Abstract
The harmful effects of trans and saturated fatty acids have attracted worldwide attention. Edible oleogels, which can structure liquid oils, are promising healthy alternatives to traditional fats. Active research on oleogels is focused on the interaction between unsaturated oils with different fatty acid compositions and low molecular weight or polymer oleogels. The unique network structure inside oleogels has facilitated their application in candies, spreads, meat, and other products. However, the micro- and macro-properties, as well as the functional properties of oleogels vary by preparation method and the system composition. This review discusses the characteristics of oleogels, serving as a reference for the application of oleogels in food products. Specifically, it (i) classifies oleogels and explains the influence of gelling factors on their gelation, (ii) describes the methods for measuring the physicochemical properties of oleogels, and (iii) discusses the current applications of oleogels in food products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Previously, researchers structured liquid oils through processes such as hydrogenation, lipid/enzyme exchange, and fractionation to obtain plastic fats with the taste and mechanical properties required for products such as margarine and cream (Yılmaz et al., 2022). However, the plastic fat produced from partially hydrogenated vegetable oil using traditional technology contains certain amounts of trans fatty acids (TFAs) and saturated fatty acids (SFAs) (Silva et al., 2023a; 2023b; Zhao et al., 2022).
According to epidemiological studies, TFAs can increase the risk of coronary heart disease and all-cause death (Qiu et al., 2022). In addition, dietary TFAs and SFAs are closely linked to chronic diseases, such as cardiovascular disease, obesity, type II diabetes, and cancer (Qiu et al., 2023a). In May 2018, the World Health Organization (WHO) released the REPLACE action package (the first global initiative to eliminate cardiovascular disease risk), aiming to eliminate industrially produced TFAs (Ghebreyesus and Frieden, 2018). This has prompted researchers to search for new structured lipids as new solid fats for food applications. Excitingly, structured lipid systems called oleogels have emerged as promising candidates for replacing unhealthy and controversial fats. The main advantage of oleogels is their potential to produce solid and semisolid lipid systems rich in unsaturated fatty acids without altering the chemical properties of the oil.
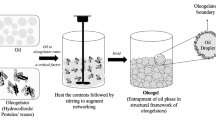
Oleogel preparation typically involves combining the solvent (oil continuous phase) with the oleogelator (structural agent) through physical mixing, which promotes the ordered or disordered arrangement of molecules in the system and finally forms a three-dimensional (3D) network structure of fixed liquid oil (Qiu et al., 2023b). During the past 10 years, researchers have scrutinized various edible oleogel formulations, their performance (including appearance, crystal structure, texture, and thermal stability) (Li and Liu, 2023a; Qiu et al., 2023b), and their value as food fat substitutes (oleogel not only has the characteristics of solid fat but can also reduce the content of TFAs and SFAs in the product and improve the stability and shelf life of the product) to deepen the understanding of oleogels and expand their application (Silva et al., 2023a; 2023b; Zhao et al., 2022).
Research on oleogels has shown breakthrough results in both basic exploration and practical application. During the gelation process, the structure of the oleogelator (including its molecular weight [Mw] and differences in noncovalent interactions and interactions between multicomponent molecules), the chemical properties of the continuous phase (the differences in carbon chain length, saturation, viscosity coefficient, and fatty acid content of triglycerides [TAGs]), and the processing method (direct and indirect methods) will all affect the physical, chemical, microstructure, and macroscopic properties of the oleogel (Han et al., 2023b; Qiu et al., 2023b) and its application as a fat substitute in food products (including spreads, baked goods, confectionery, and meat and dairy products) (Airoldi et al., 2022; Badem and Baştürk, 2023; Ghorghi et al., 2023; Gómez-Estaca et al., 2019; Igenbayev et al., 2023; Li et al., 2023a; Ma et al., 2023; Malvano et al., 2022; Moon et al., 2021; Pușcaș et al., 2021). Therefore, it is essential to classify edible oleogels in detail. In addition, it is imperative to integrate a complete set of methods for characterizing the physicochemical properties of oleogels.
Based on the benefits of oleogels, this review endeavors to promote their improvement and application as an acceptable fat replacement in food products. First, a detailed classification of popular oleogel systems is provided. Subsequently, the methods for characterizing the physicochemical properties of oleogels are summarized. Finally, the latest progress in the research on oleogel application in food is briefly introduced. This review helps deepen the knowledge and understanding of oleogels. Simultaneously, it identifies the direction for future product categories to design oleogels with improved nutritional and functional properties.
Classification of oleogels
Edibility of the oleogelator
Oleogels can be classified based on their properties and requirements. Edible and inedible oleogels are defined based on the edibility of the oleogelator. In terms of application, oleogels are concentrated in the fields of material sciences, drug delivery, analytical chemistry, and cosmetics (Li et al., 2022a; Sivakanthan et al., 2022). In particular, the edible oleogels are mainly used in food products, the production and processing of oleogels should not negatively affect the texture, oil binding capacity (OBC), sensory properties, and physical stability of the product (Silva et al., 2023a; 2023b). In addition, the price of the oleogelator should be reasonable, and the constituents and concentration of the oleogelator added should be suitable for the equilibrium point (affinity and incompatibility) of the oil phase (Shakeel et al., 2021).
Internal microstructure and overall organization
Based on the differences in the internal microstructure and overall organization of the system, oleogels can be roughly divided into two categories: (1) monocomponent oleogels prepared using only one oleogelator and (2) multicomponent/element oleogels prepared with two or more oleogelators (Fig. 1) (Li et al., 2022a). Multicomponent/element oleogels can be subdivided into three categories (Sivakanthan et al., 2022): Class 1 oleogels in which multiple components are necessary drivers of gelation but a single component cannot gel the liquid phase; Class II oleogels in which the various components are themselves oleogelators and can form self-assembling or self-sorting network structures when used alone, and Class III oleogels in which all ingredients are not oleogelators but can form an oleogel system through co-assembling structures. Note: multiple component ingredients refer to oleogelator or non-oleogelator additives. Mixed oleogelators β-sitosterol + lecithin (S/L) belong to Class I of the multicomponent/element classification, a combination that forms a crystalline structure of hollow double-walled tubules that vary with enthalpy (Qiu et al., 2023b). β-Sitosterol + lecithin oleogel (S/LO) not only has the characteristics of high strength and transparency but can also lower blood cholesterol. Therefore, S/LO is also known as the most nutritious oleogel system among all oleogel systems (Qiu et al., 2021; Qu et al., 2022). Combinations of fatty alcohols and fatty acids belong to Class II of the multicomponent/element classification. In certain ratios, fatty alcohols and fatty acids have a synergistic effect and can change the crystal structure of oleogels (Callau et al., 2020). Interestingly, when one of the two is used alone as an oleogelator, the internal crystal structure of the formed oleogel is “sheet-like,” whereas, when used in combination, it presents “needle-like” crystal characteristics (Yao et al., 2021). Combinations of lecithin + fruit wax/sucrose esters/α-tocopherol/sorbitan stearate mainly belong to the category of multicomponent Class III. In lipids with this structure, the latter is often a nonpolar medium, and lecithin, as a crystal modifier or emulsifier, can change the hydrogen bonds between hydrophilic monomers in the system, thereby increasing the viscosity of oleogels (Aguilar-Zárate et al., 2019; Guo et al., 2020). It should be noted that additives can promote the structural arrangement of the gelling factor to form a “bridge” connecting the oleogelator and strengthening the structural network of the oleogel but may hinder the self-assembly or co-assembly behavior of the oleogel (Perta-Crisan et al., 2023). The appropriate oleogelator-to-oleogelator ratio or oleogelator–oil affinity can ensure the formation of a 3D network structure that captures liquid oil inside the oleogel, whereas an incompatible ratio will lead to precipitation and separation of gelling factors (Du et al., 2022; Wang et al., 2022a). This was proved during early research on oleogels, which was focused on the development of an oleogelator, the interaction of the oleogelator with solvents, and gelation mechanisms.
