and four cross-sectional) reported associations between MSFC and BV [18–20, 22, 23, 25, 27–29]. The mean age ranged from 36.9 to 55.7 years. The studies varied on disease characteristics: three (33 %) studies included only PPMS, two (22 %) studies included only RRMS, three (33 %) studies included only SPMS, and one (11 %) study included RRMS, SPMS, and PPMS patients. Of these nine studies, seven (78 %) reported the correlation of BV and MSFC and three examined the relationship in linear regression (Table 5).
Among the seven studies correlating BV and MSFC scores, six (86 %) studies found significant positive associations with correlations ranging from 0.31 to 0.52 (all p < 0.05). Linear regressions were performed in three studies to examine the associations between BV and MSFC. In a longitudinal study with only RRMS patients, the authors found that a decrease in MSFC score from baseline to year 2 was associated with a 0.57 % (95 % CI 0.06–1.08) decrease in BPF. The same study also found that a decrease in MSFC score from year 0 to 8 was associated with a 0.29 % (95 % CI 0.06–0.53) decrease in BPF [25]. In addition, one study among SPMS patients demonstrated in a multivariable regression adjusting for age, gender, and disease duration that NBV was a significant predictor of MSFC (p < 0.0001) [20]. Another study among SPMS patients also demonstrated that NBV was a significant predictor of MSFC (R p = 0.44, p = 0.001) [19].
LV and patient outcomes
Eight studies correlated LV with patient outcomes [13, 15, 17–19, 22, 24, 30]. Total T2LV was correlated with SDMT in four studies with statistically significant negative correlations ranging from −0.36 to −0.55 (all p < 0.05), demonstrating that higher T2LV is associated with lower SDMT scores [13, 15, 17, 30]. In addition, two of the three studies reporting correlations between T2LV and MSFC found a statistically significant correlation (p < 0.05), demonstrating that an increase in T2LV is associated with a decrease in MSFC [18, 19, 22]. One cross-sectional study found a statistically significant negative correlation between T2LV and MSFC (r = −0.31, p < 0.01) [19]. One longitudinal study found a statistically significant positive correlation between change in T2LV and MSFC over 5 years (r = 0.31, p = 0.04), while a second longitudinal study found no significant correlation over 2 years (r = −0.11, p = 0.45) [18, 22]. T2LV was correlated with PASAT in six studies [13, 17–19, 22, 30]. Of these studies, two cross-sectional studies found statistically significant correlations ranging from −0.37 to −0.42 (all p < 0.05) demonstrating that a higher T2LV is associated with lower PASAT [13, 30]. In addition, three studies correlated T2LV and EDSS [19, 22, 24]; one longitudinal study found a statistically significant negative correlation between change in T2LV over 5 years and EDSS at baseline (r = −0.35, p = 0.02), but no correlation between change in both T2LV and EDSS over 5 years [22]. One of the two cross-sectional studies found a significant positive correlation between T2LV and EDSS (p = 0.39, p = 0.03) [24].
Among the four studies correlating total T1LV with patient outcomes, the results were similar to those with T2LV. The two studies correlating T1LV and SDMT found statistically significant negative correlations ranging from −0.49 to −0.54 [13]. One study correlated T1LV with MSFC and found a significant correlation (r = −0.35, p < 0.01) [19, 30]. Three studies correlated T1LV and PASAT; two studies found significant negative correlations [17, 19, 30]. Of these two studies, one correlated T1LV and PASAT-3 (r = −0.41, p < 0.01) and the second correlated T1LV with both PASAT-3 (r = −0.31, p < 0.01) and PASAT-2 (r = −0.33, p < 0.01) [19, 30].
BV and LV
In addition to examining the relationship between BV and patient outcomes, seven studies reported the correlations between BV and LV [15, 16, 19, 20, 22, 24, 28]. Six studies reported correlations between BV and T2LV and four studies correlated BV and T1LV. Among the six studies examining the associations between BV and T2LV, five reported statistically significant correlations. The one longitudinal study demonstrated that change in BV over 5 years was associated with both change in T2LV over 5 years (r = 0.36, p = 0.017) and T2LV at baseline (r = 0.39, p = 0.009) [22]. Among the five cross-sectional studies, four studies reported significant correlations ranging from −0.29 to −0.53 (all p < 0.05) [16, 19, 20, 24, 28]. One study reported a statistically significant correlation between BPF and T2LV (r = −0.29, p = 0.05) [24].
