Abstract
Animals can adapt their reward expectancy to changes in delays to reward availability. When temporal relations are altered, associative models of interval timing predict that the original time memory is lost due to the updating of the underlying associative weights, whereas the representational models render the preservation of the original time memory (as previously demonstrated in the extinction of conditioned fear). The current study presents the critical test of these theoretical accounts by training mice with two different intervals in a consecutive fashion (short → long or long → short) and then testing timing behaviors during extinction where neither temporal relation is in effect. Mice that were trained with the long interval first clustered their anticipatory responses around the average of two intervals (indirect higher-order manifestation of two memories in the form of temporal averaging), whereas mice trained with the short interval first clustered their responses either around the short or long interval (direct manifestation of memory representations by their independent indexing). We assert that the original memory representation formed during training with the long interval “metrically affords” the integration of subsequent experiences with a shorter interval, allowing their co-activation during extinction. The original memory representation formed during training with the short interval would not metrically afford such integration and thus result in the formation of a new (mutually exclusive) time memory representation, which does not afford their co-activation during extinction. Our results provide strong support for the representational account of interval timing. We provide a new theoretical account of these findings based on the “metric affordances” of the original memory representation formed during training with the original intervals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Surfacing of Latent Time Memories: Support for the Representational Basis of Timing Behavior
The experienced temporal relations between event occurrences robustly shape animal behavior. For instance, animals learn to cluster their reward or aversive-stimulus-related anticipatory responses around experienced delays to such biologically critical occurrences (Buhusi and Meck 2005). Furthermore, these behaviors are time-adaptive in that when the temporal relations change (e.g., outcome delay becoming shorter or longer than the original delay), the timing of anticipatory responses adapts to the new temporal contingency (e.g., Coleman and Gormezano 1971; Matell et al. 2016). These adaptations can even result from higher-order temporal inferences in rodents (Barnet and Miller 1996; De Corte et al. 2018). Such adaptation can be manifested gradually (Bush and Mosteller 1953; Pavlov 1927; Rescorla and Wagner 1972; Sutton 1988), abruptly (Balcı et al. 2009; Simen et al. 2011; including supplementary material), immediately (Miller and Barnet 1993; Simen et al. 2011), or over multiple steps (Meck et al. 1984).
To this end, two theoretical approaches can be considered. One of these accounts relies on association formation between time-dependent states and responses. A prime example of this approach is the Learning to Time model (LeT, Machado 1997; Machado et al. 2009). LeT assumes states with time-dependent activation dynamics (akin to the domino effect). When a response leads to a reward, the associations between the active states and the response are strengthened (maximal associative update occurs for the most active state - akin to Hebbian learning). When the interval is altered, the association between new states and response is strengthened, and the original associations are weakened (now based on their lower activation level at the new time of reward delivery). This property gives an adaptive power to the underlying timing process but at the expense of erasing the original temporal information (unless an entirely new network is trained for the new interval).
An alternative theoretical approach asserts that as a result of such time-dependent experiences, the agent registers a temporal representation in its memory system (representational approach: Gür, Duyan & Balcı, 2018). A prime example of this approach is the Scalar Timing Theory (STT; Gibbon et al. 1984; Gibbon & Gallistel, 2001), where the outputs of the clock stage are registered at working memory (i.e., accumulator) and then the long-term memory. The functional architecture of this model enables the formation of different time memory representations for different epochs or experiences. Thus, this approach is also inherently capable of adapting to changes in temporal experiences. The main difference between these two theoretical approaches is that the former does not preserve the original temporal information, whereas the latter can enable the preservation of the original information (although this property has not been explicitly defined and integrated into STT).
The different predictions of these theoretical approaches deem the question of “What happens to the memory representation of the original temporal relationship when the temporal statistics of the environment change?” theoretically critical. Is the original memory representation of time updated by the new temporal relationship (akin to using the “save” function to update the content of a text file), or does the animal end up with a new (additional) memory representation for the new temporal relationship (akin to using the “save as” function generating two different files with different indices)?
Although they did not focus on interval timing per se, a relevant indication comes from decades-long research on extinction learning that suggests that the animal ends up with two different representations, both of which can be manifested differentially depending on the task conditions (e.g., spontaneous recovery following extinction learning, context dependency of fear response). We investigated this research question in the context of interval timing behavior by training mice successively on two different time schedules and then extinguishing conditioned responses to both schedules. If the memory representation for the original interval is conserved, one would expect reinstatement of anticipatory response around the original (initially trained) schedule or migration (central tendency) effect during extinction.
