Abstract
This study aimed to investigate the probiotic potential of Pediococcus pentosaceus L1 isolated from paocai, a Chinese fermented vegetable. In vitro analysis revealed that P. pentosaceus L1 had the capability to tolerate simulated gastrointestinal juices. Adhesion of P. pentosaceus L1 to HT-29 intestinal epithelial cells (IEC) was also observed. L1 was sensitive to ampicillin, gentamycin, kanamycin, strepomycin, clindamycin, tetracycline and chloramphenicol. L1 showed effective inhibition against Escherichia coli, Salmonella typhimurium, and Shigella flexneri. P. pentosaceus L1 also exhibited the abilities of auto-aggregation and co-aggregation with Shigella flexneri. Pre-treatment of HT-29 IEC with P. pentosaceus L1 prior to tumor necrosis factor-alpha (TNFα) challenge down-regulated the expression of pro-inflammatory genes, such as IL8, CCL20, CXCL10, and CXCL1. The level of IL-8 released in culture supernatant of TNFα-challenged HT-29 IEC was reduced by strain L1, confirming the observed decrease in TNFα-induced IL-8 mRNA expression. These results indicate the probiotic potential of P. pentosaceus L1, and that this strain could be used to produce functional foods.
Similar content being viewed by others
Introduction
Probiotics are defined by the Food and Agriculture Organization/World Health Organization (FAO/WHO 2006) as “live microorganisms, which when administered in adequate amounts confer a health benefit on the host”. Mechanisms by which probiotics influence host health have been suggested to act at three levels: interacting with other microorganisms present on the site of action, strengthening mucosal barriers, and affecting the immune system of the host (Leroy et al. 2008). The gastrointestinal tract (GIT) is the site where probiotics are believed to exert most health-modulating activities. Thus, probiotics should have the ability to survive the harsh conditions of GIT such as low pH in the stomach (Maragkoudakis et al. 2006), digestive enzymes, and the bile of the small intestine (Begley et al. 2005). Tolerance to GIT conditions is an important selection criterion for probiotic candidates. In addition to surviving transit through the GIT, adherence to intestinal epithelial cells (IECs) should be assessed as probiotics may provide health benefits by competitive exclusion of enteric pathogens and interaction with intestinal mucosa (Collado et al. 2007; Lebeer et al. 2008).
Lactic acid bacteria (LAB), particularly certain strains of the genera Lactobacillus and Bifidobacterium, are the most commonly used probiotics (Saxelin et al. 2005). Pediococcus pentosaceus are categorized as LAB because they utilize sugar and produce lactic acid as a major end product. They are found commonly in naturally fermented foods and beverages including fermented sausage (Cocolin et al. 2011), cheese (Carafa et al. 2015), pickles (Halami et al. 2005) and wine (García-Ruiz et al. 2014), for their roles in rapid acidification (Abrams et al. 2011) and control of spoilage as bacteriocin producers (Carafa et al. 2015). Most of these organisms are also known to have the ability to survive in the gastrointestinal conditions in vitro (Maragkoudakis et al. 2006; Monteagudo-Mera et al. 2012; Garsa et al. 2014) and in vivo (Fernandez et al. 2003; Hwanhlem et al. 2010). More than those properties, P. pentosaceus have received increasing attention with recently reported healthy effects on the immune system (Masuda et al. 2010), enteric pathogens invasion (Vidhyasagar and Jeevaratnam 2013), obesity and lipid liver (Zhao et al. 2012), cholesterol metabolism (Ilavenil et al. 2015), and cancer (Shukla and Goyal 2014).
Paocai (artisan pickled radish) is a Chinese fermented vegetable rich in LAB (Feng et al. 2012). P. pentosaceus is found commonly in paocai (Huang et al. 2009; Gong et al. 2014). However, little is known about the probiotic potential of P. pentosaceus derived from paocai. The objective of this study was to evaluate in vitro the probiotic potential of P. pentosaceus strain L1 derived from paocai. This strain showed good survival in simulated gastrointestinal juices and effective adhesion to HT-29 IEC. P. pentosaceus L1 also had antimicrobial activity on indicator organisms as well as immunomodulatory capability at the intestinal mucosal level. These observations suggest that P. pentosaceus L1 exhibits probiotic properties and suggests its potential for incorporation into functional foods.
Materials and methods
Isolation and identification of P. pentosaceus L1 from paocai
Artisan paocai (pickled radish; 10 g) from Kunming, China, was homogenized in phosphate buffer saline (PBS) for 3 min using JC-T homogenizer (Luoheiintian Test Equipment Institute). The homogenized suspension was serially diluted in PBS and poured into sterile Petri dishes onto de Man-Rogosa-Sharpe (MRS) agar (Oxoid; http://www.oxoid.com) containing 3 mg ml−1 CaCO3 and subjected to incubation at 37 °C for 24 h. Bacterial colonies that exhibited a clear zone on the plates were individually picked and streaked on MRS agar containing 3 mg mL−1 CaCO3. Based on morphological differences in colony color, shape and gloss, LAB strains were selected for further tests. Each isolates was first tested for catalase by placing a drop of 3 % (w/v) hydrogen peroxide solution on the cells. Immediate formation of bubbles indicated the presence of catalase in the cells. Only those isolates that were catalase-negative were stained using the Gram staining method, and then Gram-positive isolates were stored in MRS broth containing 25 % (v/v) glycerol at −20 °C.
