Abstract
The selected strain, TAP041, showing an excellent ability to reduce the glyoxal and methylglyoxal levels, was identified by 16S rRNA gene-based phylogenetic analysis as Pediococcus pentosaceus. It demonstrated probiotic properties, including acid, bile salt, pancreatin, lysozyme tolerance, gut adhesion, and auto/coaggregation. In RAW264.7 macrophages, both live and heat-killed strains induced nitric oxide production and activated inducible nitric oxide synthase. RAW264.7 treated with P. pentosaceus TAP041 increased the expression level of tumor necrosis factor-α, interleukin (IL)-1β, IL-6, and cyclooxygenase-2, and regulated the expression of c-Jun amino-terminal kinase, p38, and extracellular signal-regulated kinase. These findings suggest that both live and heat-killed P. pentosaceus TAP041 can be used as potential immunostimulatory agents in functional food additives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
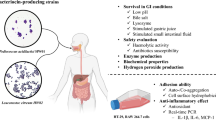
According to the Food and Agriculture Organization/World Health Organization (FAO/WHO, 2002), probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.” The guidelines presented by the FAO/WHO (2002) for in vitro testing of probiotic strains include: resistance to gastric acidity, bile acid resistance, adherence to mucus and/or human epithelial cells and cell lines, antimicrobial activity against potentially pathogenic bacteria, and the ability to reduce pathogen adhesion to surfaces. To function as probiotics in the human gastrointestinal tract, strains must survive extreme conditions, such as low acid, bile salt, and digestive enzymes (Cao et al., 2015; Cho et al., 2023; Pramanik et al., 2023).
Some strains of Lactobacillus and Bifidobacterium are known as probiotics (García-Ruiz et al., 2014). P. pentosaceus belongs to the family Lactobacillaceae and the genus Pediococcus, and is a homofermentative lactic acid bacterium (LAB) that is facultatively anaerobic, Gram-positive, and cocci-shaped. Many studies have confirmed that P. pentosaceus has potential probiotic effects, including cholesterol-lowering capacity, immune system enhancement, and prevention of cardiovascular diseases (Kwon et al., 2018; Shin et al., 2016; Zhang et al., 2022).
Glyoxal (GO) and methylglyoxal (MGO), reactive α-dicarbonyl compounds, are commonly found in food and are precursors of advanced glycation end products (AGEs). They are produced primarily during the Maillard reaction, lipid oxidation, and sugar degradation (Gaens et al., 2013). MGO accumulation has been shown to induce oxidative stress in rats and MC3T3-E1 cells, and continuous accumulation may lead to neurological disorders, such as Alzheimer’s and Parkinson's disease (Sena et al., 2012; Suh et al., 2014). Since GO and MGO are first encountered in the human gut, inhibition of their activity should occur primarily in the gut. The accumulation of glycated proteins strongly correlates with the progression of neurodegenerative diseases and diabetes complications; thus, developing a dietary agent aimed at attenuating glycated proteins is important (Lin et al., 2023).
This study aimed to isolate LAB with the ability to reduce the levels of GO and MGO, validate the probiotic attributes of the selected strain, and investigate their potential impacts on immune-related responses.
Materials and methods
Bacterial strains and culture media
To isolate the MGO-tolerant strains, 987 strains stored at the Korean Culture Collection of Probiotics (KCCP; Seongnam, Korea) were screened. Lactococcus lactis KF140 (KCCM 11673 1028P) was provided by the Korean Culture Center of Microorganisms (KCCM; Seoul, Korea). Lactobacillus rhamnosus GG ATCC 53103 was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). All LAB strains were cultured in de Man, Rogosa, and Sharpe broth (MRS; BD, Franklin Lakes, NJ, USA).
The pathogenic bacteria Salmonella Typhimurium ATCC 14028 and Escherichia coli ATCC 10536 were provided by ATCC, while Bacillus cereus KCCM 11204, Listeria monocytogenes KCCM 40307, Staphylococcus aureus subsp. aureus KCCM 40881, and Klebsiella pneumoniae subsp. pneumoniae KCCM 41433 were obtained from KCCM. B. cereus, S. Typhimurium, S. aureus subsp. aureus, and K. pneumoniae subsp. pneumoniae were cultured in nutrient broth (BD Biosciences), E. coli was cultured in tryptic soybean broth (BD Biosciences), and L. monocytogenes were cultured in brain heart infusion broth (BD Biosciences).
Screening of methylglyoxal-resistant lactic acid bacteria
The 987 LAB strains were cultured in 1 mL MRS broth at 37 ℃ for 20 h (seed culture) followed by inoculation at a concentration of 1% (v/v) into MRS broth added with 5 mM MGO (Sigma-Aldrich, St. Louis, MO, USA). To monitor the growth of the LAB strain selected from the screening, the seed culture was inoculated at a concentration of 2% into MRS broth containing four different concentrations of MGO (0, 0.5, 1.0, and 2.0 mM). Subsequently, these cultures were incubated at 37 ℃ for 7 h. Cell growth was measured every hour at an absorbance wavelength of 600 nm using an Epoch microplate reader (BioTek Instruments Inc., Winooski, VT, USA).