Of the four studies examining the relationship between BV and T1LV, all four found a statistically significant correlation. One longitudinal study reported a statistically significant correlation between change in BV over 5 years and T1LV at baseline (r = 0.464, p = 0.001); however, the study found that change in T1LV over 5 years was not associated with change in BV over 5 years (r = 0.259, p = 0.696) or BV at baseline (r = 0.285, p = 0.60) [22]. Three cross-sectional studies reported correlations ranging from −0.29 to −0.52 [16, 19, 24].
BV and EDSS
Since EDSS is widely reported as a measure of disability in MS patients, further evaluation of correlations between BV and EDSS was made. Seven studies (three longitudinal and four cross-sectional) reported associations between BV and EDSS [19, 21–24, 27, 29]. Mean age of patients ranged between 36.9 and 55.7 years. The studies differed by disease characteristics: four (57 %) studies included only RRMS patients, one (14 %) included only PPMS patients, one (14 %) included only SPMS patients, and one (14 %) included RRMS, SPMS, and PPMS patients.
Three cross-sectional studies demonstrated the association between BV and EDSS with correlations ranging from −0.39 to −0.27 (all p < 0.05). In addition to correlating BV and EDSS, one cross-sectional study also analyzed the association of BV and EDSS adjusting for age and disease duration, and found that EDSS was associated with BPF (r = −0.51, p = 0.005) [24].
Three longitudinal studies found that change in BV was associated with EDSS at different time points. One study found that the rate of reduction in BV over 5 years was significantly associated with EDSS at baseline (r = 0.43, p < 0.01); however, the same study also found that the rate of change in BV was not associated with change in EDSS (r = 0.12, p = 0.41) over the 5 year period [22]. Similarly, one study found that PBVC over 12 months was statistically significantly associated with EDSS at baseline (r = −0.51, p < 0.01), while another study found that PBVC over 2 years was statistically significantly associated with EDSS at the 2-year follow-up (r = −0.32, p < 0.01) [23, 27].
Discussion
Our systematic literature review documented the extent to which the relationship between BV and patient outcomes has been studied and qualitatively synthesized the findings. In particular, our study focused on patient outcomes measuring cognitive impairment, including SDMT, PASAT, and MSFC, to demonstrate that BVL is associated with a decline in cognitive function in MS patients. The findings for whole BV summarized in this review are in line with multiple studies demonstrating that regional BV is associated with cognitive outcomes. For instance, Batista et al. found that multiple regional BV measurements were all significantly correlated with PASAT and SDMT, after controlling for age [31]. Similarly, Amato et al. has published multiple studies demonstrating the statistically significant association between neocortical volume and cognitive outcomes [32–35]. The findings of our review have implications for the use of BV in the monitoring and treatment of MS patients to prevent cognitive decline. Cognitive impairment is a key factor that may adversely affect a patient’s disease progression and quality of life [9, 36]. It is important to use measures of cognitive decline to identify cognitive impairment which may be evident early in the MS disease course.
In this study, we found that the relationship between BV and SDMT is more pronounced than BV and PASAT. Furthermore, the reported correlation estimates were consistent across the studies that reported SDMT. The strength of the correlations between BV and SDMT is supported by the literature suggesting that there is an underlying link between BV and cognitive impairment. The SDMT instrument is sensitive to cognitive impairment because it evaluates a number of domains, including visual scanning, visual attention and processing speed, and episodic memorization [37]. In addition, SDMT has been proven to be reliable when administered over multiple time points [37, 38]. Ease of use and test–retest validity of the instrument is important to the longitudinal study of cognitive decline in patients with MS.
Associations of BV and EDSS reported in the studies were examined because EDSS has been widely used and incorporates measures of physical disability based on individual functional system scores of CNS [39]. The results of our review suggest that cognitive outcomes such as PASAT and MSFC demonstrate a greater association with BV than EDSS. For example, Furby et al. found significant correlations between NBV and both PASAT-3 (r = 0.37, p < 0.01) and MSFC (r = 0.47, p < 0.01) but not between NBV and EDSS (r = −0.13, p = 0.17) [19].