According to the composition and Mw of the oleogelator, oleogels can be divided into low molecular weight oleogels (LMWOs) and high molecular weight polymer oleogels (HMWPOs) (Pascuta et al., 2022). Among them, LMWOs include low single molecular weight oleogels (LSMWOs) and low multicomponent/composite molecular weight oleogels (LMMWOs) (Li et al., 2022a). The gelation process inside the LMMWOs systems is more complex and diverse (with adjustable features) than that inside the LSMWOs systems. Natural vegetable waxes, monoacylglycerols (MAGs), diacylglycerols, hydroxylated fatty acids (12-hydroxystearic acid and ricinoleic acid), ceramides, fatty acids/alcohols, and oligopeptides are typical LMMWOs (Li et al., 2022a). The most researched LMMWOs are phytosterol + γ-oryzanol (P/Y) and lecithin + β-sitosterol/sorbitan tristearate/sucrose ester/α-tocopherol (Wang et al., 2022b). The reason for the name LMWOs is that their systemic molecular structure is small and can be induced to the critical concentration required for gelling even at low concentrations of oleogelators (Marangoni and Garti, 2018). The gelling ability of LMWOs depends on the solubility and self-assembled network structure of the oleogelator in the oil phase (Pascuta et al., 2022). Notably, the lipophilicity of the oleogelator is the distinguishing feature of LMWOs (Han et al., 2023b).
Currently, wax-based oleogels are the most widely studied LMWSOs (Fig. 2) (Sivakanthan et al., 2022). Unlike other oleogelators, waxes are multicomponent mixtures that contain a predominant component and several minor compounds (Doan et al., 2017). As a result, the gelation mechanism of waxes is complex, and the gelling behavior of wax-based oleogels is closely linked to the composition of the wax, including the alkyl chain length and unsaturation degree of wax esters, free fatty alcohols/acids, or other components in the wax (Choi et al., 2020). Doan et al. (2017) evaluated the differences and correlations between the gelling abilities of oleogels formed using rice bran oil and seven different types of waxes. The oleogels formed using candelilla wax, sunflower wax (SW), beeswax (BW), and berry wax had higher rheological properties than oleogels formed using rice bran wax (RBW), carnauba wax (CW), and wild CW, and Pearson’s correlation analysis showed that the strength of the oleogel was positively correlated with the content of wax esters, hydrocarbons, free fatty acids, and chain length and negatively correlated with the content of free fatty alcohols (Doan et al., 2017). It should be noted that the crystal structure of a wax-based oleogel is highly dependent on the gelation mechanism. Some studies investigated the structuring capacity of waxes from a microscopic standpoint. Blake et al. (2014) suggested that candelilla wax oleogel, RBW oleogel, and carnauba wax oleogel (CWO) exhibited different OBCs due to variations in the spatial distribution and size of wax crystals. Crystal morphology with a high aspect ratio, such as the fibrous morphology of RBW, is considered beneficial for oleogelation. The reason may be that compared with low aspect ratio morphology (platelets or spheres), high aspect ratio morphology (fibers) has a larger surface area, which enhances solvent − gelator interactions (Blake et al., 2014). Liu et al. (2020) confirmed the beneficial impact of smaller crystals on the gelling behavior of fish oil oleogels prepared with three different sorghum waxes. Sorghum distillers’ soluble and dried grains wax exhibited a more homogenous crystal network and stronger OBC than sorghum kernel wax and sorghum bran wax because of the smaller crystals. The larger surface area of smaller crystals increased the possibility of gelator–gelator interactions, which increased the mechanical strength of oleogels.
Relative proportions of articles published on oleogel development using different gelators based on literature search in Web of Science (Sivakanthan et al., 2022)
Various HMWPOs have been successfully produced from cellulose polysaccharides (ethyl cellulose [EC] and hydroxypropyl methyl cellulose [HPMC]) (Espert et al., 2021; Gómez-Estaca et al., 2019), natural gums (pectin, chitin, xanthan gum, gelatin, guar gum, alginate, and chitosan) (Abdollahi et al., 2020; Abdolmaleki et al., 2020; Luo et al., 2019; Pan et al., 2021; Willett and Akoh, 2019) and proteins (caseinate, soy protein isolate, and β-lactoglobulin) (Feichtinger and Scholten, 2020; Yu et al., 2022).
Mechanism of oleogel gelation
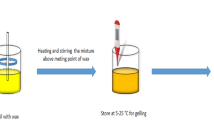
Depending on the combination strategy of oleogel and oil, the gelation method of oleogels can be divided into (i) a direct method and (ii) indirect methods. In the direct method, liquid oil is first mixed with an oleogelator (mostly a low Mw oleogelator), heated above the glass transition temperature (CWOs, 85 °C; monoacylglycerol oleogels [MAGOs] or S/LOs, 90 °C), and then cooled to form a solid fat (Li and Liu 2023a; Qiu et al., 2023a). Based on the gelation mechanisms, the oleogels prepared using the direct method can be further classified into (i) microcrystalline conformations and (ii) self-assembled structures. The indirect methods for preparing HMWPOs (which generally have hydrophilic properties) include two-way emulsion templating (da Silva et al., 2023a; 2023b), hydrogel templating (Ghorghi et al., 2023), solvent exchange (Li and Lu et al., 2023a), and foam templating (Abdollahi et al., 2020). These methods are discussed in detail in Sect. 2.6.
Crystallite conformation
Based on the conformational arrangement and crystal type of the oleogelator, oleogels with microcrystalline conformation can be broadly classified into four categories (Marangoni and Garti, 2018): (i) crystalline particles, (ii) crystalline fibers, (iii) liquid crystal mesophases, and (iv) inert particles. The synthesis of oleogels in a microcrystalline conformation has become one of the most common methods for preparing oleogels because they resemble the way solid lipids are constructed using traditional techniques. The whole gelation process of oleogels with microcrystalline conformation can be roughly divided into three stages, namely nucleation and crystal growth, aggregation, and network formation (Pinto et al., 2021). Specifically, in the first step, the primary particles are precipitated and/or crystallized through the interaction between the oleogelator (weak solute) and the oil phase. In the second step, individual particles are assembled to form different crystalline particles, including (i) rods, (ii) crystals, (iii) fibers or needle-like structures, (iv) platelets, (v) ribbons, (vi) tubules, and (vii) polymer strands (Fig. 3). Molecular clusters are then stabilized by hydrophobic interactions and hydrogen bonds, π − π covalent bonds, Van der Waals forces, and/or electrostatic interactions between small molecules. During this period, mesoscale structures are formed inside the system. Mesoscale structure refers to the asymmetric crystal structure of the oleogel in three dimensions (crystals take on a “needle-like” structure when grown in a one-dimensional fibrous structure and a “sheet-like” structure when grown in two dimensions) (Li et al., 2022a). Finally, the interaction between particles induces anisotropic diffusion and/or growth of mesoscale structures, leading to the formation of thermally reversible oleogel systems with a continuous network or a space-spanning network (Sivakanthan et al., 2022). Representatives of microcrystalline conformation oleogels include MAGs, waxes, and fatty alcohols/acids mentioned in Sect. 2.2 (Pinto et al., 2021).