Quantitative measurement of glyoxal and methylglyoxal by high-performance liquid chromatography
The quantitative evaluation of GO and MGO was performed using a modified method of Uğur et al. (2022). The LAB seed culture was inoculated at 1% (v/v) into MRS broth supplement with 200 μg/mL GO and 720 μg/mL MGO, respectively, followed by incubation at 37 ℃ for 48 h. The culture was centrifuged at 14,000 g for 5 min, and the initial incubation time (0 h) served as a control. The supernatant (0.5 mL) was taken into a 5 mL screw tube, to which 1 mL of sodium acetate buffer (0.1 M; pH 3.6) and 0.5 mL of derivatization solution (4-nitro-1,2-phenylenediamine in 1% methanol) were sequentially added. The mixture was incubated at 70 ℃ for 20 min and was filtered through a 0.22 μm polyvinylidene fluoride filter (SMARTiLAB, Seoul, Korea).
High-performance liquid chromatography (HPLC) analysis was performed using a DIONEX UltiMate 3000 HPLC system (Thermo Fisher Scientific Inc., Waltham, MA, USA). A C-18 column (Model number: TC-C18(2), 250 × 4.6 mm, ID 5 μm; Agilent Technologies, Inc., Santa Clara, CA, USA) was used while detection occurred via an ultraviolet detector to wavelength of 255 nm. The column oven temperature was maintained at 30 ℃ while the sample injection volume used was 20 μL. The mobile phase was a methanol:water:acetonitrile mixture with a ratio of 42:56:2 (v/v/v), and the samples were eluted isocratically at a flow rate of 1 mL/min.
Identification of lactic acid bacteria
16S rRNA gene sequencing and phylogenetic tree
The selected strain was cultured in MRS broth, and genomic DNA was extracted using the AccuPrep Genomic DNA Extraction Kit (Bioneer, Seoul, Korea). The 16S rRNA sequence was amplified using the universal primers 27F and 1492R in a TurboCycler 2 Thermal Cycler (BLUE-RAY Biotech, New Taipei City, Taiwan). The polymerase chain reaction (PCR) conditions involved pre-denaturation at 94 ℃ for 2 min and 35 cycles of denaturation at 94 ℃ for 30 s, annealing at 50 ℃ for 1 min, and extension at 72 ℃ for 1 min. The post-extension step was conducted at 72 ℃ for 7 min. Phylogenetic analyses were performed as described by Lee et al. (2022).
Scanning electron microscopy
The cellular morphology of the strain was examined using a H-7600 scanning electron microscope (SEM; Hitachi, Tokyo, Japan) located at Eulji University (Seongnam, Korea). This process was performed as described by Lee et al. (2022).
The carbohydrates fermentation profiling of lactic acid bacteria
The colonies were suspended to reach a concentration of 0.5 McFarland (0.05 mL of 1.175% barium chloride dihydrate with 9.95 mL of 1% sulfuric acid) (cell density at 1.5 × 108 CFU/mL). This suspension was inoculated into API 50 CHL medium (BioMérieux, I’ Etole, France) and dropped onto API 50 CH strips. These strips were incubated at 37℃ for 48 h, and carbohydrate utilization was interpreted using APIweb (http://apiweb.biomerieux.com).
Tolerance of selected strain to acid, bile salt, pancreatin, and lysozyme
To determine acid tolerance, the seed culture of the strain was inoculated into MRS broth at a 1% (v/v) concentration and allowed to grow at 37 ℃ for 16 h. The culture was centrifuged at 16,000 × g for 1 min and then washed thrice with 0.88% (w/v) NaCl. The pellets were resuspended in 4 mL glycine–HCl buffer (0.1 M, pH 2.5), with this cell resuspension incubated at 37 ℃ for 2 h without stirring. One hundred microliters of decimal dilutions were spread onto an MRS agar plate and incubated at 37 ℃ for 20 h. Viable cells were quantified as colony-forming units per mL (CFU/mL). Acid tolerance was calculated using the following equation:
To determine bile salt and pancreatin tolerance, the seed culture was inoculated at a concentration of 1% (v/v) to MRS supplemented with 0.3% (w/v) oxgall (BD) or 0.5% (w/v) pancreatin (Sigma-Aldrich), respectively, and followed by incubation at 37 ℃ for 24 h. Viable cell counts were determined similarly to the acid tolerance test, and bile salt or pancreatin tolerance was calculated as follows:
The lysozyme tolerance of the strain was examined using a modified method of Tarique et al. (2022). The seed culture of the strain was inoculated into lysozyme solution (0.22 g CaCl2, 6.2 g NaCl, 2.2 g KCl, 1.2 g NaHCO3, and 0.1 g lysozyme (Sigma-Aldrich), per liter) to achieve a concentration of 10% (v/v). After incubation of the bacterial suspensions at 37 °C for 90 min, decimal dilutions of the bacterial suspensions were performed as described above. Lysozyme tolerance was calculated using the following equation:
Adherent ability to Caco-2 cells
Caco-2 cells were grown at a density of 5 × 105 cells/well and cultured until 80–90% covered surface areas at 37 °C with 5% CO2. Caco-2 cells were then exposed to bacterial cells at a multiplicity of infection (MOI; the ratio of the number of LAB to the number of macrophage cells) of 1000 (5 × 108 CFU) in each well, followed by incubation at 37 °C for 2 h. Post-incubation, any non-adherent bacteria on the cell surface were rinsed off twice using Dulbecco’s phosphate buffered saline (DPBS; Welgene Inc., Gyeongsan, Korea) and detached from plates using a solution of 0.25% trypsin–EDTA. To release the adherent bacterial cells, 0.1% (v/v) Triton X-100 diluted in DPBS was added to the cells. Finally, the suspension was spread onto MRS agar plate, which was subsequently incubated at 37 °C for 20 h. A sample without Caco-2 cells in a microcentrifuge tube served as a control. The adhesion ability was determined as the percentage ratio between viable cell numbers in the sample (log CFU/mL) and those in the control (log CFU/mL).