The adaptation of timing behavior to changing temporal relations has been investigated in earlier research. In an early investigation of this question, Meck et al. (1984) trained rats in the peak interval procedure with one criterion duration and then altered the criterion duration in the next phase of training (i.e., 10s to 20s vs. 20s to 10s) to characterize the nature of behavioral adaptation between two intervals. After suddenly updating the criterion duration, they observed that rats adapted to the new schedule by aiming at the geometric mean of the two criterion durations and then at the second criterion duration. These results suggest that rats either had two separate time memory representations and made an online computation to determine the target interval when the task conditions posed ambiguity or they updated the value of the same memory representation in two steps. However, this study did not run the critical test regarding the fate of temporal information by introducing a high level of ambiguity as in the case of extinction training that does not produce any differential reinforcement of behaviors. Different from this relevant earlier work, we tested the timing behavior of mice during extinction following sequential training with two intervals. Our reasoning is that the high ambiguity introduced by extinction phase would set the occasion for the manifestation of temporal memories and reflect their full complexity.
The notion that temporal information might be preserved during extinction comes from an independent set of studies on interval timing. To this end, one of the earlier studies that investigated the extinction of timed responding in the Pavlovian variant of the peak interval responding in goldfish (in response to aversive stimulus) suggested that the content of the temporal memory was preserved since the extinction decreased the amplitude of responding without altering its temporal features (Drew et al. 2005). Another example from Ohyama and colleagues (1999) demonstrated that the pecking response of ring doves in the extinction phase peaked nearly at the same time point in the acquisition phase, with a reduced amplitude (Experiment 3). Similarly, Guilhardi and colleagues (2006a) demonstrated similar temporal features for the response peaks during extinction and the acquisition phase (for similar effects see also Guilhardi and Church 2006b; Experiment 3 of Galtress and Kirkpatrick 2009; Delamater et al. 2018; Neuringer et al. 2001). Together, these results suggest that the temporal features of the acquired responses are preserved through the extinction training (but see Roberts 1981 where leftward shift is observed in extinction), which supports the idea that the temporal representations of the acquired responses are not erased as a result of extinction training.
Siegel and colleagues (2015) tested mice in a trace fear conditioning paradigm. In the training phase, trials started with a 200-ms baseline interval followed by 50-ms blue light (CS). Following 250-ms, 350-ms, or 450-ms long stimulus-free (trace) interval (varied between groups), a 20-ms air puff was presented as the US. A break period (7–14 days) separated the training and test phases, following which mice were tested in the extinction phase for three days, where only the CS was presented. Although the response rate was drastically lower, the CR timing of mice matched the stimulus-free interval that was experienced in the training phase. Balsam et al. (2002) argue for a similar invariance of temporal characteristics of the anticipatory responding during the initial acquisition of temporal relationships (see also Balcı et al. 2009).
One of the studies that is most directly related to our research question by showing the preservation of multiple time memory representations after the temporal statistics are altered was conducted using eye-blink conditioning in rabbits (Ohyama and Mauk 2001). The rabbits were initially trained with a 700-ms delay between the conditioned stimulus and unconditioned stimulus, but this training with the initial interval was terminated before the conditioned eye-blink response emerged, and the inter-stimulus interval was decreased to 200-ms. The training with the second interval continued until the rabbits’ emitted eye-blink conditioning. Finally, when rabbits were tested with a much longer conditioned stimulus (i.e., 1250-ms), they eye-blinked twice, once at the updated inter-stimulus interval (i.e., 200-ms) and once at the original inter-stimulus interval (i.e., 700-ms). This result suggests that the altered temporal relations did not result in the erasure of the original memory content.
None of the studies outlined above have addressed the fate of the original memory representation of time intervals following a change in the temporal statistics of the experience. Extinction learning (after training with a second schedule) allows one to study the state of the original memory representations. Suppose the ambiguity posed by the extinction experience sets the occasion for the manifestation of alternative (e.g., previously learned) temporal relations. In that case, the resultant timing behavior of the mice should contain a marker of those memories. To this end, we trained mice on one criterion duration and then replaced the original criterion duration with another criterion duration (15s → 30s–30s → 15s between subjects). The two-phase training was followed by an extinction phase, during which the reward was omitted. We predicted two potential outcomes if the original time memory is retained: (a) Over the course of extinction, mice should start to respond around the original criterion time or (b) in between the original and new criterion times (similar to migration or temporal averaging effect; De Corte and Matell 2016aa; for a detailed discussion see De Corte and Matell 2016b). We also expect an asymmetry between those cases when the criterion duration was increased vs. decreased due to the containment of the original metric relation by the memory content in the latter but not the former condition. This prediction is derived from the affordances of representational approaches in general and the variant of the time-adaptive opponent-Poisson drift-diffusion model (TopDDM) of timing (Balcı & Simen, 2016; Simen et al. 2011; 2013) developed by De Corte (2022). This variant of TopDDM enables the co-activation of different cue units (that determine the temporal accumulation rate) in cases of ambiguity (see Discussion for details). The same prediction can also be derived from a meta-theoretical perspective that assumes Bayesian integration of multiple sources of temporal information in case of ambiguity (for discussion, see De Corte and Matell 2016a, b, c). Alternatively, if the memory content is updated with the new criterion interval, the timed responses are expected to retain the same temporal characteristics as the second phase of the training phase (with the new criterion duration).