Self-assembled structure
Oleogels developed from self-assembled structures generally include four types: LMWOs, ethyl cellulose oleogels (ECOs), colloidal silica particles, and lecithin-containing oleogel systems (Li et al., 2022a; Sivakanthan et al., 2022). The combination of P/Y is one of the typical cases of LMWOs. The P/Y mixture forms a self-assembled fibril network inside the system, and the length of the fibers is determined by the cooling rate and storage temperature during the preparation process (Dong et al., 2020). Because the oleogel produced from this combination does not affect the visual and nutritional properties of the product even at high concentrations, it has been approved by the European Food Safety Authority (EFSA) for food applications (Dong et al., 2020).
To date, ECO is the only HMWPO found to form gels in the same manner as LMWOs. The gelation mechanism of ECO is attributed to its semicrystalline conformation, hydrophobic character, and the number of hydrogen bonds formed between polymer chains (Patil and Patel, 2021). Specifically, when EC is heated above 140 °C, it completely melts in the oil, and during cooling, the system recovers its rigid structure, and restructuring of the hydrogen bonds occurs, eventually forming a polymer network to capture liquid oil (Qiu et al., 2023b).
When the food additive, colloidal silica, is combined with oil as an oleogelator (at least 10% content needs to be added) for inorganic particles, fractal aggregates are generated inside the system, forming a stable 3D network structure through hydrogen bonds and electrostatic interactions (Whitby, 2020).
Oleogels containing lecithin usually have a self-assembled network structure (Qu et al., 2022). Lecithin is amphiphilic. It forms inverted “spherical” micelles when combined with oil (a nonpolar solvent). However, when it is surrounded by a polar solvent (water), it promotes the uniaxial growth of micelles and forms intertwined “tubular” structures to trap flowing solvents (Qiu et al., 2023b). It is worth mentioning that multicomponent/element oleogels containing lecithin have better rheological properties and physical structure stability than single-component oleogels (Li et al., 2019; Qu et al., 2022). However, too much lecithin can destroy the extreme branching of the crystal structure and hinder the development of the oleogel network structure (Qiu et al., 2023a).
Indirect methods
Emulsion template method
To broaden the types of edible oleogels, the development of oleogels using emulsion templates has become a research hotspot. An emulsion contains two immiscible liquids—a polar water phase and a nonpolar oil phase. Therefore, emulsion template oleogels can be divided into two categories: (i) oil-in-water, wherein oil droplets are dispersed in the water phase to create a fluid-like system; (ii) water-in-oil, wherein water droplets are dispersed in the oil phase, maintaining the texture profile of the semirigid structure (Okuro et al., 2020).
In the emulsion template method, the hydrophilic oil coagulant is allowed to interact with liquid oil molecules through hydration to form an open or extended conformational polymer stacking network inside the system, and then the water phase in the hydrogel is removed to finally obtain an oleogel [Fig. 4(a)] (Puşcaş and Mureşan, 2022). In this process, the network interface layer inside the system must be strong enough to resist dehydration during the drying process. In addition, during the gelation process of the polymer oleogel, a suitable surfactant can be added to improve the degree of gelation (Liu et al., 2020; Qiu et al., 2023b). For example, chitin is highly biocompatible and degradable. When it is used alone as an oleogelator in combination with oil, it directly disperses in the system and has poor component efficiency, resulting in an unstable oleogel system. However, when used in combination with surfactants (sorbitan monostearic acid ester and phosphatidylcholine), it can significantly increase the strength of oleogels (Perța-Crișan et al., 2023). Similarly, the insufficient gel strength of oleogels prepared, for example, with propyl methyl cellulose, carboxymethylcellulose, and regenerated cellulose, can be improved by adding surfactants (Jiang et al., 2018; Lee, 2018).
A protein oleogel is another oleogel system widely prepared using the emulsion template method (Zheng et al., 2023). In these systems, synthetic proteins are usually used as emulsifiers to prepare emulsions and remove the aqueous phase. Note: the storage modulus (G′) and apparent viscosity of the emulsion are positively correlated with the polarity of the oil (Wang et al., 2022a). If additional polysaccharides or gelatin, xanthan gum, and other substances are added to the protein oleogel systems, the interfacial tension that prevents the coalescence of oil droplets can be strengthened, thereby improving the stability of oleogels (Scholten, 2019).
Solvent-exchange method
In this method, a protein network is formed within the aqueous medium (hydrogel) by introducing an intermediate organic solvent (such as tetrahydrofuran/acetone) to reduce the polarity of the gelling factor. Then, the organic solvent is replaced with oil through a dipping step to form an oleogel system [Fig. 4(b)] (Feichtinger et al., 2022).
Feichtinger et al. (2022) investigated the physical properties of oleogels prepared from three different vegetable proteins (potato, pea, and soybean protein isolates) and two animal proteins (egg protein isolates and whey protein isolates) using the solvent-exchange method. At the same native protein concentration, the smaller aggregate size of whey and potato protein oleogels led to higher gel strengths owing to more efficient network formation. In an apolar environment, the higher hydrophobicity of potato protein aggregates resulted in weaker attractive interactions compared to the more hydrophilic whey protein aggregates. These results show that the gel strength of protein oleogels can be controlled by both protein aggregate size and protein characteristics.
Jung et al. (2023) characterized oleogels prepared from mesoporous whey and potato protein-based aerogel microparticles. The results suggested that for both whey and potato protein isolates, different aerogel microstructures are induced by different gelation pH rather than by protein nature; the firmest oleogels for aerogel templates were produced at pH 9.0. In addition, low macropore volumes, high mesopore fractions, and high specific surface areas were found to increase the oil holding capacity. Overall, it showed that the strength of a protein oleogel is controlled by the size and nature of protein aggregates.
Foam template method
Because oleogels prepared using the foam template method do not require high-temperature drying or solvent-exchange processes like most hydrogel or emulsion templates, this method is promising for the preparation of oleogels (Alavi et al., 2020). In the foam template method, first, the protein solution is whipped and foamed using a mixer, quickly shaped under the support of liquid nitrogen, and then freeze-dried to remove water [Fig. 4(c)]. Note: an essential condition for system formation is the use of ideal “air–water interface” biopolymers.
The main feature of the oleogel prepared using the foam template method is its super-high oil absorption capacity (Alavi et al., 2020; Wei et al., 2022). Wei et al. (2022) developed rice bran protein oleogels for the first time through a facile low-temperature process using foam templates. It was observed that the foamability and the foam stability of rice bran protein were significantly enhanced when the pH was far from the isoelectric point (pH 4.6), which was mostly determined by changes in the secondary structure and surface hydrophobicity of rice bran protein. The foam prepared at pH 3.0 showed particularly high foam stability due to the formation of a strong interfacial film filled with tiny bubbles. Moreover, the gel strength of the oleogel was inversely correlated to the oil absorption rate of the corresponding cryogels.
Characterization of the physicochemical properties of oleogels
Characterization of structured lipid phases involves understanding the oleogel itself or determining the properties relevant to its application in food products (Flöter et al., 2021). The suitability of an oleogel depends on its critical gel concentration, solid fat content (SFC), texture, fatty acid composition, sensory properties, and spreadability (Pușcaș et al., 2021). Therefore, mastering the methods for detecting the physicochemical properties of oleogel is essential.