Auto-/coaggregation
The auto- and coaggregation capacities of the strains were measured using the method described by Ohn et al. (2020). Pellets of cultured LAB and pathogenic bacteria were washed twice with phosphate-buffered saline (PBS), and the optical density (OD600) was adjusted to 0.3 at PBS in a cuvette. The OD600 was measured at 0, 2, 5, 10, 24, 36, and 48 h at 37 °C using a spectrophotometer (BioSpec-mini; Shimadzu Co., Kyoto, Japan).
The coaggregation assay was performed using the same method described for the auto-aggregation assay. The OD600 of a mixture of LAB and pathogenic bacteria at a ratio of 1:1 was measured at 0, 2, 5, 8, 12, 24, 36, and 48 h at 37 ℃. Auto-/co-aggregation was calculated as described by Tarique et al. (2022):
where “Atime” is the absorbance at the different incubation times and “Ainitial” is the absorbance at the initial time.
Immunostimulating activity
Cell culture and lactic acid bacteria preparation
RAW264.7 macrophage cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin (Gibco) and incubated at 37 ℃ with 5% CO2 atmosphere. The seed culture of LAB was inoculated into MRS broth at a concentration of 1% (v/v) and cultivated at 37 °C until it reached a density of 5 × 108 CFU/mL. This culture was then centrifuged at 16,000 × g, 4 ℃ for 1 min, and the collected cells were rinsed twice with DPBS to prepare the live LAB sample. These harvested cells were heat-killed at 121 °C for 15 min to prepare the heat-killed LAB sample used in subsequent experiments.
Cell viability
The RAW264.7 macrophages were seeded into a 96-well plate at a density of 5 × 104 cells/well and incubated at 37 ℃ for 20 h. These cells were exposed to both live and heat-killed P. pentosaceus TAP041 at MOI 62.5–1000. Following a 24 h incubation at 37 ℃, the supernatant was removed, and the plate was washed twice with DPBS. The plate was incubated to DMEM along with the addition of EZ-cytox (DoGenBio Co., Ltd., Seoul, Korea) for 40 min. The absorbance of each well was measured at 450 nm using a microplate reader, and cell viability was calculated.
Nitric oxide assay
RAW264.7 cells were seeded into a 24-well plate at a density of 5 × 105 cells/well and incubated at 37 ℃ for 20 h. The RAW264.7 cells were treated with live and heat-killed LAB and 1 μg/mL of lipopolysaccharides (LPS; Sigma-Aldrich) as a positive control. After incubation for 24 h, NO levels were determined using the Griess reagent. The supernatants were collected into a new tube, and 100 μL supernatant was transferred to a 96-well plate. Griess reagents A (0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in water) and B (1% sulphanilamide in 2.5% phosphoric acid) were added to be equivalent to supernatants. The produced nitric oxide was detected at 540 nm using a microplate reader, and the 0–250 μM sodium nitrite (Samchun chemicals, Seoul, Korea) was used to generate a standard curve.
Real-time quantitative polymerase chain reaction
The mRNA expression level of inducible NO synthase (iNOS), tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β, and cyclooxygenase (COX)-2 on the cells was evaluated by real-time quantitative PCR (RT-qPCR). The seeded RAW264.7 cells in a 6-well plate at 1 × 106 cells/well cultured for 20 h, and live LAB (MOI 250), heat-killed LAB (MOI 500), and LPS (1 μg/mL) were treated each for 24 h. After washing the cells twice with DPBS, total RNA was isolated using an Easy-BLUE Total RNA extraction kit (iNtRON Biotechnology Inc., Seongnam, Korea). The isolated total RNA was quantified by nanodrop (Epoch microplate reader, BioTek Instruments Inc.), and 1 μg RNA was used to prepare cDNA. Template DNA was prepared using CellScript™ cDNA master mix (CellSafe, Suwon, Korea), and RT-qPCR analysis was performed with QuatStudio™ 1 Real-Time PCR system (Thermo Fisher Scientific Inc.) using the QgreenBlue™ 2X PCR master mix (CellSafe) according to the manufacturer’s instructions. The detailed primer sequences are listed in Table S1.