Genomic DNA extracted using a genomic DNA extraction reagent kit of bacteria (SK8225, Sangon Biotech, Shanghai, China) following lysozyme treatment served as template for PCR; 16S rDNA was amplified by PCR using the universal 16S rDNA primers 27f (AGAGTTTGATCCTGGCTCAG) and 1492r (TACGGCTACCTT GTTACGACTT). PCR products electrophoresed through 1 % agarose gels and DNA bands corresponding to 1450 bp of the16S rDNA amplicon were extracted and sent to Sangon Biotech for DNA sequencing. The partial 16S rDNA sequence was submitted to the GenBank database and compared to similar sequences by BLAST analysis. The 16S rDNA sequences were aligned with the most similar sequences and the sequences of other representative bacteria using Clustal X (Thompson et al. 1997). A phylogenetic tree of the 16S rDNA sequences was constructed with MEGA 4.0 software using the neighbor-joining method (Tamura et al. 2007). Based on morphology, physiological and chemical assays, and 16S rDNA sequences, 35 LAB strains were isolated. In pilot studies, six LAB strains showed greater resistance to simulated gastrointestinal juices. Of these six LAB isolates, P. pentosaceus strain L1 was chosen to assess probiotic potential, and its 16S rDNA sequence was deposited with GenBank (http://www.ncbi.nlm.nih.gov/genbank/) under accession number KP792278.
In vitro resistance to simulated gastrointestinal juices
Cells of P. pentosaceus L1 from a 24-h incubation were harvested by centrifuging at 10,000 g for 10 min at 4 °C. Cells were washed twice with PBS and resuspened in the same buffer. Then, 40 μL 108 CFU mL−1 bacterial suspension was mixed with 3960 μL simulated gastric juice [125 mmol L−1 NaCl, 7 mmol L−1 KCl, 45 mmol L−1 NaHCO3, and 0.3 % (w/v) pepsin pH 2.0 adjusted with HCl]. Tolerant LAB were assessed in terms of viable colony counts and enumerated after incubation at 37 °C in water bath for 1, 2 or 3 h, which mimicked the gastric transit time for humans.
After 3 h exposure to stimulated gastric juice, the strain was harvested by centrifugation at 10,000 g for 10 min at 4 °C and washed twice with PBS before being resuspended in 4 mL simulated small intestinal juice [0.1 % (w/v) pancreatin and 0.3 % (w/v) bovine bile salts]. Tolerance was assessed in terms of viable colony counts, and enumerated after incubation at 37 °C in water bath for 1, 2, 3 or 4 h.
In vitro adhesion assay
The human cell line HT-29 IEC (ATCC HTB-38) was cultured in RPMI 1640 supplemented with 10 % (v/v) fetal bovine serum, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (Applied Biosystems, Foster City, CA). HT-29 IEC were propagated routinely in 25-cm2 tissue culture flasks at 37 °C in a humidified, 5 % CO2 incubator (Thermo Scientific, Waltham, MA) until they approached 80–90 % confluence, and were used between passages 10 and 25. To prepare for treatment with P. pentosaceus L1 strain, 2 × 104 HT-29 IEC were inoculated into a six-well culture plate (Corning, Sigam, St. Louis, MO) and incubated for 48 h at 37 °C in a humidified, 5 % CO2 incubator. The broth culture of P. pentosaceus L1 was centrifuged at 10,000 g for 10 min at 4 °C and the pellets were resuspended in RPMI 1640 medium (HyClone, http://www.gelifesciences.com) without serum and antibiotics. Then, 2 ml of the bacterial suspension (2 × 107 CFU mL−1) was added to each well of a six-well cell culture plate and allowed to incubate with HT-29 IEC for 1 h at 37 °C, 5 % CO2. HT-29 human IEC were then washed with DPBS to remove non-adherent bacteria and lysed by incubation for 15 min with 0.1 % (v/v) Triton X-100. The lysates were then diluted and plated onto MRS agar to determine the number of adherent bacteria. The adhesion capacity was recorded as the percentage of adherent bacteria of the bacteria applied.