Methods
Subjects
Eighteen male C57BL6 mice bred in Koç University Animal Research Facility were used in the experiment. Seventeen animals were used for the formal analyses (one discarded since it did not learn the criterion duration). All subjects were experimentally naive. Mice were divided into two experimental groups (“long first” group: 30 s; “short first” group: 15 s). Mice were approximately 8–10 weeks old at the onset of the first experimental session. Animals were group housed (4 mice per cage) in 12-hour dark/light cycle. Experimental sessions took place in the light period and lasted one hour. Deprivation started three days before the first experimental session and aimed to keep the animals’ weight at 85–90% of their ad-lib weight. Water was not restricted.
Apparatus
Subjects were tested in conventional operant chambers (21.6 cm × 17.8 cm × 12.7 cm, ENV-307 W; Med Associates). Each operant chamber was contained by a sound-attenuated cubicle (40 cm × 69 cm × 41.5 cm). One of the two metal walls of the chamber had two retractable levers placed at the extreme ends of the wall and a central hopper between the two levers. The opposite wall contained three food hoppers equipped with an IR-beam break detector and a house light. Throughout all experimental phases, 0.01 mL of liquid food was delivered as a reward from only the central food hopper. The liquid food was prepared with a 1:1 ratio of Coffee Mate powder coffee creamer and tap water mix.
Procedure
Lever Press Training (FR1FT60 Phase)
The FR1-FT60 phase was applied to familiarize mice with the lever press and magazine. At the beginning of each trial, one of the levers was inserted along with the illuminated house light to signal the beginning of the trial. The lever was freely available for the subject for 60 s. If the mouse pressed the lever within 60 s or after 60 s of no lever press, the lever was retracted, and the house light was turned off. The central food hopper was illuminated and activated for 6 s (0.01 mL of liquid food). The active lever was counterbalanced across mice. Each session lasted 60 min. Each animal was tested in FR1FT60 sessions for three consecutive days and proceeded to FR1 phase.
Lever Press Training (FR1)
The procedure was the same as in FR1FT60, except the reward was delivered only after the lever press. The lever was available until it was pressed. The FR1 phase continued until mice received 40 or more rewards in two consecutive sessions.
FI-PI, training for the target duration
The FI-PI training sessions consisted of two trial types. The first response after the target duration had elapsed was reinforced in the FI trials. The reinforcement was omitted in the PI trials, which were three times longer than the target duration. The FI trials were two times more frequent than the PI trials. The order of trial types was randomized.
Mice were trained in the first target duration for at least 16 sessions (first duration), after which the target duration was changed (second duration). Mice were trained in the second target duration for at least eight sessions. The order of the target duration training was counterbalanced across mice. The intertrial interval (ITI) was a random value drawn from an exponential distribution with a mean that is equivalent to the target duration plus the target duration (left truncated exponential distribution).
Extinction
During extinction, the FI trials were eliminated for three sessions.
Analytical Approach
Peak Location
We calculated the peak location for the last three sessions of each phase (i.e., PI15, PI30, extinction). The peak location refers to the global maxima of the response rate. We used the peak location to index the duration where the subjects expected the reward. We used Matlab “findpeaks” function in the Signal Processing Toolbox for peak location calculation. Data were analyzed in Jamovi (version 2.4.11; The jamovi project 2023; R Core Team 2022).
Single Trial Start-Stop Analysis
We also estimated the start and stop times in individual trials for the last three sessions of each phase where the start time refers to the first time point at which the response rate abruptly switches to an up-state and the stop time refers to the time point at which the response rate abruptly returns of the down state (Balcı and Freestone 2020; Gibbon & Church, 1990; Gibbon et al., 1984). Our search algorithm also included a second start time, which is commonly observed in responding during the peak interval trials. Accordingly, the start and stop times can capture when the subject starts anticipating reward delivery and terminates its reward anticipation when the reward is omitted in individual trials.