Western blot
Sample-treated RAW264.7 cells were prepared for RT-qPCR as described above. After washing the cells twice with DPBS, cell lysis was performed using PRO-PREP Protein extraction solution (iNtRON Biotechnology). Protein contents in the lysate were quantified using the Pierce™ BCA protein assay kit (Thermo Fisher Scientific Inc.). Each sample containing 50 μg of protein was loaded onto a 9% SDS-PAGE and transferred onto a nitrocellulose membrane. This membrane was blocked for 30 min at 25 ℃ with a solution of 3% (w/v) bovine serum albumin fraction V (Roche, Basel, Switzerland) in Tris-buffered saline with Tween-20 (TBST, iNtRON Biotechnology). The membrane was then exposed to rabbit primary antibodies targeting p-c-Jun amino-terminal kinase (JNK), JNK, p-extracellular signal-regulated kinase (ERK), p-p38, p38, and β-actin (Cell Signaling Technology Inc., Danvers, MA, USA) at 25 ℃ for 1 h and washed with TBST for 45 min. The membrane was incubated with anti-rabbit IgG-HRP-conjugated secondary antibody (Cell Signaling Technology) at 25 ℃ for 1 h and washed four times with TBST. EzWestLumi Plus (ATTO Corporation, Tokyo, Japan) was used as the chemiluminescent substrate. The protein bands were visualized using Amersham™ Imager 600 (GE Healthcare, Chicago, IL, USA) and quantified using Image J software (National Institutes of Health, Bethesda, MD, USA). The protein expression levels were determined by comparison to β-actin.
Statistical analysis
All results are expressed as mean ± standard deviation for experiments performed in triplicate. Statistical analyses were performed using IBM SPSS Statistics version 28.0 (SPSS Inc. Chicago, IL, USA). Statistical significance was estimated using a one-way analysis of variance (ANOVA) with Duncan’s multiple-range test. Statistical significance was set at p values < 0.05.
Results and discussion
Strain selection and GO and MGO degradation
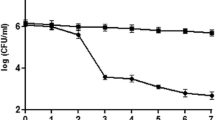
The growth of the 987 LAB strains was assessed in MRS broth supplemented with 5 mM MGO, and the strain TAP041 with the highest MGO reduction ability was selected (data not shown). L. lactis KF140 exhibited an ability to reduce the level of Nε-(carboxymethyl)lysine (CML), a type of AGE derived from GO, and served as a positive control (Park et al., 2022). When the growth of strain TAP041 and L. lactis KF140 was monitored in co-incubation with varying concentrations of MGO from 0 to 2.0 mM, both strains exhibited the highest growth rates in MRS broth without MGO for 7 h (Fig. S1). However, adding MGO to the broth resulted in dose-dependent growth inhibition. At a concentration of 2 mM MGO, the growth of L. lactis KF140 was inhibited more than that of strain TAP041.
As shown in Fig. S2, the initial GO level at 95.2 ppm was reduced by co-incubation with L. lactis KF140 and strain TAP041 by 25.4% and 31.4%, respectively. Similarly, the initial MGO level at 523 ppm decreased to 8.4% when co-incubated with L. lactis KF140 and to 5.6% when co-incubated with strain TAP041. Compared to L. lactis KF140, strain TAP041 demonstrated higher efficacy in reducing MGO levels. However, these results alone do not conclusively establish the in vivo AGE-reducing activity of strain TAP041; thus, further investigation is required to elucidate the anti-glycation mechanism of this strain.
Identification of strains
As shown in Fig. 1, a phylogenetic analysis was performed using the nucleotide sequence of the 16S rRNA gene of strain TAP041. The morphology of strain TAP041 is characterized by the diameter of 1.0–1.2 μm forming tetrads, a characteristic feature of Pediococcus spp. The genus Pediococcus is spherical, 1.0–2.0 μm in diameter, and tetrads form (Holt et al., 1994). When the carbohydrate utilization of strain TAP041 was compared with that of the type strain P. pentosaceus KCCM 11902, it was observed that strain TAP041 could utilize D-melibiose, D-saccharose, D-raffinose, and potassium gluconate, not used by type strain KCCM 11902, which was similar to the type strain P. pentosaceus KCCM 11902 (Table S3). Based on these results, strain TAP041 was identified as P. pentosaceus.