Antibiotic sensitivity testing
The antibiotic susceptibility of P. pentosaceus L1 was evaluated according to the technical guidelines of the European Food Safety Authority (EFSA 2012). The minimal inhibitory concentrations (MIC) of nine antibiotics, including ampicillin, vancomycin, gentamycin, kanamycin, streptomycin, clindamycin, tetracycline and chloramphenicol (Sigma-Aldrich), were determined as described by Ryu and Chang (2013). Overnight culture of P. pentosaceus L1 in MRS broth were centrifuged at 10,000 g for 10 min at 4 °C and resuspended in Mueller-Hinton (MH) broth (Oxoid) containing 0.5 % (w/v) dextrose. The resultant cell suspensions were then further diluted in the same medium to a final concentration of 5.0 log CFU/mL. Each antibiotic was added to aliquots of the diluted cell suspension, which were incubated at 30 °C for 24–48 h without shaking. Cell growth was observed visually and measured based on the turbidity of the suspensions at 600 nm (UNICO 2100, UNICO Instruments, Shanghai, China). MIC values were determined using the serial antibiotic dilution procedure in MH broth containing 0.5 % (w/v) dextrose.
Antimicrobial activity
Effects on the growth of indicator enteropathogen were determined using the agar well diffusion method according to Tejero-Sariñena et al. (2012) with simple modifications. P. pentosaceus L1 was incubated in MRS broth at 37 °C for 24 h. The broth culture was centrifuged at 10,000 g for 10 min at 4 °C, and cell-free culture supernatant (CFCS) was harvested. CFCS was then filtered through a Millex® 0.22 μm filter (Millipore; https://www.emdmillipore.com) and divided into four aliquots. One aliquot was not treated. One aliquot was adjusted to pH 6.5. One aliquot was adjusted to pH 6.5 followed by being heated at 100 °C for 15 min. One aliquot was adjusted to pH 6.5 followed by being incubated with 1 mg mL−1 proteinase K at 37 °C for 3 h. Then, 20 mL 1.2 % (w/v) of LB agar (Oxoid) or TSA (Oxoid) was mixed vigorously with 20 μL of an overnight culture of the indicator pathogen including Escherichia coli CMCC44825, Shigella flexneri CMCC(B)51592 and Salmonella typhimurim CMCC(B)50115 (Guangdong Huankai Microbial SCI. & TECH., Guangdong, China), poured into a Petri dish. Wells 8 mm in diameter were made in the agar using sterile steel cylinders. Then, 90 μL non-treated CFCS or treated CFCS was placed into each well and incubated at 37 °C for 24 h. The inhibition zone (in mm) was measured around each well, and the antimicrobial activity was expressed as the mean inhibition zone diameter.
Auto-aggregation and co-aggregation
Auto-aggregation and co-aggregation were tested as described by Zhang et al. (2011) with simple modifications. An overnight culture of P. pentosaceus L1 in MRS broth at 37 °C was centrifuged at 12,000 g for 10 min at 4 °C. The pellet was washed twice with PBS and then resuspened in PBS to approximately 108 CFU mL−1. The mixture was vortexed for 10 s followed by incubation for 4 h at 37 °C and the absorbance was read at 600 nm. The auto-aggregation percentage was calculated as follows (Kos et al. 2003):
where At is the absorbance at time t = 4 h ,and Ao is the absorbance at time t = 0 h.
For the co-aggregation assay, 2 mL aliquots of pairs of bacterial suspensions (P. pentosaceus L1 and Shigella flexneri) were vortexed for 10 s. Samples from the auto-aggregation were also used as control tubes (4 mL aliquots of a single bacterial suspension). Co-aggregation was also determined at 4 h, as described above. Co-aggregation percentage was calculated as follows (Kos et al. 2003):
where Ax and Ay are the individual aggregation properties of P. pentosaceus L1 and Shigella flexneri, and A(x + y), is the combined aggregation of P. pentosaceus L1 and Shigella flexneri.
IEC pre-treated with P. pentosaceus L1
A broth culture of P. pentosaceus L1 was centrifuged at 10,000 g for 10 min at 4 °C and the pellets were resuspended in RPMI 1640 medium without serum and antibiotics. To prepare for treatment with P. pentosaceus L1 strain, 2 × 106 HT-29 IEC was inoculated into 25-cm2 flasks and incubated for 48 h to establish confluent monolayers. Four million HT-29 IECs were treated for 16 h with 2 × 105 CFU P. pentosaceus L1. TNFα (Pepro Tech, Rocky Hill, CT) was added to a final concentration of 50 ng mL−1, and incubation continued for 3 h, at which point HT-29 IEC were collected for RNA extraction. Six hours later, cell supernatants were collected and frozen at −80 °C until analysis of IL-8 concentrations.