Characterization of the physical (macroscopic) properties of oleogels
Critical concentration
Determining the critical concentration of the oleogelator is the primary consideration in the preparation of oleogels. In general, the critical gel concentration refers to the minimum amount of oleogelator required for combining with oil to form a nonfluidizing soft solid that does not flow or leak (Pușcaș et al., 2021). Currently, the most widely used method for assessing the formation of oleogels is the “visual observation” method. In this method, the container containing the mixture is inverted or tilted to observe gelation. The conclusion is based on whether the resulting sample is freestanding or flowable (Hong et al., 2022).
Integrity
Another essential property used to characterize oleogel is the ability of the gel network to trap oil, known as the OBC. OBC refers to the amount of oil loading remaining in the oleogel structure after accelerated stability testing. Specifically, after centrifugation (usually at > 8000 × g for more than 20 min) of the structured lipids, the centrifuge tube containing the oleogel is inverted for approximately 30 min to drain the released supernatant. Then, the total ratio of the volume of the remaining sample to the original volume is calculated (Flöter et al., 2021; Qiu et al., 2023b). Omonov et al. (2010) developed another method for determining OBC. They evaluated the ability of the oleogelator to bind oil by measuring the weight gain of the filter paper on which a piece of structured fat was placed. Either way, the results of the oleogel’s OBC require rigorous scrutiny.
SFC
The SFC of an oleogel generally refers to the proportion of SFC in structured fat at a specific temperature. The SFC of oleogels is affected by their fatty acid composition and saturation as well as temperature (Borriello et al., 2022). Determining the SFC of the oleogel is important because it affects the appearance, plasticity, and sensory properties of the product (Sivakanthan et al., 2022). Although SFC was traditionally determined using the “expansion method,” the official method for determining the SFC of oleogels is the NMR method, which has been standardized (AOCS Cd 16b-93; ISO 8292-1 and IUPAC 2.150) (Pușcaș et al., 2021).
Texture and viscoelasticity
The texture profile analysis (TPA) test for textural evaluation of oleogels typically consists of a two-cycle penetration of the sample using a texture analyzer with a cylindrical P5 probe (Luo et al., 2019). Stickiness, chewiness, spreadability, cohesion, and hardness are the main textural parameters displayed by TPA. Among them, chewiness and hardness are important parameters for a measure of the strength or mechanical properties (firmness) of oleogels or oleogel products (Qiu et al., 2023b). Oleogel stickiness is defined as the force required to pull or remove a probe or knife from the sample (Qu et al., 2022). Cohesion is the degree of internal bonding that makes up the oleogel, with lower values indicating a more brittle product (Qu et al., 2022).
The deformation characteristics of oleogel are also important and cannot be ignored. The rheological analysis helps understand the breakdown properties of oleogel systems (Wang et al., 2022c). From a rheological point of view, an oleogel is a semisolid system with viscoelasticity. The rheological analysis of oleogel mainly includes temperature, frequency, amplitude sweep, and long-term monitoring (Flöter et al., 2021). Specifically, (i) it helps determine the gelling point by performing decreasing heat treatment or isothermal treatment on the oleogel (Sivakanthan et al., 2022); (ii) in the oscillatory rheological test, according to the G′ and loss modulus (G′′) values, an oleogel is classified as a viscous sol (G′′/G′ ≥ 1), weak coagulation oleogel (0.1 < G′′/G′ < 1), and strong oleogel (G′′/G′ ≤ 0.1) (Qiu et al., 2023b); (iii) thixotropic behavior is usually monitored by the three-interval thixotropy test (3ITT) time test. In the 3ITT, the sample is exposed to a low shear interval, a high shear interval, and then a repeat of the first low shear setting, where the recovery fraction of the last interval is used as the parameter for the viscosity change in the oleogel (Pușcaș et al., 2021). The viscosity change of oleogels can also be determined using the Casson model equation or flow curve fitting of the power law model (Li et al., 2023; Qu et al., 2022). Note: the SFC and microstructure of the oleogel can be used to determine the rheological parameter information (Meng et al., 2019).
Appearance
A visual inspection (color) of the overall appearance of the oleogel is required to determine whether the use of the oleogel in food preparation will negatively impact consumer acceptance. In addition, the color change of oleogels in the fresh state or during storage must be characterized by reflectance measurements using a colorimeter for the analysis of the opacity or lightness (L*), redness/greenness (a*), and blueness/yellowness (b*) in the color spectra.
Characterization of the physical (micrometric) properties of oleogels
Microstructure
Discussing the construction of the network structure of oleogel systems is a challenging task. Different types of microscopes can be used for microscopic characterization due to the birefringence of structural fat crystals (Pușcaș et al., 2021). Different devices allow visualization of the structure of oleogels at different depths and resolutions. The formation of network structures during gelation can be observed at the micron scale using polarized light microscopy (PLM) and ordinary optical inverted fluorescence microscopy (Qiu et al., 2023b). The structure and structural arrangement of molecules within a system can be studied at the nanoscale using atomic force microscopy (AFM) and scanning electron microscopy (SEM) (Flöter et al., 2021). The self-assembly or self-aggregation of gelling factors during the initial stages of the gelation of oleogels, a process that is affected by the surface roughness or surface area of the oleogelator, can also be imaged using SEM. Moreover, light microscopy is often used in conjunction with SEM (Qiu et al., 2022). Furthermore, cryo-transmission electron microscopy (cryo-TEM) allows analysis of the morphology of fundamental building blocks (Jansen-van Vuuren et al., 2023). In summary, ordinary light microscopy and PLM can provide detailed information on crystal morphology, whereas SEM, AFM, and cryo-TEM can provide better visualization of crystal network structures.
Building unit structure
During the gelation of oleogels, TAGs assemble into nanosheets at the nanoscale, which leads to the crystallization behavior of the lipid structures within the system (Li et al., 2022a). In addition, crystals undergo polymorphic transitions depending on changes in the cooling rate and shear force during gelation (Brykczynski et al., 2022).
X-ray diffraction (XRD) techniques (including wide-angle XRD) and small-angle X-ray scattering (SAXS) can be used to examine the details of crystal morphology, size, alignment, and internal molecular packing within oleogel systems (Qu et al., 2022). The detection results of XRD are usually given in the form of a longitudinal intermolecular packing arrangement, which is determined by the long and short distances of the crystal (Bragg peak location) (Pușcaș et al., 2021).
Crystal types include hexagonal α- and γ-forms that are less stable and have slightly larger lamellar thickness dimensions of TAG chains. After heat treatment, the α- and γ-forms transform into the β′-form, which is orthorhombically aligned and has metastable properties, or the β-form, which is a stable parallel triclinic form of MAGs. Among them, the β′-crystal form mainly determines the structure and rheological parameters of the oleogels and indirectly imparts the plasticity and mouthfeel of the oleogels. The β-polymorphic form is used to assess the strength of the oleogel as well as the density of the gelling factor (Qiu et al., 2023b).
Interactions between gelling factors
Because the gelation process of oleogels involves individual aggregate − aggregate interactions, such as hydrogen bonding, Van der Waals interactions, π − π stacking, or metal coordination, exploring the interactions between structurant molecules and between the oleogelator and liquid oil is essential (Li et al., 2022a).
The gelation mechanism of oleogels can be studied using Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy. In addition, the specific chemical bonds determined using Raman spectroscopy and the absorbance read obtained using FTIR in a specific wavelength region can help determine the influence of external factors on the oleogel and track the changes in the oleogel particle size and particle aggregation during storage (Zhang et al., 2023a).