We compared start, stop and middle times of the subjects across the experimental groups for first and second target durations as well as the extinction phase by linear mixed-effects model. For each dependent variable, we ran separate linear mixed effects models with the following structure:
where DV is either start, stop or middle times (mean of start and stop times); experimental group and phase are the fixed effects and “1|subject” stands for “random intercept across subjects”. We used the Restricted Maximum Likelihood (REML) method for parameter estimations. For the sake of simplicity in the reporting, we only present the results for the most relevant DV, namely the middle times, in the main manuscript. The results for the start and stop times and spread values can be found in the Supplementary Online Materials (SOM).
Results
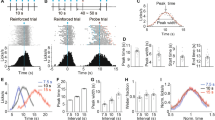
Figure 1 shows the normalized response curves for individual mice categorized as those that learned first the short criterion and then the long criterion and vice versa. Visual inspection of the response curves shows that the location of the peak response curve changed during the extinction phase, and the amplitude of response curves clustered at a point between short and long criterion intervals.
Peak Location Comparison
We compared the extinction peak locations to the peak locations in short and long FI-PI phases across training groups. Mixed ANOVA revealed only the main effect of different phases on peak location (Greenhouse-Geiser F(1.20, 18.01) = 17.72, p < 0.001, partial η2 = 0.54). Post hoc comparisons revealed that short-duration peak locations were significantly lower than the long-duration (t(15) = -11.68, p < 0.001; Mshort = 11.7, SDshort = 3.06; Mlong = 25.8, SDlong = 4.67), which points that the two target durations were well distinguished by mice. The difference between extinction and short-duration peak locations was also statistically significant (t(15) = -2.29, p = 0.037; Mextinction = 17.3 SDextinction = 9.04), pointing to a shorter peak location for the short criterion duration. The peak locations for extinction were also significantly shorter than long duration (t(15) = 2.74, ptukey = 0.038; Mextinction = 17.3, SDextinction = 9.04; Mlong = 25.8, SDlong duration = 4.67). However, this difference did not change across the two groups as there was no significant interaction between the training group and different phases (Greenhouse-Geisser p = 0.74; partial η2 = 0.0104). Together, the observed migration effect suggests that the representation of the original duration is maintained and factored into the temporal organization of the behaviour throughout the extinction phase regardless of the order of training with the target durations.
Figure 2 illustrates the peak locations for different phases across the training groups. An important observation is that when mice were first trained with the short interval and then with the long interval, their anticipatory responses during extinction either clustered around the short criterion or the long criterion (varied between mice - see Fig. 2, diamond markers during extinction). Thus, the migration effect in this group might be an artifact of these two patterns that differed between individual mice. This important observation is discussed in light of our predictions in the Discussion section.
Middle Times Comparison
The difference between the first duration and extinction in terms of the middle values was larger for the “long first” group (Mshort first − long first * first duration − extinction = -9.29, SE = 0.79, 95% CI = [-10.84 -7.73], p < 0.0001). On the other hand, the difference between the second duration and extinction was larger for the “short first” group (Mshort first − long first * second duration − extinction = 5.94, SE = 0.81, 95% CI = [-7.52 -4.35], p < 0.00001; estimated marginal differences: for “long first” group: Mlong dur − extinction = 3.747, SE = 0.60, 95% CI = [2.57 4.927], p < 0.00001; Mshort dur − extinction = -4.63, SE = 0.50, 95% CI = [-5.60 -3.66], p < 0.00001, by transitivity Mlong > Mextinction > Mshort; for “short first” group: Mshort dur − extinction = -5.54, SE = 0.52, 95% CI = [-6.55 -4.529], p < 0.00001; Mlong dur − extinction = 1.30, SE = 0.64, 95% CI = [0.05 2.55], p = 0.04; by transitivity Mlong > Mextinction > Mshort). Figure 3 illustrates the middle times across experimental groups and phases.
Note that the results gathered from the single trial analysis show the migration (temporal averaging) effect both for the short-first and long-first groups.
In the Supplemental Material, we present the distribution of each mouse’s start and stop times. The dip test of unimodality (using Holm-Bonferroni correction) showed that the start and stop times of only one mouse from the short-first (4–23) group were not unimodal, suggesting that the extinction distributions did not come from the mice using different target times on different trials (a mixture of short and long trials).