Relative RT-qPCR
Transcript abundance of differentially expressed and reference genes was measured by RT-qPCR on cDNA. Prior to reverse transcription, 4 μg RNA was treated with RQ1 DNase (Promega, Madison, WI) as per the manufacturer’s instructions. DNase-treated RNA (1 μg) was reverse transcribed using Superscript III (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The resulting cDNA was diluted 1:9 before amplification, and 2 μL diluted cDNA was used as template in RT-qPCR using 300 nmol gene-specific primers (Table S1) in a 20 μL reaction volume with SYBR® Premix Ex Taq TM (TaKaRa Biotechnology, Dalian, China). An initial incubation of 30 s at 95 °C was followed with 40 cycles consisting of template denaturation (5 s at 95 °C) and one-step annealing and elongation (30 s at 60 °C) with an ABI7500 Real-Time PCR System (Applied Biosystems). Melting curve analysis was used to determine amplification specificity. Reaction efficiency was determined with LinRegPCR (Ramakers et al. 2003), normalizing genes were evaluated with BestKeeper (Pfaffl et al. 2004), and relative expression and statistical analysis were conducted with REST2009 (Pfaffl et al. 2002).
Enzyme-linked immuno-sorbant assay
Levels of IL-8 were determined in tissue culture supernatants from HT-29 IEC (4 × 106) incubated for 16 h with 2 × 105 CFU P. pentosaceus L1 and then challenged with TNFα for 6 h. IL-8 concentrations were measured by enzyme-linked immuno-sorbant assay (ELISA) using a Human IL-8 ELISA Kit [Multisciences (LIANKE) Biotech, Hangzhou, China]. The optical density was read with a microplate reader (Sunrise, http://www.tecan.com) at 450 nm.
HT-29 IEC viability assay
HT-29 IEC viability was measured using trypan blue staining. The cells were washed with DPBS and detached from the surface using 0.25 % trypsin with EDTA. The cells were then resuspended in RPMI medium supplemented with 10 % fetal bovine serum. Trypan blue solution (0.4 %, w/v) was added in cell suspensions and incubated for 30 s at room temperature. Trypan blue/cell mixture (10 μl) was applied to the hemocytometer and unstained cells (live cells) were counted under the inverted microscope (XDS-37; Shanghai Optic Instrument Co., Shanghai, China).
Statistical analysis
Experiments were conducted in triplicate. Results are presented as the means ± standard deviations (error bars) of replicate experiments. Microsoft Office Excel 2013 and GraphPad Prism were used to create graphs. The results from adhesion ability, auto-aggregation and co-aggregation and RT-qPCR assays were analyzed using Student’s t-test. One-way ANOVA with Duncan’s post-test was performed to determine statistical significance of the differences of data from ELISA and cell viability assay. P < 0.05 was considered statistically significant.
Results
In vitro resistance to simulated gastrointestinal juices of P. pentosacerus L1
The ability to survive through gastrointestinal transit is one of the main desirable characteristics required for a probiotic. P. pentosaceus L1 isolated from artisan pickled radish survived exposure to simulated gastrointestinal juices for 7 h with a survival rate of 92.1 % (Fig. 1). P. pentosaceus L1 retained its viability with a negligible reduction (< 0.3-log) for up to 3 h exposed to simulated gastric juice. P. pentosaceus L1 showed a minor reduction (< 0.3-log) 4 h after exposure to simulated intestinal juice. Compared to before exposure to simulated gastrointestinal juice, viability reduced slightly with a minor 0.5-log decrease. The reference strain Lactobacillus rhamnosus GG showed less tolerance (survival rate of 43.9 %) in simulated gastrointestinal juice during all the tested period than did P. pentosaceus L1.
Adhesion of P. pentosaceus L1 to HT-29 IEC
The adhesion of LAB to intestinal cells is an essential prerequisite for probiotic strains. In this study, P. pentosaceus L1 could adhere to HT-29 IEC. Compared to reference strain LGG (12.53 ± 0.58), L1 showed significantly less capability to adhere to HT-29 IEC (6.24 ± 0.46).
Antibiotic susceptibility
Although resistance of potential probiotics to antibiotics allows them to survive antibiotic treatment, the presence of potentially transferable antibiotic resistances to pathogenic bacteria in potential probiotics is of concern (Ammor et al. 2007). According to the microbiological breakpoints for antimicrobials defined by the Panel on Additives and Products or Substances in Animal Feed (FEEDAP) of the European Food Safety Authority (EFSA 2012), P. pentosaceus L1 were susceptible to ampicillin, gentamycin, kanamycin, streptomycin, clindamycin, tetracycline and chloramphenicol, except vancomycin (Table 1).
Antimicrobial activity of P. pentosaceus L1
The non-treated CFCS of P. pentosaceus L1 in MRS showed antibacterial activity against all the three indicator pathogens (E. coli, Shigella flexneri, Salmonella typhimurium) in the agar well diffusion assay (Table 2). The highest inhibitory activity was observed against the indicator strain Shigella flexneri with inhibition halo of 9.9 mm (data not shown). The CFCS of P. pentosaceus L1 adjusted to pH 6.5 showed antimicrobial activity against all three indicators comparable to non-treated CFCS. Proteinase K treatment diminished inhibitory impact of CFCS on growth of these pathogen strains. Moreover, the heated CFCS of P. pentosaceus L1 remained antimicrobial activity.