Thermal stability and phase change analysis
Usually, the gelation of oleogels prepared using the direct method requires heating and cooling cycles. In addition, the shelf life of oleogel products and the temperature conditions they can withstand during transportation are also important aspects that need to be verified (Sivakanthan et al., 2022).
DSC can be used to determine the “thermal behavior” of oleogels, including onset melting and crystallization points (Puscas et al., 2021). The “falling ball method” is among the most commonly used methods for determining the melting point of oleogels (Ash et al., 2019). Because oleogels become more fluid-like when heated, the gel − sol transition temperature can be recorded during heating by sliding a ball with a melting point apparatus. In addition, the thermal behavior of the oleogels can be calorimetrically analyzed by obtaining the crystallization and melting enthalpy values corresponding to the exothermic and endothermic peak areas (Ash et al., 2019). In general, the ratio between the pure oleogelator and the enthalpy of fusion of the oil determines the percentage crystallization of the oleogels, and lower entropy values are generally associated with thermally stable oleogels (Fayaz et al., 2020).
Characterization of the chemical properties of oleogels
Fatty acid composition
Because oleogels are lipid products, the fatty acid composition of oleogels or oleogel products can be determined using GC or GC–MS techniques (Pușcaș et al., 2021). Due to the complexity of the interaction between gelation factors and the effect of shear force or temperature on the quality of fatty acids and lipophilic bioactive active substances during gelation, different assays have been developed for different oleogel products. Sobolev et al. (2022) revealed the effects of different chemical compositions of waxes, free fatty acids, and fatty alcohol chain lengths on oleogels; the fatty acid composition of isolated samples was determined using HPLC with evaporative light scattering detection. Puşcaş et al. (2021) evaluated the changes in the fatty acids of oils and oleogels during oxidation by measuring the content of harmful compounds using HPLC with diode array detection.
Oxidation properties
In the traditional detection of lipid oxidation, the peroxide value (PV) represents the concentration of the primary oxidation products of lipid products, and Codex Alimentarius has approved this method for the detection of primary oxidation products in oleogels (Qiu et al., 2021). PV can be determined through titration (sodium thiosulfate as titration solvent) or spectrophotometry (oxidation of ferrous to ferric ions). Typical methods for detecting the secondary oxidation products of lipid products corresponding to PV include thiobarbituric acid-reactive substances (TBARS) and p-anisidine value (p-AV). Regardless of the method used, although test results can provide individual oxidation product values, they cannot provide complete information on major or minor degradation compounds (Qiu et al., 2022). In addition, traditional lipid oxidation assays require the use of large quantities of high-purity reagents and, for some variants, high-speed centrifugation. Therefore, there are potential safety hazards, environmental pollution, and time-consuming and labor-intensive disadvantages (Qiu et al., 2023a). Moreover, it has been demonstrated in our previous studies that the 2-thiobarbituric acid value cannot be used to represent the oxidation value of oleogels (Qiu et al., 2021, 2023a).
To improve the stability and accuracy of oleogel oxidation detection, Wang et al. (2019) used HPLC to monitor the propionaldehyde content in an oleogel. Hwang et al. (2018) developed a method to judge oleogel oxidation based on color penetration. The method was based on the assumption that the rate of dye diffusion was related to the diffusion of oxygen in the sample; that is, more oxidized samples showed more dye “bleeding.” Furthermore, proton nuclear magnetic resonance spectroscopy (pNMR) is a powerful technique for studying the degradation of fatty acids in oleogels and detecting the primary or secondary oxidation products present therein (Qiu et al., 2021, 2022). pNMR records the oxidation of oleogels in terms of the absorption intensity of electromagnetic radiation induced by the covalent electrons of the oleogel and the chemical shift (ppm) of functional groups representing the corresponding chemical products. It should be noted that some compounds detected using pNMR may exhibit overlapping peaks (Alexandri et al., 2017). Moreover, we developed a fast discriminant model for determining the oxidation of oleogels using an electronic nose combined with cluster analysis (CA), principal component analysis (PCA), discriminant factor analysis (DFA), and linear discriminant analysis (LDA) (Qiu et al., 2023a). Our results showed that combining an electronic nose with GC can improve the accuracy of detecting oxidation products in oleogels.
In vitro simulated digestion process of oleogels
Oleogels have been proven to be effective vehicles for incorporating active ingredients, such as curcumin (Kavimughil et al., 2022; Li et al., 2019; Palla et al., 2022; Xia et al., 2022), astaxanthin (Wang et al., 2022b, 2022d; Zhao et al., 2023), β-carotene (Barragán-Martínez et al., 2022a; Zhang et al., 2022, 2023b), and resveratrol (Qiu et al., 2021, 2023b; Qu et al., 2022). Oleogels impart specific structural and textural properties to products, and the structure of food, including the gel strength and network density of the internal structure, OBC, and rheological behavior, impact the nutritional properties, accessibility of lipophilic bioactive substances, and overall digestive fate of the product (Qiu et al., 2023b; Wang et al., 2022b).
The digestibility of a lipid system is related to its lipid formulation, so its digestibility can be expressed in terms of the degree and rate of lipolysis (Cofrades et al., 2022). Specifically, in vitro digestion of simulated food (oleogels) goes through three steps from ingestion to absorption. (i) Digestion begins with the oral phase. This stage occurs in a physicochemical environment with a near-neutral pH and the presence of salivary amylase (α-amylase), which plays a major role in the cleavage of starch (Wojtunik-Kulesza et al., 2020). However, in the mouth, food hardly dissolves but rather breaks down into clumps after chewing (Pugh et al., 2023). (ii) The chewed food is then swallowed through the coordinated movement of the tongue, pharynx, and esophagus, and it reaches the stomach to enter the second stage of digestion. This phase is characterized by the presence of mechanical forces (peristalsis) and gastric enzymes at low pH (pH ≈ 1.0–3.0) (Okuro et al., 2021). Among them, gastric lipase, an acid-resistant enzyme, is responsible for initiating approximately 10–30% of lipid hydrolysis and preferentially esterifying it to fatty acids at the sn-3 carbon position of TAG. Furthermore, the combined effect of the emulsifying properties of lipid digestion products and shear forces leads to the formation of lipid droplet emulsions with small diameters (1–50 μm) (Okuro et al., 2021). Notably, the subsequent hydrolysis during digestion is improved due to the initial cleavage of the fat and the formation of an emulsion (Wang et al., 2022d). (iii) Finally, the mass is digested in the gastric phase for 0.5–4 h and then moves into the intestinal digestion phase (Li et al., 2019). This stage is characterized by the presence of the mass in a near-neutral pH environment, with high ionic strength, bile salts, and pancreatic enzymes (Qiu et al., 2023b). Bile salts anchor lipase at the interface of fat droplets, remove other components from the surface of fat globules, and promote the migration of the agglomerates to the intestinal mucosa for final digestion and absorption (Okuro et al., 2021). Therefore, the digestion of oleogels composed of TAG and oleogelator occurs mainly in the stomach and intestine (Li et al., 2019; Qu et al., 2022). The intestinal phase is especially responsible for the breakdown of most components of liposomes, and immune signaling pathways usually run through the entire intestinal phase, which also determines the final absorption of lipophilic bioactive substances (Liu et al., 2020; Okuro et al., 2021; Qiu et al., 2023b).