Discussion
Animals can adapt their timed responses to changes in the temporal relations in their environment. For instance, animals learn to wait longer before initiating responses when the delay to reward availability is increased (e.g., Matell et al. 2016). One of the fundamental questions that stems from the flexibility of interval timing behavior is the cognitive fate of the original predictive temporal relationship that was in effect before the change in environmental statistics. Associative models of interval timing, such as the Learning to Time model (Machado 1997) assert that the connections of the presumed associative network that underlies the temporal modulation of anticipatory responses are modulated such that previously updated associative weights between “time states” (corresponding to original interval) and outcome-related response are weakened while weights between new “time states” (corresponding to new interval) and response are strengthened. The functional outcome of such an associative approach is that the original memory (encoded via associative weights) is lost and is not recoverable. On the contrary, a representational approach to timing behavior affords the preservation of the original time memory and the formation of a second memory representation for the new temporal relationship, which results in dual time memory representations. Within this framework, the original time memory might become latent and simply lose control of anticipatory responses since it no longer predicts reward availability. However, within the framework, since the original time memory is preserved, it can regain control of anticipatory responses when ambiguity regarding the temporal relations is introduced (e.g., when the new temporal relation is not functional anymore, as in the case of extinction training).
To test these predictions, we trained mice in the peak interval procedure successively with two different temporal relations (15s → 30s and 30s → 15s) and then examined the temporal characteristics of anticipatory responses during extinction (during which neither the original nor the new temporal relation was in effect). If the original time memory is preserved (as predicted by the representational account), we expected its manifestation during extinction such that the new responses would be clustered either around the original time criterion (a direct manifestation of original memory) or migrate to the mean of the original and new time criteria (an indirect manifestation of original time memory). Ohyama and Mauk’s (2001) findings with the double timing of eye-blink responses after subsecutive training with two intervals support this prediction. In that study, rabbits were trained with a long interval but not enough for the emergence of the conditioned eyeblink response. When the conditioned eyeblink response emerged during the second training phase with a shorter interval, rabbits eye-blinked twice; once at the short and another time at the long interval. On the other hand, if the original time memory is lost (as predicted by the associative models), one would expect the recent temporal relation to continue guiding anticipatory responses despite their decreasing frequency. The latter behavioral pattern was observed in goldfish during extinction following training with a single interval (Drew et al. 2005).
Our findings strongly supported the predictions of the representational approach on multiple fronts. The anticipatory responses of the mice (particularly those trained first with the long interval) peaked around the middle points between the two-time criteria. Although the descriptive statistics also supported this pattern for peak times (estimated from average response curves) for the mice first trained with the short interval, a closer inspection of individual subjects’ peak times provided independent and more direct support for the representational approach. Specifically, as a result of this training procedure, the anticipatory responses of half of the mice clustered around the short interval, whereas the anticipatory responses of the other half of the mice clustered around the long interval.
In addition to support provided for the representational accounts, our results do not necessarily contradict the Pavlovian-like associative accounts that leaves room for renewal/new inhibitory learning (e.g., Pearce-Hall model; Pearce and Hall 1980). Still, it should be noted that our results contradict the accounts that rely mainly on the unlearning of the behavior during extinction training such as LeT (Machado 1997; Machado et al. 2009) and the classical Rescorla-Wagner model of associative learning (Rescorla and Wagner 1972), irrespective of whether they are associative accounts or not.
One existing account could explain the obtained results in the current study: De Corte (2021) proposed a model of temporal averaging (during compound stimuli each of which is associated with two different intervals - e.g., Swanton et al. 2009; for review see De Corte and Matell 2016a, b, c) based on the functional neural architecture of time-adaptive, opponent Poisson Drift Diffusion Model (TopDDM; Balcı & Simen, 2016; Simen et al. 2011; 2013). TopDDM assumes that the cue units send input to a second layer with recurrent excitation (tonic units), which in turn drives the ramping units (again with balanced recurrent excitation). The rate of temporal integration (i.e., the slope of ramping activity) is determined by the amount of net excitation received from the tonic layer (e.g., the number of tonic units excited by cue units). Different from the original model, De Corte (2021) assumed both excitatory and inhibitory connections between cue units and tonic units on compound responding. In other words, cue units excite several tonic units while inhibiting the others. As a result during the compound trials, some tonic units would receive excitatory input from one cue unit while inhibitory input from the other cue unit with the net effect of intermediary activation. This would result in a ramping slope between the original two values. The same model can also account for our regression to the mean findings. It is possible that our different training protocols result in the updating of synaptic connections between cue units and a group of tonic units (weakening in case of longer interval, activation of less tonic units, and thus lower ramping slope) or the synaptic connections between a group tonic units and ramping unit. In our case, the cue units can be replaced by time memory/memories.