Phylogenetic tree of P. pentosaceus TAP041 based on the sequence of the 16S rRNA gene and the scanning electron microscopy images of P. pentosaceus TAP041. (Left panel) Neighbor-joining method was used to construct the phylogenetic tree, using 16S rRNA sequences for analysis. (Right panel) Scanning electron microscopy (× 20,000)
In vitro resistance to acid, bile salt, pancreatin, and lysozyme
When the P. pentosaceus TAP041 and LGG were exposed to acid, bile salts, pancreatin, and lysozyme, their tolerance to acidic environments (pH 2.5) of P. pentosaceus TAP041 was 63.4%, and that of LGG was 64.9% (Table 1). Survival at low-pH conditions is an important factor in selecting potential probiotics to ensure their viability and functionality. P. pentosaceus TAP041 and LGG exhibited 82.9% and 88.8% bile tolerances, respectively. Bile salts generally act as detergents that damage the lipid membranes of bacterial cells (Abdalla et al., 2021). The resistance of a strain to bile salts is related to the enzymatic activity of the bile salt hydrolase, which is involved in the hydrolysis of conjugated bile, thereby alleviating toxicity (du Toit et al., 1998). The survival rate of P. pentosaceus TAP041 did not decrease in pancreatin and lysozyme tolerance tests. Pancreatic enzymes are secreted into the small intestine and are involved in food digestion. Lysozymes present in the saliva have antimicrobial activity. In acid, bile salt, and lysozyme tolerance tests, the viable cell count of P. pentosaceus TAP041 was higher than that of P. pentosaceus CRAG3 (Shukla and Goyal, 2014). In general, probiotics are considered more effective if there is a minimal reduction in viable bacterial counts in an in vitro tolerance test (Ayyash et al., 2021). The four tolerance tests of P. pentosaceus TAP041 were similar to those of a well-known probiotic strain, LGG, suggesting the potential of P. pentosaceus TAP041 as a probiotic strain.
Gut-adhesion ability
P. pentosaceus TAP041 and LGG confirmed the same levels of adhesion rate of 88.4% (Table 1). LGG is a representative probiotic strain recognized for its high adhesion to gastrointestinal cells, and its ability to adhere to the mucosal membranes of the gastrointestinal tract is a pivotal characteristic of probiotic strains. Leuconostoc lactis CCK940, L. lactis SBC001, and Weissella cibaria YRK005 show different adhesion rates (54.2–67.6%) (Jeong et al., 2023). P. acidilactici MTCC5101 showed strong adhesion to the villi of Caco-2 cells (150%) and was observed to adhere to each other (Balgir et al., 2013). Thus, the intestinal adherence of P. pentosaceus TAP041 was similar to that of LGG and could be used as a probiotic strain.
Auto/coaggregation ability
As shown in Fig. 2A, the auto-aggregation ability of P. pentosaceus TAP041 at 48 h was 66.6%, comparable to that observed in LGG (71.2%). Whereas, pathogenic strains generally showed a higher ability to auto-aggregation, at the level of 82.2% for L. monocytogenes and that of 91.1 ± 0.8% for B. cereus. Auto-aggregation of some P. pentosaceus strains (65.2–69.1%) was exhibited higher than that of P. pentosaceus TAP041 (53.0%) at 24 h (Lee et al., 2014). It is reported that aggregation ability increases in a time-dependent manner (Joung et al., 2023). It has also been reported that strains with a strong ability to auto-aggregate have high adhesion to the intestinal tract (Ocaña and Nader-Macías, 2002). Different autoaggregation levels between strains may be due to factors such as bacterial cell surface structure and adhesion mechanisms. Autoaggregation has been frequently observed in pathogenic bacteria and can increase their tolerance to antimicrobial activity. In the aggregated state of probiotics, pathogens are neutralized and unable to colonize the mucosal surface of the host (Trunk et al., 2018).
Auto/Coaggregation of lactic acid and pathogenic bacteria over incubation time at 37 ℃. The pellets of P. pentosaceus TAP041, LGG, and six pathogenic bacterial strains were suspended in PBS, and the optical density (OD600) was adjusted to 0.3 in a cuvette. OD600 was measured at 0, 2, 5, 10, 24, 36, and 48 h. A auto-aggregation of lactic acid bacteria and pathogens, B coaggregation of P. pentosaceus TAP041 against pathogens, C coaggregation of LGG against pathogens. TAP041, P. pentosaceus TAP041; LGG, Lactobacillus GG
When coaggregation of P. pediococcus TAP041 and LGG with some pathogens was examined, P. pentosaceus TAP041 showed the best coaggregation rate with B. cereus (76.7 ± 0.4%) (Fig. 2B-C). For LGG, the best result was observed with B. cereus (78.1 ± 8.7%). B. cereus exhibits a robust survival capacity in the intestinal tract, which is attributed to factors such as biofilm formation (Lin et al., 2022). P. pentosaceus TAP041 and LGG had 74.3 ± 1.4% and 69.2 ± 1.2% coaggregation with E. coli, respectively. Strains with high auto-aggregation ability were also found to have high coaggregation ability. The two LAB strains showed coaggregation abilities with pathogenic bacteria, and the degree of coaggregation varied depending on the species or strain (Lee et al., 2014).
Immunostimulating activity
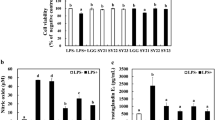
As shown in Fig. 3A, RAW264.7 cells treated with K. pneumoniae subsp. pneumoniae exhibited 52.8% of cell viability. The viability of RAW264.7 cells decreased as the cell numbers of live P. pentosaceus increased, and no cytotoxicity on RAW264.7 cells was observed up to MOI 500. Heat-killed bacteria showed no cytotoxicity up to an MOI of 1000. It was confirmed that the heat-killed LAB treatment was less toxic than the live cell treatment.