Examples of successful applications of oleogels in food products
Due to the functional properties of oleogels and their benefits to human health, much attention has been focused on increasing the practical applications of oleogels as solid fat substitutes in baked goods and cakes, meat and dairy products, spreads/sauces, and confectionery (Li et al., 2022a; Silva et al., 2023a; 2023b; Zhao et al., 2022). Table 1 summarizes the typical examples in which oleogels have been used as fat substitutes in food products.
Baked goods and cakes
Texture plays an important role in the quality of bakery food products, such as biscuits and bread, and the moisturizing effect of fat on gluten determines the softness of the product (Quilaqueo et al., 2022). Ye et al. (2019) structured EC with different viscosities (7, 20, 50, and 100 cP) and 1% glyceryl monostearate for bread processing, aiming to develop new formulations to prepare stable and soft bread ingredients. The oleogel-based bread had a specific firmness and showed a comparable volume to the control sample (Ye et al., 2019). Hwang et al. (2022) investigated the feasibility of using oleogels made from distinct types of vegetable oils and natural waxes instead of traditional margarine to make biscuits. It was found that all biscuits containing oleogel had similar physicochemical properties to biscuits made with commercial margarine, including crispness, firmness, and modulus of extension. In addition, the network structure properties and sensory evaluation results of the dough containing oleogel were better than those of the margarine dough (Hwang et al., 2022).
There is a positive correlation between the quality of a cake and the air permeability of its internal structure. Low-viscosity batters retain air bubbles during whipping, which results in less volume expansion in the product (Pușcaș et al., 2020). To reduce the fat content and calorific value of a cake and increase the viscosity of the batter, Badem and Baştürk (2023) studied the effect of safflower oil-based BW and RBW oleogel on the quality of cake samples. The acceptance of cakes made with oleogels was comparable to that of cakes made with shortening, and neither oil gelation nor baking temperature significantly changed the fatty acid content of the cake.
Fat is a key ingredient in producing satisfying gluten-free baked goods. Demirkesen and Mert (2019) compared cakes made from batters containing different beeswax oleogels (BWOs) and shortening mixtures and found that the overrun values of the batter samples decreased when BWO was used alone. After 100% replacement of shortening with BWO, the porosity and specific volume of the samples decreased. However, the replacement of 15% shortening with 45% and 30% BWO resulted in batter and baked product properties comparable to those of the control product.
Meat products
Meat products typically contain 35% SFA, which can increase the risk of cardiovascular disease in consumers who consume more than the recommended limit (Perța-Crișan et al., 2022). However, SFA is closely linked to the taste, internal structure stability, and texture of meat products (López-Pedrouso et al., 2021). Researchers have proved through a plethora of experimental results that oleogel is a feasible solution to replace plastic fat in meat products, providing a new formula that is both nutritious and can maintain the characteristics of traditional meat products.
Recently, Igenbayev et al. (2023) conducted a study on the replacement of lard with BWO in semi-smoked sausages. SFA levels were reduced by 35 and 38% in samples that partially replaced pork fat with 7 and 10% oleogel, respectively. Adding oleogel to sausages improved the fatty acid profile (decreased stearic and palmitic acids and increased linoleic acid). In addition, the uniformity of the internal crystal grain structure of the sausage increased as the amount of oleogel increased. Martins et al. (2019) developed a recipe for a hamburger pork patty using an oleogel with a mixture of linseed oil and P/Y. The results showed that the firmness and chewiness of patties formulated with 25% oleogel and subcutaneous pork fat were comparable to those of commercially available patties. Furthermore, the production of oleogel-based patties did not incur additional costs. Han et al. (2023a) improved the lubrication and sensory properties of meat analogs using a protein-stabilized oleogel. In that study, the oleogel was integrated into a soybean protein gel induced by transglutaminase to set up a composite gel model for the meat analog. The results indicated that the addition of oleogel significantly enhanced oil release and improved fat-related sensory attributes. It was concluded that the prepared oleogel might be a potential candidate to improve the juiciness of the meat analog.
Dairy products
Butterfat is composed of considerable amounts of TAGs. Fat and fat particle size distribution determine the textural properties of dairy products, including their appearance, consistency, and shape during ripening (Pușcaș et al., 2022). For example, in ice cream, the amount of fat melted during consumption can affect consumer outcomes for sensory attributes such as flavor (Genovese et al., 2022). However, the size and shape of the fat crystals determine the number of air bubbles incorporated into the composition and the amount of melted fat during freezing (Airoldi et al., 2022). In addition, cream cheese (curd cheese) has a composition and function similar to that of milk fat (Cumhur and Kilic-Akyilmaz, 2022).
To reduce the cost and milk fat content and increase the nutritional value of dairy products, different kinds of oleogels have been successfully used as cream substitutes. Airoldi et al. (2022) developed CWO for use as a fat substitute in ice cream formulations. The oleogels were structured using 6%, 8%, and 10% CW with soybean and peanut oils. The oleogel fat replacement reduced the melting rate of ice creams, and a 50% replacement of the traditional lipid phase with CWO could be performed without causing sensory impairment. However, the waxy mouthfeel could potentially affect the acceptability of the product (Airoldi et al., 2022). Moon et al. (2021) evaluated the processing performance of canola–CW oleogel as a solid fat substitute in imitation cheese. Cheese samples produced with the oleogel were firmer and more viscous/chewier than the control, with no significant difference in meltability between cheeses using 3% and 6% oleogel instead of palm oil. Furthermore, the SFA level of the oleogel cheese was reduced significantly (from 45.70 to 5.20%).
Spreads
Due to the network of fat crystals, the fat content of spreads, which can be as high as 60%, largely determines the rheology, texture, and organoleptic properties of the spread (Espert et al., 2021). Therefore, substituting solid fats for liquid oils can greatly affect the quality of the spread. Pușcaș et al. (2022) used cold-pressed walnut oil oleogels structured with 10% waxes and MAG as a milk fat replacing system for reformulating chocolate-flavored butter. The results showed that all breakfast spreads with oleogels had higher hardness values and crystallinity than the commercially available breakfast spreads. Furthermore, candelilla wax-based walnut oil oleogels were the key to incorporating the nutritional properties (reduced SFC) ideal for the preparation of chocolate spreads. Gómez-Estaca et al. (2019) used different oleogels developed using EC and BW to replace part of the back fat in pork liver pâté, improving the fatty acid composition and nutritional content of the pâté. While the pork liver pâté developed using ECO exhibited greater conformational thermostability and flexibility without altering appearance or texture, its susceptibility to oxidation was higher than that of the control group. However, consumer acceptance of pork liver pâté containing BWO was comparable to that of the control sample (Gómez-Estaca et al., 2019).
Candies
Fat has the greatest impact on the quality and nutritional properties of confectionery products associated with composite materials (Pușcaș et al., 2022). For example, in chocolate manufacturing, cocoa butter (CB) or its substitutes and butterfat determine the tempering ability, texture, and melting point of the product (Li and Lu, 2023). Additionally, chocolate may undergo oil migration during storage. Oleogels have been reported as a viable alternative to CB for partially replacing the oil phase and controlling oil migration. Sun et al. (2021) replaced CB in chocolate with oleogels developed using β-sitosterol with γ-oryzanol/lecithin/stearic acid and corn oil. Chocolate prepared using β-sitosterol/γ-oryzanol oleogel and CB (1:1) had crystal structure, rheology, texture, and sensory properties similar to those of the control dark chocolate (Sun et al., 2021). Li et al. (2023a) evaluated the effects of replacing 50% and 100% of CB with oleogels using monoglyceryl stearate oleogel [MO], S/LO, and ECO. Among the chocolates prepared by replacing 50% of CB with oleogels, the hardness of ECO chocolate was higher than that of MO and S/LO chocolates due to the higher SFC in ECO chocolate. In all oleogel chocolate samples, only MO replacing 100% of CB had a solid appearance like the control (Li and Lu, 2023). Ghorghi et al. (2023) developed a novel BWO-based hybrid gel and applied it to compound chocolate formulations. Hybrid gels are double-component systems resulting from the combination of oleogel and hydrogel. The optimum hybrid gels were used to substitute 0, 15, 30, and 45% of CB in the formulation of compound chocolate. The samples with 15% substitution were most similar to the control samples, as determined by the whiteness index and the thermal, rheological, textural, and sensory property evaluations of the samples (Ghorghi et al., 2023).