A conceptual analysis-derived reason behind the differential manifestation of the original time memory in two different groups of mice (long → short vs. short → long) could be driven by the “metric affordances” of the time memory representation established during initial training. For the mice that were trained with the long interval first, the original time memory can serve as a representational template that can metrically afford the integration of the shorter interval into it. In other words, since the second experience is contained within the first criterion and due to the metric character of time, the new shorter time interval can be integrated into the metric template formed during the original training period (e.g., I(max) or I(30) for the long interval and I(15) for the short interval, where I is the initial template - Fig. 4 - top panel). Since both indices would be activated due to the activation of the common template I, extinction would result in equal weighting of two memory states when the experimental context does not support the predictive power of either memory representation. On the other hand, the functional representational template established during initial training with the short interval would not metrically afford the integration of the new longer time criterion into it (Fig. 4 - bottom panel). This could result in independent indexing of time memories after training with two intervals (i.e., I(max) or I(15) as a result of initial training and II(max) or II(30) as a result of second training). This would result in the indexing of either the original or the new memory to take control of the anticipatory behavior during extinction, predicting anticipatory responses to cluster either around the original or new time criterion. Under this rationale, the asymmetry in the temporal characteristics of anticipatory responses during extinction in two different groups of mice provides a meta-evidence for the representational account of the timing behavior. This theoretical framework is supported by the temporal rescaling property of neural representations of time such that the transition between different units underlying the progression of subjective time is altered depending on the interval between the predictor and the outcome (e.g., Mello et al. 2015; Wang et al. 2018; Xu et al. 2014).
Conceptual illustration of the metric affordances of time memory representations for mice trained first with the long and then short interval (top panel) vs. mice trained first with the short and then the long interval (bottom panel). The original memory representation in the first group of mice metrically affords the integration of the new temporal relation into its content. This is not the case for the second group of mice, which results in the establishment of a new memory representation due to experiencing the new temporal relation
There are differences between the two groups in terms of extinction profile expected in short-first vs. long-first groups. In the short-first group, when the time interval is elongated during second interval training, the response to the short interval is inadvertently extinguished. In terms of the neurobiological events, one would expect a negative prediction error to occur at the time of the short target interval (with the omission of reward). On the other hand, no extinction to long time interval would be expected to occur in second interval training for “long first” group and in terms of neurobiological events one would expect a positive prediction error to occur at the short target interval (because of an increase in the value of the predictor). These differential extinction responses and prediction error coding might be why temporal information is retained in one case and not in the other case.
In the context of fear extinction, Salinas-Hernandez et al. (2018) showed that positive reward prediction error is both necessary and sufficient for fear extinction learning; possibly signaling a change in the state of the world and thereby resulting in the opening of a new time file in short-first condition. When this prediction error was inhibited, no extinction occurred, while its enhancement enhanced extinction learning. When these results are evaluated in terms of the value of the cues, the reverse can be expected to occur in our experimental setting. Specifically, one would expect extinction learning to occur in the long-first condition and not in the short-first condition. The formation of a second memory in the long-first condition would set the occasion for the manifestation of both time memories and thus central tendency effect. In the case of no extinction learning, the new time interval would replace the old value in the same time file (akin to object file), resulting in the updating of the temporal control of anticipatory responses.
Earlier findings that showed immediate and/or abrupt adaptation to changing temporal relations already provided indirect support for the representational view (e.g., Guilhardi and Church 2005; Meck et al. 1984; Simen et al. 2011 - supplemental material; Wynne and Staddon 1988). For instance, Meck et al. (1984) showed that when the target interval is changed from one phase to another, rats adjusted their timing behaviors in two steps (first around the geometric mean of two intervals and then around the second interval). The migration of peak time to the middle of the two time criteria has also been previously observed in experimental setups in which two different intervals were associated with two different stimuli, and then the two stimuli were presented as a compound (e.g., Swanton et al. 2009; Delamater and Nicolas 2015, - see Gür et al. 2021; for similar results in the numerosity domain). These findings were interpreted in terms of the Bayesian integration of the two time memories (De Corte and Matell 2016a; Corte and Matell 2016c) in case of ambiguity resulting from the simultaneous presentation of both stimuli.
Our findings provide independent support for this behavioral phenomenon in a context where the temporal relations were part of the training history rather than being cue-dependent as in the case of the temporal averaging paradigm. Within the theoretical framework outlined above, stimulus-dependent temporal averaging can be accounted for by establishing two separate time memories due to their different stimulus correlates. In our experiments, establishing a single or two separate memory representations is determined purely by the metric features of training since there are no differential stimulus correlates of temporal relations. Combined with these other sources of evidence summarized above, our findings suggest that the cognitive architecture of the mice allows for multiple time representations, the indexing of which, depends on the metric properties of experiences.