Auto-aggregation and co-aggregation
Auto-aggregation of probiotic strains is known to be a prerequisite for adhesion to intestinal epithelium. Our results showed that the auto-aggregating ability of P. pentosaceus L1 strain (18.3 ± 3.15) was significantly less than that of LGG (25.2 ± 2.03) after 4 h. Co-aggregation of P. pentosaceus L1 with Shigella flexneri was examined. P. pentosaceus L1 aggregated Shigella flexneri at 12.8 ± 2.25, significantly greater than that of LGG with Shigella flexneri at 3.9 ± 1.09.
Immunomodulatory effect of P. pentosaceus L1
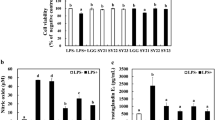
In this study, P. pentosaceus L1 down-regulated gene expression of IL8, CCL20, CXCL10 and CXCL1 encoding pro-inflammatory chemokines stimulated by TNFα in HT-29 IEC (Fig. 2). Furthermore, we examined the level of IL-8 released in cell-free supernatant of HT-29 IEC pre-incubated with P. pentosaceus L1 prior to TNFα treatment. As shown in Fig. 3, strain L1 resulted in a significant decrease in IL-8 production relative to TNFα challenged control. P. pentosaceus strain L1 did not affect IL-8 production by HT-29 IEC co-cultured with P. pentosaceus alone (Fig. 3), suggesting that strain L1 does not induce an inflammatory response in IECs. To detect any toxic effect of tested P. pentosaceus L1 on HT-29 IEC, cell viability after incubation with the P. pentosaceus L1 was determined. P. pentosaceus L1 had no observed effect on viability relative to TNFα-challenged IECs (Fig. 4).
Effects of pre-incubation P. pentosaceus L1 on TNFα-induced gene expression in HT-29 intestinal epithelial cells (IEC). Expression of genes such as IL8, CCL20, CXCL10, CXCL1 in TNFα-challenged HT-29 IEC following incubation with P. pentosaceus L1 were analyzed by RT-qPCR. HT-29 IEC cultured in RPMI1640 served as controls. Dark-gray filled bars Ratio of change in gene expression between HT-29 IEC treated with P. pentosaceus L1 prior to TNFα challenge versus those exposed to TNFα only. Open bars Ratio of change in gene expression between TNFα-treated HT-29 IEC versus untreated control HT-29 IEC. Data are presented as mean ± SD (n = 3, *P < 0.05)
Discussion
Fermented vegetables have proved a good source of potential LAB probiotics since LAB naturally occupy these plant matrices as well as showing good gastrointestinal tolerance (Peres et al. 2012). Several studies have reported that P. pentosaceus originating from fermented vegetables showed probiotic potential such as higher resistance to acid and bile as well stronger adhesive ability to IEC (Ryu and Chang 2013), as well as antimicrobial activity against Salmonella species in mice (Chiu et al. 2008), and immunomodulation through affecting cytokine production (Jonganurakkun et al. 2008). In this study, we reported the probiotic potential of P. pentosaceus strain L1 isolated from paocai, suggesting that paocai could be a resource of potential LAB probiotics.
P. pentosaceus L1 showed the ability to survive when exposed to simulated gastrointestinal juices, in keeping with recent studies demonstrating that certain P. pentosaceus strains originated from fermented vegetables have the ability to tolerate the gastrointestinal environment in vitro (Jonganurakkun et al. 2008; Ryu and Chang 2013; Shukla and Goyal 2014) and in vivo (Chiu et al. 2008). Acid adaption of LAB during vegetable fermentation may account for the reason why P. pentosaceus derived from these matrices show gastrointestinal tolerance (McDonald et al. 1990). A recent study conducted using genomic analysis has shown that the transit tolerance capability of P. pentosaceus strains may be attributed to genes associated with acid and bile tolerance present in the genome as found in P. pentosaceus strain LI05 isolated from the human GI tract (Lv et al. 2014), suggesting that molecular and genomic-based studies will likely provide useful insight into the mechanisms underlying adaptation to the GI environment in P. pentosaceus derived from fermented vegetables.
In addition to surviving passage through the GIT, adhesion of bacteria to epithelial cells is one of the key features for the selection of probiotics. P. pentosaceus strain L1 was able to adhere to HT-29 IEC with less potential than the reference strain LGG. Previous studies have reported that the adhesion abilities of P. pentosaceus vary with IEC cell line, source and strain (Ryu and Chang 2013; Vidhyasagar and Jeevaratnam 2013; Varsha et al. 2014). It will be important to evaluate effects of P. pentosaceus L1 in the context of different IEC cell lines in future study.