We also reported the relationship between LV and patient outcomes to observe if there was a similar relationship when compared with the associations between BV and patient outcomes. T2LV has been used in the real-world setting as a measure of disease progression as well as a secondary endpoint in clinical trials [40]. It has been demonstrated that LV is associated with both physical and cognitive disability. In one review, the authors found that both BV and T2LV correlated well with information processing speed as measured by SDMT and PASAT and that there was a stronger relationship between T2LV and SDMT than T2LV and PASAT [11]. Another study analyzing the ratio of T1LV to T2LV and work productivity demonstrated an association between increased T1LV and lower chance of employment, which may be mediated through the cognitive domain [41]. However, studies also demonstrated that LV has a plateau relationship with disability over time [42]. In this study, we found that the correlations between BV and selected cognitive outcomes were greater when compared with those between LV and selected cognitive outcomes. The higher correlations found among studies correlating BV with cognitive outcomes are consistent with the literature demonstrating that BVL provides a more complete understanding of the full extent of tissue damage and its impact on patient outcomes [5]. Our systematic review further provides support for BV as a measure of disease activity and progression because of the strong correlations observed between BV and selected cognitive outcomes. In addition, a recent meta-analysis demonstrated that the size of the treatment effect of disease modifying therapies (DMTs) on brain atrophy is closely related to the size of the treatment effect on disability progression. In conjunction with the treatment effect on lesions, the two MRI measures could explain up to 75 % of the variance in disability progression [43]. The findings of these studies indicate that it is important to measure BV in patients in addition to LV to fully measure axonal loss and to monitor patient’s stability status. As a predictor of disability, BVL is an important endpoint to consider in the treatment of patients in both clinical trials as well as the real-world clinical setting.
The findings of this study highlight important characteristics of disability in MS that have implications for the on-going debate regarding the timing of treatment for patients with CIS and early stage clinically diagnosed MS. Patients with MS begin with a focal inflammatory disease and longer disease duration is associated with an accumulation of diffused brain inflammation, neuronal loss, cortical demyelination, and progressive axonal injury in white matter [44]. Studies have shown that BVL is present even among patients with CIS and early RRMS, and that overall cognitive performance begins to decline prior to the onset of clinical disease symptomology [9, 44–46]. The US National MS Society recommends that early therapy should be given without interruption, particularly in patients at high risk for disease progression [2]. Furthermore, the early initiation of DMTs in clinically diagnosed MS has demonstrated improved effectiveness of treatment by reducing relapses as well as slowing disease progression in patients with RRMS [2]. Based on the demonstrated correlations between BV and patient outcomes and the evidence demonstrating that early treatment of MS reduces BVL and disease progression, literature suggests that MS patients may benefit from early treatment to prevent further disability. Withholding treatment from patients until the disease progresses may lead to reduced efficacy of DMTs and irreversible axonal and neuronal damage [47]. By treating early in the disease course and reducing BVL, the early initiation of effective DMTs may help to further improve patient outcomes and delay the onset of both cognitive and physical disabilities.
This study has a few notable limitations. We included studies published from January 2003 to September 2013 to adequately capture the use of MRIs in MS and focus on the technological advancement in imaging in the past decade. This restriction may limit the generalizability of the results to older studies published before 2003. In addition, we only included English studies which limited us from making inferences on studies published in other languages.
In our study, we were limited to reporting ranges of the correlation estimates instead of quantitatively synthesizing the relationships due to the high level of heterogeneity that exists across the studies. Cohen and Rudick reported that studies assessing cognitive impairment comprise different MS populations, variability of symptom onset, severity, and disease duration as well as instruments to reliably capture cognitive impairment [6]. One of the drivers of the variability is that there is no standardized method of measurement of BVL which complicates the evaluation of study results from various studies of BV and patient outcomes. Locatelli et al. correlated BPF and BPAV with PASAT and found that the correlation with BPF was significant, while the correlation with BPAV was not significant [24]. As demonstrated by Locatelli et al., the correlation of BV and patient outcomes varies greatly based on the BV measurement used; the normalized BPF consistently showed stronger correlations when compared to the non-normalized BPAV [24]. The variation in correlations between different BV measures and patient outcomes provides support for the establishment of a standard BV measurement to be used in studies examining BVL. BPF was the most frequently used measure reported in our study and studies have shown that BPF reduces variability between individuals and the high test–retest reproducibility improves the power to predict significant changes in a longitudinal setting [48, 49].