One limitation of the current study is that the obtained data cannot directly reveal the baseline cognitive dynamics of how each trained duration was represented in the absence of second duration interference. While potentially an interesting issue to be addressed, this question does not directly fall under the scope of the current study’s main motivation: The current study aimed at investigating the fate of original temporal representations as a result of override by changing in the temporal relations in the environment. Nevertheless, to disentangle this, future studies should introduce additional conditions where animals are trained in a single duration prior to the extinction phase.
Data Availability
Data will be available upon request.
References
Balcı F, Freestone D (2020) The peak interval procedure in rodents: a tool for studying the neurobiological basis of interval timing and its alterations in models of human disease. Bio-protocol 10(17):e3735. https://doi.org/10.21769/BioProtoc.3735
Balcı F, Freestone D, Gallistel CR (2009) Risk assessment in man and mouse. Proceedings of the National Academy of Sciences, 106(7), 2459–2463
Balsam PD, Drew MR, Yang C (2002) Timing at the start of associative learning. Learn Motiv 33(1):141–155. https://doi.org/10.1006/lmot.2001.1104
Barnet RC, Miller RR (1996) Second-order excitation mediated by a backward conditioned inhibitor. J Exp Psychol Anim Behav Process 22:279–296. https://doi.org/10.1037/0097-7403.22.3.279
Buhusi CV, Meck WH (2005) What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6(10):755–765
Bush RR, Mosteller F (1953) A stochastic model with applications to learning. Annals Math Stat 24(4):559–585. https://doi.org/10.1214/aoms/1177728914
Coleman SR, Gormezano I (1971) Classical conditioning of the rabbit’s (Oryctolagus cuniculus) nictitating membrane response under symmetrical CS-US interval shifts. J Comp Physiological Psychol 77(3):447–455. https://doi.org/10.1037/h0031879
R Core Team (2022) R: A Language and environment for statistical computing. (Version 4.1) [Computer software]. Retrieved from https://cran.r-project.org. (R packages retrieved from CRAN snapshot 2023-04-07)
De Corte BJ (2021) What Are the Neural Mechanisms of Higher-Order Timing? Complex Behavior from Low-Level Circuits. Laurel Hollow, New York: ProQuest Dissertations Publishing. https://doi.org/10.17077/etd.006279
De Corte BJ, Matell MS (2016a) Temporal averaging across multiple response options: insight into the mechanisms underlying integration. Anim Cogn 19(2):329–342
De Corte BJ, Matell MS (2016b) Interval timing, temporal averaging, and cue integration. Curr Opin Behav Sci 8:60–66
De Corte BJ, Matell MS (2016c) Interval timing, temporal averaging, and cue integration. Curr Opin Behav Sci 8:60–66. https://doi.org/10.1016/j.cobeha.2016.02.004
De Corte BJ, Della Valle RR, Matell MS (2018) Recalibrating timing behavior via expected covariance between temporal cues. eLife 7:e38790
Delamater AR, Nicolas DM (2015) Temporal averaging across stimuli signaling the same or different reinforcing outcomes in the peak procedure. International journal of comparative psychology/ISCP; sponsored by the International Society for Comparative Psychology and the University of Calabria, p 28
Delamater AR, Chen B, Nasser H, Elayouby K (2018) Learning what to expect and when to expect it involves dissociable neural systems. Neurobiol Learn Mem 153:144–152
Drew MR, Zupan B, Cooke A, Couvillon PA, Balsam PD (2005) Temporal control of conditioned responding in Goldfish. J Exp Psychol Anim Behav Process 31(1):31–39. https://doi.org/10.1037/0097-7403.31.1.31
Galtress T, Kirkpatrick K (2009) Reward value effects on timing in the peak procedure. Learn Motiv 40(2):109–131
Guilhardi P, Church RM (2005) Dynamics of temporal discrimination. Learn Behav 33(4):399–416
Guilhardi P, Church RM (2006b) The pattern of responding after extensive extinction. Learn Behav 34(3):269–284
Guilhardi P, Yi L, Church RM (2006a) Effects of repeated acquisitions and extinctions on response rate and pattern. J Exp Psychol Anim Behav Process 32(3):322
Gür E, Duyan YA, Balcı F (2021) Numerical averaging in mice. Anim Cogn 24(3):497–510
Machado A (1997) Learning the temporal dynamics of behavior. Psychol Rev 104:241–265