Cytotoxicity and nitric oxide production of live and heat-killed P. pentosaceus TAP041 on RAW264.7 macrophages. A Viability of RAW264.7 cells. RAW264.7 cells were treated with live or heat-killed P. pentosaceus TAP041 at the concentrations (MOI 62.5–1000) or K. pneumoniae subsp. pneumoniae as a positive control for 24 h. B NO production was measured by Griess reagent in RAW264.7 cells treated with live P. pentosaceus TAP041. C NO production was measured by Griess reagent in RAW264.7 cells treated with heat-killed P. pentosaceus TAP041. N.C., negative control; P.C., K. pneumonia subsp. pneumoniae KCCM41433. Data represent the mean ± SD of three independent experiments
NO production in the live or heat-killed strains increased in an MOI-dependent manner (Fig. 3B–C). In the live P. pentosaceus TAP041-treated RAW264.7 cells (Fig. 3B), it was confirmed that the maximum production of NO produced by the macrophage cells treated with live P. pentosaceus TAP041 was 26.5 μM at MOI 62.5. At an MOI of 500, macrophages highly susceptible to NO-induced apoptosis may be affected by NO production (Coleman, 2001). Meanwhile, the production of NO in cells treated with heat-killed bacteria showed a significant increase of 19.1 ± 1.8 μM at MOI 500 (Fig. 3C). Although excessive NO production is known to cause tissue damage due to an inflammatory response, an appropriate amount of NO can control microorganisms and cancer cells that cause intracellular infections (MacMicking et al., 1997). Thus, the increase in NO secretion following treatment with live or heat-killed P. pentosaceus TAP041 shows that P. pentosaceus TAP041 may stimulate immune activity, especially in heat-killed bacteria. NO, an indicator of macrophage activation, is a reactive nitrogen species produced by macrophages and is a gas molecule produced from L-arginine by NOS. The production of iNOS by LAB-treated macrophages with LAB showed similar to that of NO (Fig. 4A). It has been suggested that P. pentosaceus TAP041 regulates NO production in macrophages via NO- and iNOS-related signaling pathways and is involved in immune stimulation.
The mRNA expression level of iNOS, TNF-α, IL-6, IL-1β, and COX-2-related immunostimulating activity of P. pentosaceus TAP041 in RAW 264.7 macrophages. The levels of A iNOS, B TNF-α, C IL-6, D IL-1β, and E COX-2 mRNA expression were determined by real-time qPCR, and the relative expressions of these genes were normalized to GAPDH. CNT, negative control; LPS, LPS-treated positive control; TAP, treated with live P. pentosaceus TAP041 (MOI 250); TAPK, treated with heat-killed P. pentosaceus TAP041 (MOI 500). Data depict the mean ± SD of three independent experiments
Among various cytokines, TNF-α and IL-6 are pro-inflammatory cytokines produced by activated immune cells during inflammation, and these cytokines can influence each other’s secretion levels. As shown in Fig. 4B-C, when the gene expression levels of TNF-α and IL-6 in the untreated RAW264.7 cells were used as a negative control, and the cells treated with 1 μg/mL LPS showed a 16.8- and 259.5-fold increase, respectively. When RAW264.7 macrophages were treated with live LAB, the expression levels of TNF-α and IL-6 were 11.5- and 137.2-fold higher than those of heat-killed LAB (3.8- and 69.4-fold). The productions of TNF-α and IL-6 in macrophages treated with live and heat-killed LAB tended to increase in a similar way.
IL-1β and cyclooxygenase-2 (COX-2) expressions were also shown in Fig. 4D-E, and the expressions of IL-1β and COX-2 in LPS-treated macrophages were 306.5 and 423.4 fold, respectively, compared to non-treated cells. RAW264.7 cells treated with live LAB at MOI 250 displayed a significant capacity to induce IL-1β and COX-2 (334.9- and 704.9-fold, respectively) compared to the positive control. Heat-killed LAB at MOI 500 also induced IL-1β and COX-2, although lower than live LAB. IL-1β and COX-2 are known to be pro-inflammatory cytokines secreted by the inflammatory response. Thus, the mRNA expression levels of these cytokines indicate that P. pentosaceus TAP041 dosage significantly impacts the immunostimulatory effects, whether live or heat-killed. Considering cell cytotoxicity, heat-killed P. pentosaceus TAP041 may be deemed safe for further applications (Xu et al., 2023).
When RAW264.7 cells were treated with live and heat-killed P. pentosaceus TAP041, the protein expression levels of JNK, p38, and ERK were significantly increased (Fig. 5A-C). The JNK, p38, and ERK expression levels were markedly higher in LPS-induced macrophages than in untreated cells (40.4%, 35.0%, and 61.2%, respectively). Live P. pentosaceus TAP041 increased the levels of JNK, p38, and ERK phosphorylation in RAW264.7 cells compared to untreated cells. Moreover, there was no induction of phosphorylation of these proteins by heat-killed LAB relative to the untreated control group.