In addition, factors such as age and education (cognitive reserve) introduce considerable variability when examining the impact of BVL on cognitive function. Some patients can retain cognitive function while sustaining considerable disease burden due to higher levels of cognitive reserve [50]. Achiron et al. reported that the prevalence of cognitive impairment among MS patients ranged between 20 and 65 % and the wide range was due to the disease subtypes, disease duration, and level of disability of the MS population studied, as well as differences in the cognitive assessment scales, procedures, and tools used [9]. The studies included in our review varied greatly in both clinical and patient characteristics. Study populations included all disease courses and the mean age of patients varied by over 20 years across studies. As a result, we did not examine the relationship between BVL and cognitive impairment as a function of age because of the variability that existed in the studies. However, the studies reporting results that controlled for patient characteristics such as age and education demonstrated that BV was associated with cognitive decline.
Conclusion
Our study demonstrated that BVL is consistently associated with a decrease in SDMT score. Among the studies included in this review, lower BV was associated with lower SDMT, PASAT, and MSFC scores. Furthermore, correlations between BV and selected cognitive outcomes reported in the studies were higher when compared with those between LV and selected cognitive outcomes. This finding has implications for the use of BV in monitoring disease progression as well as early initiation of DMTs to minimize BVL and downstream effects of cognitive impairment. Further prospective studies examining the relationship between treatment, BVL, and cognitive functions may offer additional validations on the findings from this systematic literature review.
References
Chaudhuri A (2013) Multiple sclerosis is primarily a neurodegenerative disease (Vienna, Austria: 1996). J Neural Transm 120(10):1463–1466. doi:10.1007/s00702-013-1080-3
Gold R, Wolinsky JS, Amato MP, Comi G (2010) Evolving expectations around early management of multiple sclerosis. Ther Adv Neurol Disord 3(6):351–367. doi:10.1177/1756285610385608
Dutta R, Trapp BD (2007) Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 68(22 suppl 3):S22–S31
Weinshenker BG (1994) Natural history of multiple sclerosis. Ann Neurol 36(Suppl):S6–S11
Miller DH, Barkhof F, Frank JA, Parker GJ, Thompson AJ (2002) Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 125(Pt 8):1676–1695
Cohen JA, Rudick RA (2007) Multiple sclerosis therapeutics, 3rd edn. Taylor & Francis, New York
Benedict RHB (2011) Cognitive dysfunction in multiple sclerosis. Clinical Bulletin Information for Health Professionals. National Multiple Sclerosis Society, Professional Resource Center. http://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Clinical-Bulletin-Cognitive-Dysfunction-Benedict.pdf
Amato MP, Ponziani G, Siracusa G, Sorbi S (2001) Cognitive dysfunction in early-onset multiple sclerosis: a reappraisal after 10 years. Arch Neurol 58(10):1602–1606
Achiron A, Chapman J, Magalashvili D, Dolev M, Lavie M, Bercovich E, Polliack M, Doniger GM, Stern Y, Khilkevich O, Menascu S, Hararai G, Gurevich M, Barak Y (2013) Modeling of cognitive impairment by disease duration in multiple sclerosis: a cross-sectional study. PLoS One 8(8):e71058. doi:10.1371/journal.pone.0071058
Achiron A, Polliack M, Rao SM, Barak Y, Lavie M, Appelboim N, Harel Y (2005) Cognitive patterns and progression in multiple sclerosis: construction and validation of percentile curves. J Neurol Neurosurg Psychiatry 76(5):744–749. doi:10.1136/jnnp.2004.045518