Machado A, Malheiro MT, Erlhagen W (2009) Learning to Time: a perspective. J Exp Anal Behav 92(3):423–458. https://doi.org/10.1901/jeab.2009.92-423
Matell MS, De Corte BJ, Kerrigan T, DeLussey CM (2016) Temporal averaging in response to change. Timing Time Percept 4(3):223–247
Meck WH, Komeily-Zadeh FN, Church RM (1984) Two-step acquisition: modification of an internal clock’s criterion. J Exp Psychol Anim Behav Process 10(3):297
Mello GB, Soares S, Paton JJ (2015) A scalable population code for time in the striatum. Curr Biology: CB 25(9):1113–1122. https://doi.org/10.1016/j.cub.2015.02.036
Miller RR, Barnet RC (1993) The role of time in elementary associations. Curr Dir Psychol Sci 2(4):106–111. https://doi.org/10.1111/1467-8721.ep10772577
Neuringer A, Kornell N, Olufs M (2001) Stability and variability in extinction. J Exp Psychol Anim Behav Process 27(1):79
Ohyama T, Mauk MD (2001) Latent acquisition of timed responses in cerebellar cortex. J Neurosci 21(2):682–690
Ohyama T, Gibbon J, Deich JD, Balsam ED (1999) Temporal control during maintenance and extinction of conditioned keypecking in ring doves. Anim Learn Behav 27:89–98
Pavlov IP (1927) Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford University Press, Oxford
Pearce JM, Hall G (1980) A model for pavlovian learning: the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev 87:532–552
Rescorla RA, Wagner AR (1972) A theory of pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF (eds) Classical conditioning II. Appleton-Century-Crofts, New York, NY, pp 64–99
Salinas-Hernández XI, Vogel P, Betz S, Kalisch R, Sigurdsson T, Duvarci S (2018) Dopamine neurons drive fear extinction learning by signaling the omission of expected aversive outcomes. eLife, 7. e38818. https://doi.org/10.7554/eLife.38818
Siegel JJ, Taylor W, Gray R, Kalmbach B, Zemelman BV, Desai NS, Chitwood RA (2015) Trace Eyeblink conditioning in mice is dependent upon the dorsal medial prefrontal cortex, cerebellum, and amygdala: behavioral characterization and functional circuitry. ENeuro, 2(4)
Simen P, Balcı F, deSouza L, Cohen JD, Holmes P (2011) A model of interval timing by neural integration. J Neurosci https://doi.org/10.1523/JNEUROSCI.3121-10.2011
Sutton RS (1988) Learning to predict by the methods of temporal differences. Mach Learn 1:9–44. https://doi.org/10.1023/A:1022633531479
Swanton DN, Gooch CM, Matell MS (2009) Averaging of temporal memories by rats. J Exp Psychol Anim Behav Process 35:434–439. https://doi.org/10.1037/a0014021
The jamovi project (2023) jamovi. (Version 2.4) [Computer Software]. Retrieved from https://www.jamovi.org
Wang J, Narain D, Hosseini EA, Jazayeri M (2018) Flexible timing by temporal scaling of cortical responses. Nat Neurosci 21(1):102–110. https://doi.org/10.1038/s41593-017-0028-6
Wynne CDL, Staddon JER (1988) Typical delay determines waiting time on periodic-food schedules: static and dynamic tests. J Exp Anal Behav 50(2):197–210
Xu M, Zhang SY, Dan Y, Poo MM (2014) Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex. Proceedings of the National Academy of Sciences, 111(1), 480–485. https://doi.org/10.1073/pnas.1321314111
Acknowledgements
This study was supported by the Scientific and Technological Research Council of Turkey - TÜBİTAK (117K370) and NSERC Discovery (RGPIN-2021-03334) grants to FB. The authors would like to thank Merve Erdoğan and Ezgi Gür for their help during the pilot experiments.
Funding
This study was supported by the Scientific and Technological Research Council of Turkey - TÜBİTAK (117K370) and NSERC Discovery (RGPIN-2021-03334) grants to FB.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the conception and design of the study. TÖ performed material preparation, data collection, and analysis. TÖ and FB wrote the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors have no conflict of interest to declare.
Ethical Approval
All procedures described in this study were approved by the Koç University Animal Research Local Ethics Committee (ethical approval number: 2016.034).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Öztel, T., Balcı, F. Surfacing of Latent Time Memories Supports the Representational Basis of Timing Behavior in Mice. Anim Cogn 27, 57 (2024). https://doi.org/10.1007/s10071-024-01889-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10071-024-01889-z