P. pentosaceus L1 was sensitive to all antibiotics tested except vancomycin. Bacteria from the genus Pediococcus are known to be intrinsically resistant to vancomycin due to a modified peptidoglycan precursor ending in d-Ala-d-lactate (Billot-Klein et al. 1994). Intrinsic resistance is not horizontally transferable and poses no risk in nonpathogenic bacteria (Ammor et al. 2007). Susceptibility of P. pentosaceus L1 to other antibiotics should be tested by further study. The non-treated CFCS of P. pentosaceus L1 exerted antimicrobial activities against three tested pathogenic strains. The non-treated CFCS of P. pentosaceus L1 showed greater antimicrobial activity than did the reference strain LGG. It is well documented that P. pentosaceus strains produce bacteriocin against Escherichia and Salmonella (Uymaz et al. 2009; Ryu and Chang 2013; Vidhyasagar and Jeevaratnam 2013). Neutralized CFCS of L1 strain treated with proteinase K lost inhibitory activity against indicator strains, suggesting that bacteriocin may contribute to the antimicrobial activity of P. pentosaceus L1. Heat treatment for 15 min at 100 °C had no significant effect on antimicrobial activity. To our knowledge, this is the first report showing the antimicrobial activity of P. pentosaceus against Shigella flexneri.
Auto-aggregation of probiotic strains is known to be a prerequisite for adhesion to intestinal epithelium. Our results showed that the auto-aggregating ability of P. pentosaceus L1 strain was less than that of LGG after 4 h, which is lower than that of earlier reports of P. pentosaceus (Ruas-Madiedo et al. 2005; Osmanagaoglu et al. 2010; Vidhyasagar and Jeevaratnam 2013; Ilavenil et al. 2015). For example, Osmanagaoglu et al. (2010) reported that P. pentosaceus OZF originated from human breast milk auto-aggregated at 85.71 % after 5 h. Six strains of P. pentosaceus isolated from Idly batter also exhibited significant auto-aggregation properties of 42 % or more after 5 h (Vidhyasagar and Jeevaratnam 2013). Notably, auto-aggregation of P. pentosaceus strains in these latter studies were shown to be independent of incubation time, suggesting that further study to examine the auto-aggregation of P. pentosaceus L1 at different time points may be of interest. Co-aggregation of P. pentosaceus L1 with Shigella flexneri was examined. P. pentosaceus L1 aggregated Shigella flexneri at 12.8 ± 2.25. Co-aggregation of probiotics with pathogens enables them to eliminate pathogens from colonizing the intestinal epithelium (Aslim et al. 2007). Therefore, our results suggest that P. pentosaceus L1 could prevent Shigella flexneri infection through co-aggregation with those cells as well as production of antimicrobial substances.
IECs are the initial point of contact with ingested probiotics at the intestinal mucosal interface. Probiotics have been shown to enhance epithelial cell functions (Thomas and Versalovic 2010). Although several reports have shown immunomodulating activities of P. pentosaceus originated from varied sources in vitro and in vivo, little is known of the immunomodulatory activities of P. pentosaceus at the IEC level. In our study, P. pentosaceus strain L1 down-regulated expression of pro-inflammatory genes in TNFα-challenged HT-29 IEC. Similar to our results, feeding P. pentosaceus strain LAB4012 (isolated from cobia intestine) in Vibrion anguillarum challenged groups increased gene expression of the anti-inflammatory cytokine TGF-β1 and decreased expression of pro-inflammatory genes such as TNF1, TNF2, and IL1B in the head-kidney phagocytes of groupers (Huang et al. 2014). A few studies have also reported immunomodulatory activities of P. pentosaceus through affecting the Th1/Th2 immune response. For example, P. pentosaceus NB-17 originated from Hokkaido pickle induced secretion of interferon-γ and IL-12 p70 and decreased IL-4 production by ovalbumin-sensitized mouse spleen cells (Jonganurakkun et al. 2008). Similar results that feeding heat-killed P. pentosaceus Sn26 derived from Japanese Sunki pickle increased interferon-γ and IL-12 p70 levels in Peyer’s patch of BALB/c mice and down-regulated IgE production by splenocytes of ovalbumin-induced allergic diarrheic mice, were reported in a recent study (Masuda et al. 2010). A recent study using an animal model reported that feeding P. pentosaceus OZF—a strain derived from human breast milk—in BALB/c mice up-regulated IL-6 production by spleens cells and peritoneal exudates cells (Osmanagaoglu et al. 2013). Our study showed that pre-incubation of HT-29 IEC with P. pentosaceus L1 prior to TNFα challenge reduced the expression of TNFα-induced pro-inflammatory genes, suggesting that P. pentosaceus L1 has immunomodulatory activity at the IEC level. To our knowledge, this is the first study reporting effects of P. pentosaceus derived from fermented vegetables on pro-inflammatory chemokine expression at the intestinal mucosal level.