Salama and Hashim (2022) developed four functional spreadable oleogels based on milk protein and canola oil and used them as an innovative model for the preparation of confectionery gummies. The results showed that the impact properties, oxidative stability, and sensory acceptance of the gummies developed using the spreadable oleogel were higher than those of the control samples.
In conclusion, in the past decade, although liquid oils have been successfully structured by different methods and the successful application of oleogels in food has been proven feasible, understanding the gelation mechanism of oleogels with different structurants remains challenging. Moreover, no food product currently available in the market uses oleogels as raw material. While the current scientific research and alternative fat formulations are encouraging, continued innovation is needed to meet the “clean fat product” requirement for reformulated foods that do not contain TFAs and are low in SFAs.
As mentioned in this report, there are many kinds of gelling methods and organic oleogelators. Thus, even if the oleogels developed with the same structurant are used in different products, there will be differences in their physicochemical and sensory properties. In the future, it is necessary to plan, classify, and sort out the developed oleogels and accurately identify the processing parameters and nutritional characteristics of specific foods based on existing research. Importantly, the large-scale production of oleogels should be facilitated, and consumer acceptance and consumption of foods containing oleogels should be promoted. In short, there are still gaps in the exploration of oleogels, and consequently, there is infinite room for improvement.
Abbreviations
- 3D:
-
Three-dimensional
- 3ITT:
-
Three-interval thixotropy test
- AFM:
-
Atomic force microscopy
- BW:
-
Beeswax
- BWO:
-
Beeswax oleogel
- CA:
-
Cluster analysis
- CB:
-
Cocoa butter
- CW:
-
Carnauba wax
- CWO:
-
Carnauba wax oleogel
- Cryo-TEM:
-
Cryo-transmission electron microscopy
- DFA:
-
Discriminant factor analysis
- EC:
-
Ethyl cellulose
- ECO:
-
Ethyl cellulose oleogel
- FTIR:
-
Fourier transform infrared spectroscopy
- G′:
-
Storage modulus
- G′′:
-
Loss modulus
- HMWPO:
-
High molecular weight polymer oleogel
- HPMC:
-
Hydroxypropyl methyl cellulose
- LDA:
-
Linear discriminant analysis
- LMMWO:
-
Low multicomponent/composite molecular weight oleogel
- LMWO:
-
Low molecular weight oleogel
- LSMWO:
-
Low single molecular weight oleogel
- MAG:
-
Monoacylglycerol
- MAGO:
-
Monoacylglycerol oleogel
- MO:
-
Monoglyceryl stearate oleogel
- Mw:
-
Molecular weight
- OBC:
-
Oil binding capacity
- p-AV:
-
p-Anisidine value
- PCA:
-
Principal component analysis
- PLM:
-
Polarized light microscopy
- pNMR:
-
Proton nuclear magnetic resonance spectroscopy
- PV:
-
Peroxide value
- P/Y:
-
Phytosterol + γ-oryzanol
- RBW:
-
Rice bran wax
- S/L:
-
β-Sitosterol + lecithin
- S/LO:
-
β-Sitosterol + lecithin oleogel
- SEM:
-
Scanning electron microscopy
- SFA:
-
Saturated fatty acid
- SAXS:
-
Small-angle X-ray scattering
- SW:
-
Sunflower wax
- TAG:
-
Triglyceride
- TBARS:
-
Thiobarbituric acid-reactive substances
- TFA:
-
Trans Fatty acid
- TPA:
-
Texture profile analysis
- XRD:
-
X-ray diffraction
References
Abdollahi M, Goli SAH, Soltanizadeh N. Physicochemical properties of foam‐templated oleogel based on gelatin and xanthan gum. European Journal of Lipid Science and Technology. 122: 1900196 (2020) https://doi.org/10.1002/ejlt.201900196
Abdolmaleki K, Alizadeh L, Nayebzadeh K, Baranowska HM, Kowalczewski PŁ, Mousavi KA. Potential application of hydrocolloid-based oleogel and beeswax oleogel as partial substitutes of solid fat in margarine. Applied Sciences. 12: 12136 (2022) https://doi.org/10.3390/app122312136
Abdolmaleki K, Alizadeh L, Nayebzadeh K, Hosseini SM, Shahin R. Oleogel production based on binary and ternary mixtures of sodium caseinate, xanthan gum, and guar gum: optimization of hydrocolloids concentration and drying method. Journal of Texture Studies. 51: 290-299 (2020) https://doi.org/10.1111/jtxs.12469
Adili L, Roufegarinejad L, Tabibiazar M, Hamishehkar H, Alizadeh A. Development and characterization of reinforced ethyl cellulose based oleogel with adipic acid: its application in cake and beef burger. LWT. 126: 109277 (2020) https://doi.org/10.1016/j.lwt.2020.109277
Aguilar-Zárate M, Macias-Rodriguez BA, Toro-Vazquez JF, Marangoni AG. Engineering rheological properties of edible oleogels with ethylcellulose and lecithin. Carbohydrate Polymers. 205: 98-105 (2019) https://doi.org/10.1016/j.carbpol.2018.10.032
Airoldi R, da Silva TLT, Ract JNR, Foguel A, Colleran HL, Ibrahim SA, da Silva RC. Potential use of carnauba wax oleogel to replace saturated fat in ice cream. Journal of the American Oil Chemists’ Society. 99: 1085-1099 (2022) https://doi.org/10.1002/aocs.12652
Alavi F, Tian Z, Chen L, Emam-Djomeh Z. Effect of CaCl2 on the stability and rheological properties of foams and high-sugar aerated systems produced by preheated egg white protein. Food Hydrocolloids. 106: 105887 (2020) https://doi.org/10.1016/j.foodhyd.2020.105887
Alexandri E, Ahmed R, Siddiqui H, Choudhary MI, Tsiafoulis CG, Gerothanassis IP. High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules. 22: 1663 (2017) https://doi.org/10.3390/molecules22101663
Alvarez-Ramirez J, Vernon-Carter EJ, Carrera-Tarela Y, Garcia A, Roldan-Cruz C. Effects of candelilla wax/canola oil oleogel on the rheology, texture, thermal properties and in vitro starch digestibility of wheat sponge cake bread. LWT. 130: 109701 (2020) https://doi.org/10.1016/j.lwt.2020.109701
Ash D, Majee SB, Biswas GR. Span 40/Tween 80-based soybean oleogels: Modeling of gelation kinetics and drug release. IJPSR. 10: 1000-09 (2019) https://www.researchgate.net/profile/Dipanjana-Ash/publication/334536825_SPAN_40TWEEN_80-BASED_SOYBEAN_OLEOGELS_MODELING_OF_GELATION_KINETICS_AND_DRUG_RELEASE/links/5d30395a458515c11c39523e/SPAN-40-TWEEN-80-BASED-SOYBEAN-OLEOGELS-MODELING-OF-GELATION-KINETICS-AND-DRUG-RELEASE.pdf