Rao SM, Martin AL, Huelin R, Wissinger E, Khankhel Z, Kim E, Fahrbach K (2014) Correlations between MRI and information processing speed in MS: a meta-analysis. Mult Scler Int 2014:975803. doi:10.1155/2014/975803
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj 339. doi:10.1136/bmj.b2700
Benedict RH, Bruce J, Dwyer MG, Weinstock-Guttman B, Tjoa C, Tavazzi E, Munschauer FE, Zivadinov R (2007) Diffusion-weighted imaging predicts cognitive impairment in multiple sclerosis. Mult Scler 13(6):722–730. doi:10.1177/1352458507075592
Benedict RH, Bruce JM, Dwyer MG, Abdelrahman N, Hussein S, Weinstock-Guttman B, Garg N, Munschauer F, Zivadinov R (2006) Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol 63(9):1301–1306. doi:10.1001/archneur.63.9.1301
Benedict RH, Ramasamy D, Munschauer F, Weinstock-Guttman B, Zivadinov R (2009) Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry 80(2):201–206. doi:10.1136/jnnp.2008.148403
Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R (2004) Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 61(2):226–230. doi:10.1001/archneur.61.2.226
Calabrese M, Agosta F, Rinaldi F, Mattisi I, Grossi P, Favaretto A, Atzori M, Bernardi V, Barachino L, Rinaldi L, Perini P, Gallo P, Filippi M (2009) Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol 66(9):1144–1150. doi:10.1001/archneurol.2009.174
Furby J, Hayton T, Altmann D, Brenner R, Chataway J, Smith KJ, Miller DH, Kapoor R (2010) A longitudinal study of MRI-detected atrophy in secondary progressive multiple sclerosis. J Neurol 257(9):1508–1516. doi:10.1007/s00415-010-5563-y
Furby J, Hayton T, Anderson V, Altmann D, Brenner R, Chataway J, Hughes R, Smith K, Miller D, Kapoor R (2008) Magnetic resonance imaging measures of brain and spinal cord atrophy correlate with clinical impairment in secondary progressive multiple sclerosis. Mult Scler 14(8):1068–1075. doi:10.1177/1352458508093617
Hayton T, Furby J, Smith KJ, Altmann DR, Brenner R, Chataway J, Hughes RA, Hunter K, Tozer DJ, Miller DH, Kapoor R (2009) Grey matter magnetization transfer ratio independently correlates with neurological deficit in secondary progressive multiple sclerosis. J Neurol 256(3):427–435. doi:10.1007/s00415-009-0110-4
Hildebrandt H, Hahn HK, Kraus JA, Schulte-Herbruggen A, Schwarze B, Schwendemann G (2006) Memory performance in multiple sclerosis patients correlates with central brain atrophy. Mult Scler 12(4):428–436
Ingle GT, Stevenson VL, Miller DH, Thompson AJ (2003) Primary progressive multiple sclerosis: a 5-year clinical and MR study. Brain 126(Pt 11):2528–2536. doi:10.1093/brain/awg261
Jasperse B, Vrenken H, Sanz-Arigita E, de Groot V, Smith SM, Polman CH, Barkhof F (2007) Regional brain atrophy development is related to specific aspects of clinical dysfunction in multiple sclerosis. Neuroimage 38(3):529–537. doi:10.1016/j.neuroimage.2007.07.056
Locatelli L, Zivadinov R, Grop A, Zorzon M (2004) Frontal parenchymal atrophy measures in multiple sclerosis. Mult Scler 10(5):562–568
Marrie RA, Fisher E, Miller DM, Lee JC, Rudick RA (2005) Association of fatigue and brain atrophy in multiple sclerosis. J Neurol Sci 228(2):161–166. doi:10.1016/j.jns.2004.11.046
Mineev KK, Prakhova LN, Il’ves AG, Kataeva GV, Petrov AM, Reznikova TN, Pozdnyakov AV, Stolyarov ID (2009) Characteristics of neurological and cognitive status in patients with multiple sclerosis in relation to the location and volumes of demyelination foci and the severity of brain atrophy. Neurosci Behav Physiol 39(1):35–38. doi:10.1007/s11055-008-9086-2
Sastre-Garriga J, Ingle GT, Chard DT, Cercignani M, Ramio-Torrenta L, Miller DH, Thompson AJ (2005) Grey and white matter volume changes in early primary progressive multiple sclerosis: a longitudinal study. Brain 128(Pt 6):1454–1460. doi:10.1093/brain/awh498