References
Abrams D, Barbosa J, Albano H, Silva J, Gibbs PA, Teixeira P (2011) Characterization of bacPPK34 a bacteriocin produced by Pediococcus pentosaceus strain K34 isolated from “Alheira”. Food Control 22:940–946
Ammor MS, Flórez AB, Mayo B (2007) Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol 24:559–570
Aslim B, Onal D, Beyatli Y (2007) Factors influencing autoaggregation and aggregation of Lactobacillus delbrueckii subsp. bulgaricus isolated from handmade yogurt. J Food Prot 70:223–227
Begley M, Gahan CGM, Hill C (2005) The interaction between bacteria and bile. FEMS Microbiol Rev 29:625–651
Billot-Klein D, Gutmann L, Sable S, Guittet E, van Heijenoort J (1994) Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VANB-type Enterococcus D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J Bacteriol 176:2398–2405
Carafa I, Nardin T, Larcher R, Viola R, Tuohy K, Franciosi E (2015) Identification and characterization of wild lactobacilli and pediococci from spontaneously fermented Mountain Cheese. Food Microbiol 48:123–132
Chiu HH, Tsai CC, Hsih HY, Tsen HY (2008) Screening from pickled vegetables the potential probiotic strains of lactic acid bacteria able to inhibit the Salmonella invasion in mice. J Appl Microbiol 104:605e612
Cocolin L, Dolci P, Rantsiou K (2011) Biodiversity and dynamics of meat fermentations: the contribution of molecular methods for a better comprehension of a complex ecosystem. Meat Sci 89:296–302
Collado MC, Meriluoto J, Salminen S (2007) Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett Appl Microbiol 45:454–460
EFSA (2012) Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10:2740–2749
Food and AgricultureOrganization/World Health Organization (2006) Probiotics in food: health and nutritional properties and guidelines for evaluation. FAO Food and Nutrition Paper 85. FAO, Rome
Feng M, Chen X, Li C, Nurgul R, Dong M (2012) Isolation and identification of an exopolysaccharide-producing lactic acid bacterium strain from Chinese Paocai and biosorption of Pb(II) by its exopolysaccharide. J Food Sci 77:T111–T117
Fernandez MF, Boris S, Barbes C (2003) Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J Appl Microbiol 94:449–455
García-Ruiz A, de Llano DG, Esteban-Fernández A, Requena T, Bartolomé B, Moreno-Arribas MV (2014) Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol 44:220–225
Garsa AK, Kumariya R, Kumar A, Lather P, Kapila S, Sood SK, Kapasiva M (2014) In vitro evaluation of the probiotic attributes of two pediococci strains producing pediocin PA-1 with selective potency as compared to nisin. Eur Food Res Technol 239:491–499
Gong C, Yu W, Zhang Q, Song P, Zhang B, Zhu L, You J, Li F (2014) Research of sichuan paocai and lactic acid bacteria. Adv J Food Sci Technol 6:1–5
Halami PM, Ramesh A, Chandrashekar A (2005) Fermenting cucumber, a potential source for the isolation of pediocin-like bacteriocin producers. World J Microbiol Biotechnol 21:1351–1358
Huang Y, Luo Y, Zhai Z, Zhang H, Yang C, Tian H, Li Z, Feng J, Liu H, Hao Y (2009) Characterization and application of an anti-Listeria bacteriocin produced by Pediococcus pentosaceus 05-10 isolated from Sichuan Pickle, a traditionally fermented vegetable product from China. Food Control 20:1030–1035
Huang J, Wu Y, Chi S (2014) Dietary supplementation of Pediococcus pentosaceus enhaces innate immunity, physiological health and resistance to Vibrio anguillarum in orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol 39:196–205
Hwanhlem N, Watthanasakphuban N, Riebroy S, Benjakul S, H-Kittikun A, Maneerat S (2010) Probiotic lactic acid bacteria from Kung-Son: isolation, screening, inhibition of pathogenic bactria. Int J Food Sci Technol 45:594–601
Ilavenil S, Vijayakumar M, Kim DH, Valan Arasu M, Park HS, Ravikumar S, Choi KC (2015) Assessment of probiotic, antifungal and cholesterol lowering properties of Pediococcus pentosaceus KCC-23 isolated from Italian ryegrass. J Sci Food Agric. doi:10.1002/jsfa.7128
Jonganurakkun B, Wang Q, Xu SH, Tada Y, Minamida K, Yasokawa D, Sugi M, Hara H, Asano K (2008) Pediococcus pentosaceus NB-17 for probiotic use. J Biosci Bioeng 106:69–73
Kos B, Susković J, Vuković S, Simpraga M, Frece J, Matosić S (2003) Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol 94:981–987
Lebeer S, Vanderleyden J, De Keersmaecker SC (2008) Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev 72:728–764
Leroy F, Falony G, De Vuyst L (2008) Latest developments in probiotics. In: Toldrà F (ed) Meat biotechnology. Springer New York, pp 217–229
Lv LX, Li YD, Hu XJ, Shi HY, Li LJ (2014) Whole-genome sequence assembly of Pediococcus pentosaceus LI05 (CGMCC 7049) from the human gastrointestinal tract and comparative analysis with representative sequences from three food-borne strains. Gut Pathog 6:36