Western blot analysis and relative protein levels of JNK, p38, and ERK. RAW 264.7 cells were co-cultured with P. pentosaceus TAP041 for 24 h. The relative protein expressions of A JNK, B p38, and C ERK of the MAPK pathway were measured by western blot analysis. CNT, negative control; LPS, LPS-treated positive control; TAP, RAW 264.7 cells were treated with live P. pentosaceus TAP041 (MOI250); TAPK, RAW 264.7 cells were treated with heat-killed P. pentosaceus TAP041 (MOI500). Data depict the mean ± SD of three independent experiments
The MAPK signaling pathway is usually correlated with LPS-induced inflammation in immune cells and regulates various cellular responses. The MAPK signaling pathway, which includes JNK, p38, and ERK, mediates signaling responses in the immune and inflammatory systems. These results suggested that live and heat-killed P. pentosaceus TAP041 maintain a balance between M1/M2 macrophages by regulating pro-inflammatory and anti-inflammatory mediators (Shapouri‐Moghaddam et al., 2018; Zhu et al., 2016).
Concludingly, P. pentosaceus TAP041 reduced the levels of GO and MGO in culture supernatants. The probiotic properties and immunostimulatory activities of this strain have been confirmed. This strain was demonstrated to be resistant to acid, bile salts, pancreatin, and lysozyme and to have higher gut adhesion and aggregation capacities. In the live and heat-killed state, the strain was confirmed to significantly enhance the expression levels of various cytokines such as iNOS, TNF-α, IL-6, IL-1β, and COX-2 and the expression levels of JNK, p38, and ERK protein in the MAPK signaling pathway. These findings suggest that both live and heat-killed P. pentosaceus TAP041 may act as immunostimulatory agents potentially applicable as a functional food additive and pharmacologically.
References
Abdalla AK, Ayyash MM, Olaimat AN, Osaili TM, Al-Nabulsi AA, Shah NP, Holley R. Exopolysaccharides as antimicrobial agents: Mechanism and spectrum of activity. Frontiers in Microbiology 12: 664395 (2021)
Ayyash MM, Abdalla AK, AlKalbani NS, Baig MA, Turner MS, Liu SQ, Shah NP. Invited review: Characterization of new probiotics from dairy and nondairy products—Insights into acid tolerance, bile metabolism and tolerance, and adhesion capability. Journal of Dairy Science 104: 8363-8379 (2021)
Balgir PP, Kaur B, Kaur T, Daroch N, Kaur G. In vitro and in vivo survival and colonic adhesion of Pediococcus acidilactici MTCC5101 in human gut. BioMed Research International 2013: 583850 (2013)
Cao Z, Pan H, Tong H, Gu D, Li S, Xu Y, Ge C, Lin Q. In vitro evaluation of probiotic potential of Pediococcus pentosaceus L1 isolated from paocai—a Chinese fermented vegetable. Annals of Microbiology 66: 963-971 (2015)
Cho Y, Han HT, Kim TR, Sohn M, Park YS. Immunostimulatory activity of Lactococcus lactis LM1185 isolated from Hydrangea macrophylla. Food Science and Biotechnology 32: 497-506 (2023)
Coleman JW. Nitric oxide in immunity and inflammation. International immunopharmacology 1: 1397-1406 (2001)
du Toit M, Franz CMAP, Dicks LMT, Schillinger U, Haberer P, Warlies B, Ahrens F, Holzapfel WH. Characterisation and selection of probiotic lactobacilli for a preliminary minipig feeding trial and their effect on serum cholesterol levels, faeces pH and faeces moisture content. International Journal of Food Microbiology 40: 93-104 (1998)
FAO/WHO. Guidelinces for the evaluation of probiotics in food. FAO/WHO working group, 1–11 (2002)
Gaens KH, Stehouwer CD, Schalkwijk CG. Advanced glycation endproducts and its receptor for advanced glycation endproducts in obesity. Current Opinion in Lipidology 24: 4-11 (2013)
García-Ruiz A, González de Llano D, Esteban-Fernández A, Requena T, Bartolomé B, Moreno-Arribas MV. Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiology. 44: 220-225 (2014)
Holt JG, Krieg NR, Sneath PH, Staley JT, Williams ST. Bergey’s manual of determinative bacteriology. 9th. Baltimor: William & Wilkins (1994)
Jeong S, Kwon A, Jeong H, Park YS. Synergistic immunostimulatory activities of probiotic strains, Leuconostoc lactis and Weissella cibaria, and the prebiotic oligosaccharides they produce. Microorganisms 11: 1354 (2023)
Joung JA, You JY, Lee SS, Choi JH. Potential probiotic activity of isolated Lactobacillus harbinensis VF from taditionally fermented vinegar. Food Engineering Progress 27: 155-164 (2023)
Kwon HK, Song MJ, Lee HJ, Park TS, Kim MI, Park HJ, Pediococcus pentosaceus-fermented Cordyceps militaris inhibites inflammatory reactions and alleviates contact dermatitis. International Journal of Molecular Sciences. 9: 3504 (2018).