Sastre-Garriga J, Ingle GT, Chard DT, Ramio-Torrenta L, Miller DH, Thompson AJ (2004) Grey and white matter atrophy in early clinical stages of primary progressive multiple sclerosis. Neuroimage 22(1):353–359. doi:10.1016/j.neuroimage.2004.02.008
Shiee N, Bazin PL, Zackowski KM, Farrell SK, Harrison DM, Newsome SD, Ratchford JN, Caffo BS, Calabresi PA, Pham DL, Reich DS (2012) Revisiting brain atrophy and its relationship to disability in multiple sclerosis. PLoS One 7(5):e37049. doi:10.1371/journal.pone.0037049
Lazeron RH, Boringa J, Schouten M, Uitdehaag BM, Bergers E, Lindeboom J, Eikelenboom M, Scheltens P, Barkhof F, Polman C (2005) Brain atrophy and lesion load as explaining parameters for cognitive impairment in multiple sclerosis. Mult Scler 11(5):524–531
Batista S, Zivadinov R, Hoogs M, Bergsland N, Heininen-Brown M, Dwyer MG, Weinstock-Guttman B, Benedict RH (2012) Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 259(1):139–146
Amato MP, Portaccio E, Goretti B, Zipoli V, Battaglini M, Bartolozzi ML, Stromillo ML, Guidi L, Siracusa G, Sorbi S (2007) Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol 64(8):1157–1161
Amato MP, Razzolini L, Goretti B, Stromillo ML, Rossi F, Giorgio A, Hakiki B, Giannini M, Pastò L, Portaccio E (2013) Cognitive reserve and cortical atrophy in multiple sclerosis: a longitudinal study. Neurology 80(19):1728–1733
Amato M, Bartolozzi M, Zipoli V, Portaccio E, Mortilla M, Guidi L, Siracusa G, Sorbi S, Federico A, De Stefano N (2004) Neocortical volume decrease in relapsing–remitting MS patients with mild cognitive impairment. Neurology 63(1):89–93
Amato M, Hakiki B, Goretti B, Rossi F, Stromillo M, Giorgio A, Roscio M, Ghezzi A, Guidi L, Bartolozzi M (2012) Association of MRI metrics and cognitive impairment in radiologically isolated syndromes. Neurology 78(5):309–314
Deloire M, Ruet A, Hamel D, Bonnet M, Brochet B (2010) Early cognitive impairment in multiple sclerosis predicts disability outcome several years later. Mult Scler 16(5):581–587. doi:10.1177/1352458510362819
Langdon DW, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, Hamalainen P, Hartung HP, Krupp L, Penner IK, Reder AT, Benedict RH (2012) Recommendations for a brief international cognitive assessment for multiple sclerosis (BICAMS). Mult Scler 18(6):891–898. doi:10.1177/1352458511431076
Akbar N, Honarmand K, Kou N, Feinstein A (2011) Validity of a computerized version of the symbol digit modalities test in multiple sclerosis. J Neurol 258(3):373–379. doi:10.1007/s00415-010-5760-8
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis an expanded disability status scale (EDSS). Neurology 33(11):1444
Lee MA, Smith S, Palace J, Matthews PM (1998) Defining multiple sclerosis disease activity using MRI T2-weighted difference imaging. Brain 121(Pt 11):2095–2102
Tauhid S, Chu R, Sasane R, Glanz B, Neema M, Miller J, JKim G, Signorivich J, Healy B, Chitnis T, Weiner H, Bakshi R (2014) Brain MRI lesions and atrophy are associated with employment status in patients with multiple sclerosis. Paper presented at the ACTRIMS-ECTRIMS, Boston, MA
Li DK, Held U, Petkau J, Daumer M, Barkhof F, Fazekas F, Frank JA, Kappos L, Miller DH, Simon JH, Wolinsky JS, Filippi M, Sylvia Lawry Centre for MSR (2006) MRI T2 lesion burden in multiple sclerosis: a plateauing relationship with clinical disability. Neurology 66(9):1384–1389. doi:10.1212/01.wnl.0000210506.00078.5c
Sormani MP, Arnold DL, De Stefano N (2014) Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol 75(1):43–49
Calabrese M, De Stefano N, Atzori M, Bernardi V, Mattisi I, Barachino L, Morra A, Rinaldi L, Romualdi C, Perini P, Battistin L, Gallo P (2007) Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol 64(10):1416–1422. doi:10.1001/archneur.64.10.1416
Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH (2002) Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain 125(Pt 2):327–337
Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Bruck W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM (2011) Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 365(23):2188–2197. doi:10.1056/NEJMoa1100648
Frohman E, Havrdova E, Lublin F, Barkhof F, Achiron A, Sharief M, Stuve O, Racke M, Steinman L, Weiner H (2006) Most patients with multiple sclerosis or a clinically isolated demyelinating syndrome should be treated at the time of diagnosis. Arch Neurol 63(4):614–619
Rudick R, Fisher E, Lee J-C, Simon J, Jacobs L (1999) Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Neurology 53(8):1698
Simon J, Li D, Traboulsee A, Coyle P, Arnold D, Barkhof F, Frank J, Grossman R, Paty D, Radue E (2006) Standardized MR imaging protocol for multiple sclerosis: consortium of MS Centers consensus guidelines. Am J Neuroradiol 27(2):455–461
Sumowski JF, Rocca MA, Leavitt VM, Riccitelli G, Comi G, DeLuca J, Filippi M (2013) Brain reserve and cognitive reserve in multiple sclerosis: what you’ve got and how you use it. Neurology 80(24):2186–2193. doi:10.1212/WNL.0b013e318296e98b
Losseff N, Wang L, Lai H, Yoo D, Gawne-Cain M, McDonald W, Miller D, Thompson A (1996) Progressive cerebral atrophy in multiple sclerosis: a serial MRI study. Brain J Neurol 119(6):2009–2019
Fisher E, Rudick R, Cutter G, Baier M, Miller D, Weinstock-Guttman B, Mass M, Dougherty D, Simonian N (2000) Relationship between brain atrophy and disability: an 8-year follow-up study of multiple sclerosis patients. Mult Scler 6(6):373–377
Acknowledgments
Lynn Huynh, Philip Galebach, and Caroline Kelley contributed to the acquisition, analysis, and interpretation of the data, manuscript development, and approval of the final submitted version. James Signorovitch, Rahul Sasane, Allitia DiBernardo, and Timothy Vollmer conceived and planned the work that led to the manuscript, provided substantive suggestions for revisions, and approved the final submitted version. Dr. Vollmer has received consulting fees, and his institution has received a grant and consulting fee for his participation in the BRAVO study; is the medical director for the Rocky Mountain MS Center; he or his institution has received consultancy fees from Biogen-Idec, Teva, Hoffman-LaRoche, Accelerated Cure Project, Genzyme, Acorda, Novartis, Questor, Medscape, Xenoport, and Sanofi; his institution received fees/Grants from Teva, Biogen Idec, Genzyme, Ono, Eli Lilly, Novartis, BioMS, Orasi, Sanofi-Aventis, NIH, EMD Sorono, Acorda, Accelerated Cure Project, Hoffmann-LaRoche, Jensen Research, Janssen Pharmaceutical, MedImmune, Delta Quest, and Roche/Genentech. He is a co-holder of a patent with Teva Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Lynn Huynh, Caroline Kelley, Phil Galebach, and James Signorovitch are employees of Analysis Group, Inc., a consulting company that has received research Grants from Novartis Pharmaceuticals Corporation. Rahul Sasane is an employee of Novartis Pharmaceuticals Corporation. Allitia DiBernardo was an employee of Novartis Pharmaceuticals Corporation at the time the study was conducted. Financial support for this study was provided by Novartis Pharmaceuticals Corporation.
Rights and permissions
About this article
Cite this article
Vollmer, T., Huynh, L., Kelley, C. et al. Relationship between brain volume loss and cognitive outcomes among patients with multiple sclerosis: a systematic literature review. Neurol Sci 37, 165–179 (2016). https://doi.org/10.1007/s10072-015-2400-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2400-1