Maragkoudakis PA, Zoumpopoulou G, Miaris C, Kalantzopoulos G, Pot B, Tsakalidou E (2006) Probiotic potential of Lactobacillus strains isolated from dairy products. Int Dairy J 16:189–199
Masuda T, Kimura M, Okada S, Yasui H (2010) Pediococcus pentosaceus Sn26 inhibits IgE production and the occurrence of ovalbumin-induced allergic diarrhea in mice. Biosci Biotechnol Biochem 74:329–335
McDonald LC, Fleming HP, Hassan HM (1990) Acid tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl Environ Microbiol 56:2120–2124
Monteagudo-Mera A, Rodriguez-Aparicio L, Rua J, Martinez-Blanco H, Navasa N, Garcia-Armesto MR, Ferrero MA (2012) In vitro evaluation of physiological probiotic properties of different lactic acid bacteria strains of dairy and human origin. J Funct Foods 4:531–541
Osmanagaoglu O, Kiran F, Ataoglu H (2010) Evaluation of in vitro probiotic potential of Pediococcus pentosaceus OZF isolated from human breast milk. Probiotics Antimicrob Proteins 2:162–174
Osmanagaoglu O, Kiran F, Yagci FC, Gursel I (2013) Immunomodulatory function and in vivo properties of Pediococcus pentosaceus OZF, a promising probiotic strain. Ann Microbiol 63:1311
Peres CM, Peres C, Hernández-Mendoza A, Malcata FX (2012) Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria—with an emphasis on table olives. Trends Food Sci Technol 26:31–42
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Ramakers C, Ruijte JM, Deprez RH, Moorman AF (2003) Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339:62–66
Ruas-Madiedo P, de los Reyes-Gavilan CG (2005) Invited review: methods for the screening, isolation, and characterization of exo-polysaccharides produced by lactic acid bacteria. J Dairy Sci 88:843–856
Ryu EH, Chang HC (2013) In vitro study of potentially probiotic lactic acid bacteria strains isolated from kimchi. Ann Microbiol 63:1387–1395
Saxelin M, Tynkkynen S, Mattila-Sandholm T, de Vos WM (2005) Probiotic and other functional microbes: from markets to mechanisms. Curr Opin Biotechnol 16:204–211
Shukla R, Goyal A (2014) Probiotic potential of Pediococcus pentosaceus CRAG3: a new isolate from fermented cucumber. Probiotics Antimicrob Protein 6:11–21
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tejero-Sariñena S, Barlow J, Costabile A, Gibson GR, Rowland I (2012) In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: evidence for the effects of organic acids. Anaerobe 18:530–538
Thomas CM, Versalovic J (2010) Probiotics-host communication: modulation of signaling pathway in the intestine. Gut Microbes 1:1–16
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Uymaz B, Şimşek Ö, Akkoç N, Ataoğlu H, Akçelik M (2009) In vitro characterization of probiotic properties of Pediococcus pentosaceus BH105 isolated from human faeces. Ann Microbiol 59:485–491
Varsha KK, Priya S, Devendra L, Nampoothiri KM (2014) Control of spoilage fungi by protective lactic acid bacteria displaying probiotic properties. Appl Biochem Biotechnol 172:3402–3413
Vidhyasagar V, Jeevaratnam K (2013) Evaluation of Pediococcus pentosaceus strains isolated from Idly batter for probiotic properties in vitro. J Funct Foods 5:235–243
Zhang Y, Zhang L, Du M, Yi H, Guo C, Tuo Y, Han X, Li J, Zhang L, Yang L (2011) Antimicrobial activity against Shigella sonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol Res 167:27–31
Zhao X, Higashikawa F, Noda M, Kawamura Y, Matoba Y, Kumagai T, Sugiyama M (2012) The obesity and fatty liver are reduced by plant-derived Pediococcus pentosaceus LP28 in high fat diet-induced obese mice. PLoS One 7:e30696
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 31501496), the Project Sponsored by Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (Grant No. (2013)1792), the Key Program of Scientific Research Foundation of Yunnan Provincial Department of Education (Grant No. 2013Z024), the Program supported by Yunnan Provincial Key Laboratory of Animal Nutrition and Feed Science (Grant No. DYKF2015006) and the Post-doctoral program from Dalian SEM Bio-Engineering Technology Co. Ltd..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Zhenhui Cao and Hongbin Pan contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 122 kb)
Rights and permissions
About this article
Cite this article
Cao, Z., Pan, H., Tong, H. et al. In vitro evaluation of probiotic potential of Pediococcus pentosaceus L1 isolated from paocai—a Chinese fermented vegetable. Ann Microbiol 66, 963–971 (2016). https://doi.org/10.1007/s13213-015-1182-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-015-1182-2