Lee KW, Park JY, Sa HD, Jeong JH, Jin DE, Heo HJ, Kim JH. Probiotic properties of Pediococcus strains isolated from jeotgals, salted and fermented Korean sea-food. Anaerobe 28: 199-206 (2014)
Lee MG, Joeng H, Shin J, Kim S, Lee C, Song Y, Lee BH, Park HG, Lee TH, Jiang HH, Han YS, Lee BG, Lee HJ, Park MJ, Jun YJ, Park YS. Potential probiotic properties of exopolysaccharide-producing Lacticaseibacillus paracasei EPS DA-BACS and prebiotic activity of its exopolysaccharide. Microorganisms 10: 2431 (2022)
Lin SW, Wu CH, Jao YC, Tsai YS, Chen YL, Chen CC, Fang TJ, Chau CF. Fermented supernatants of Lactobacillus plantarum GKM3 and Bifidobacterium lactis GKK2 protect against protein glycation and inhibit glycated protein ligation. Nutrients 15: 277 (2023)
Lin Y, Briandet R, Kovács ÁT. Bacillus cereus sensu lato biofilm formation and its ecological importance. Biofilm 4: 100070 (2022)
MacMicking J, Xie Qw, Nathan C. Nitric oxide and macrophage function. Annual review of immunology 15: 323-350 (1997)
Ocaña VS, Nader-Macías ME. Vaginal lactobacilli: self- and co-aggregating ability. British Journal of Biomedical Science 59: 183-190 (2002)
Ohn JE, Seol MK, Bae EY, Cho YJ, Jung HY, Kim BO. The potential probiotic and functional health effects of lactic acid bacteria isolated from traditional korean fermented foods. Journal of Life Science. 30: 581-591 (2020)
Park HY, Lee HB, Lee SY, Oh MJ, Ha SK, Do E, Lee HHL, Hur J, Lee KW, Nam MH, Park MG, Kim Y. Lactococcus lactis KF140 reduces dietary absorption of Nε - (carboxymethyl)lysine in rats and humans via β-galactosidase activity. Frontiers in Nutrition 9: 916262 (2022)
Pramanik S, Venkatraman S, Karthik P, Vaidyanathan VK. A systematic review on selection characterization and implementation of probiotics in human health. Food Science and Biotechnology 32: 423-440 (2023)
Sena CM, Matafome P, Crisóstomo J, Rodrigues L, Fernandes R, Pereira P, Seiça RM. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacological Research 65: 497-506 (2012)
Shapouri‐Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. Journal of Cellular Physiology 233: 6425-6440 (2018)
Shin JS, Jung JY, Lee SG, Shin KS, Rhee YK, Lee MK, Hong HD, Lee KT. Exopolysaccharide fraction from Pediococcus pentosaceus KFT18 induces immunostimulatory activity in macrophages and immunosuppressed mice. Journal of Applied Microbiology 120: 1390-1402 (2016)
Shukla R, Goyal A. Probiotic potential of Pediococcus pentosaceus CRAG3: A new isolate from fermented cucumber. Probiotics and Antimicrobial Proteins 6: 11-21 (2014)
Suh KS, Choi EM, Rhee SY, Kim YS. Methylglyoxal induces oxidative stress and mitochondrial dysfunction in osteoblastic MC3T3-E1 cells. Free Radical Research 48: 206-17 (2014)
Tarique M, Abdalla A, Masad R, Al-Sbiei A, Kizhakkayil J, Osaili T, Olaimat A, Liu S-Q, Fernandez-Cabezudo M, al-Ramadi B, Ayyash M. Potential probiotics and postbiotic characteristics including immunomodulatory effects of lactic acid bacteria isolated from traditional yogurt-like products. Lwt 159: 113207 (2022)
Trunk T, Khalil HS, Leo JC. Bacterial autoaggregation. AIMS Microbiology 4: 140-164 (2018)
Uğur H, Görünmek M, Çatak J, Efe E, Özgür B, Duman S, Yaman M. Determination and assessment of the most potent precursors of advanced glycation end products in baklava and Turkish delight by HPLC. Food Science and Technology 42: e08522 (2022)
Xu X, Qiao Y, Peng Q, Shi B. Probiotic properties of Loigolactobacillus coryniformis NA-3 and in vitro comparative evaluation of live and heat-killed cells for antioxidant, anticancer and immunoregulatory activities. Foods 12: 1118 (2023)
Zhang H, Wang Z, Li Z, Wang K, Kong B, Chen Q. l-glycine and l-glutamic acid protect Pediococcus pentosaceus R1 against oxidative damage induced by hydrogen peroxide. Food Microbiology 101: 103897 (2022)
Zhu Y, Li X, Chen J, Chen T, Shi Z, Lei M, Zhang Y, Bai P, Li Y, Fei X. The pentacyclic triterpene Lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. International Immunopharmacology 30: 74-84 (2016)
Acknowledgements
This study was supported by MetaCen Therapeutics.
Funding
This work received support from MetaCen Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, C., Park, YS. Probiotic and immunostimulating effects of live and heat-killed Pediococcus pentosaceus TAP041. Food Sci Biotechnol 33, 2189–2200 (2024). https://doi.org/10.1007/s10068-024-01530-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-024-01530-2