Badem Ş, Baştürk A. Oxidative stability and characterization of oleogels obtained from safflower oil‐based beeswax and rice bran wax and their effect on the quality of cake samples. Journal of the American Oil Chemists’ Society (2023) https://doi.org/10.1002/aocs.12694
Barragán-Martínez LP, Alvarez-Poblano L, Vernon-Carter EJ, Alvarez-Ramirez J. Effects of β-carotene on the color, textural, rheological and structural properties of canola oil/beeswax oleogel. Journal of Food Measurement and Characterization. 16: 3946-3956 (2022a) https://doi.org/10.1007/s11694-022-01449-4
Barragán-Martínez LP, Román-Guerrero A, Vernon-Carter EJ, Alvarez-Ramirez J. Impact of fat replacement by a hybrid gel (canola oil/candelilla wax oleogel and gelatinized corn starch hydrogel) on dough viscoelasticity, color, texture, structure, and starch digestibility of sugar-snap cookies. International Journal of Gastronomy and Food Science. 29: 100563 (2022b) https://doi.org/10.1016/j.ijgfs.2022.100563
Bascuas S, Espert M, Llorca E, Quiles A, Salvador A, Hernando I. Structural and sensory studies on chocolate spreads with hydrocolloid-based oleogels as a fat alternative. LWT 135: 110228 (2021a) https://doi.org/10.1016/j.lwt.2020.110228
Bascuas S, Morell P, Quiles A, Salvador A, Hernando I. Use of oleogels to replace margarine in steamed and baked buns. Foods. 10: 1781 (2021b) https://doi.org/10.3390/foods10081781
Blake AI, Co ED, Marangoni AG. Structure and physical properties of plant wax crystal networks and their relationship to oil binding capacity. Journal of the American Oil Chemists’ Society. 91: 885-903 (2014) https://doi.org/10.1007/s11746-014-2435-0
Borriello A, Miele NA, Masi P, Aiello A, Cavella S. Effect of fatty acid composition of vegetable oils on crystallization and gelation kinetics of oleogels based on natural wax. Food Chemistry. 375: 131805 (2022) https://doi.org/10.1016/j.foodchem.2021.131805
Brito GB, Peixoto VODS, Martins MT, Rosário DK, Ract JN, Conte-Júnior CA, Castelo-Branco VN. Development of chitosan-based oleogels via crosslinking with vanillin using an emulsion templated approach: structural characterization and their application as fat-replacer. Food Structure. 32: 100264 (2022) https://doi.org/10.1016/j.foostr.2022.100264
Brykczynski H, Wettlaufer T, Flöter E. Revisiting pure component wax esters as basis of wax‐based oleogels. Journal of the American Oil Chemists’ Society. (2022) https://doi.org/10.1002/aocs.12589
Callau M, Sow-Kébé K, Nicolas-Morgantini L, Fameau AL. Effect of the ratio between behenyl alcohol and behenic acid on the oleogel properties. Journal of Colloid and Interface Science. 560: 874-884 (2020) https://doi.org/10.1016/j.jcis.2019.10.111
Choi KO, Hwang HS, Jeong S, Kim S, Lee S. The thermal, rheological, and structural characterization of grapeseed oil oleogels structured with binary blends of oleogelator. Journal of Food Science. 85: 3432-3441 (2020) https://doi.org/10.1111/1750-3841.15442
Cofrades S, Saiz A, Pérez-Mateos M, Garcimartín A, Redondo-Castillejo R, Bocanegra A, Álvarez MD. The influence of cellulose ethers on the physico-chemical properties, structure and lipid digestibility of animal fat emulsions stabilized by soy protein. Foods. 11: 738 (2022) https://doi.org/10.3390/foods11050738
Cumhur O, Kilic-Akyilmaz M. Special processed cheeses, cheese spreads, and analogue cheeses. In Processed Cheese Science and Technology. pp. 269-295 (2022). Woodhead Publishing https://doi.org/10.1016/B978-0-12-821445-9.00009-1
da Silva TLT, Baeten V, Danthine S. Modifying sucrose esters oleogels properties using different structuration routes. Food Chemistry. 405: 134927 (2023) https://doi.org/10.1016/j.foodchem.2022.134927
Demirkesen I, Mert B. Utilization of beeswax oleogel‐shortening mixtures in gluten‐free bakery products. Journal of the American Oil Chemists’ Society. 96: 545-554 (2019) https://doi.org/10.1002/aocs.12195
Doan CD, To CM, De Vrieze M, Lynen F, Danthine S, Brown A, Patel AR. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chemistry. 214: 717-725 (2017) https://doi.org/10.1016/j.foodchem.2016.07.123
Dong L, Lv M, Gao X, Zhang L, Rogers M, Cao Y, Lan Y. In vitro gastrointestinal digestibility of phytosterol oleogels: Influence of self-assembled microstructures on emulsification efficiency and lipase activity. Food & Function. 11: 9503-9513 (2020) https://doi.org/10.1039/D0FO01642J
Du Q, Zhou L, Lyu F, Liu J, Ding Y. The complex of whey protein and pectin: Interactions, functional properties and applications in food colloidal systems–A review. Colloids and Surfaces B: Biointerfaces. 210: 112253 (2022) https://doi.org/10.1016/j.colsurfb.2021.112253
Espert M, Hernández MJ, Sanz T, Salvador A. Reduction of saturated fat in chocolate by using sunflower oil-hydroxypropyl methylcellulose based oleogels. Food Hydrocolloids. 120: 106917 (2021) https://doi.org/10.1016/j.foodhyd.2021.106917
Fayaz G, Calligaris S, Nicoli MC. Comparative study on the ability of different oleogelators to structure sunflower oil. Food Biophysics. 15: 42-49 (2020) https://doi.org/10.1007/s11483-019-09597-9
Feichtinger A, Scholten E. Preparation of protein oleogels: effect on structure and functionality. Foods. 9: 1745 (2020) https://doi.org/10.3390/foods9121745
Feichtinger A, Nibbelink DG, Poppe S, Bozzo L, Landman J, Scholten E. Protein oleogels prepared by solvent transfer method with varying protein sources. Food Hydrocolloids. 132: 107821 (2022) https://doi.org/10.1016/j.foodhyd.2022.107821
Flöter E, Wettlaufer T, Conty V, Scharfe M. Oleogels-their applicability and methods of characterization. Molecules. 26: 1673 (2021) https://doi.org/10.3390/molecules26061673
Genovese A, Balivo A, Salvati A, Sacchi R. Functional ice cream health benefits and sensory implications. Food Research International. (2022) https://doi.org/10.1016/j.foodres.2022.111858
Ghebreyesus TA, Frieden TR. REPLACE: a roadmap to make the world trans fat free by 2023. The Lancet. 391: 1978-1980 (2018) https://doi.org/10.1016/S0140-6736(18)31083-3
Ghorghi ZB, Yeganehzad S, Hesarinejad MA, Faezian A, Kutsenkova V, Gao Z, Nepovinnykh N. Fabrication of novel hybrid gel based on beeswax oleogel: application in the compound chocolate formulation. Food Hydrocolloids. 140: 108599 (2023) https://doi.org/10.1016/j.foodhyd.2023.108599
Gómez-Estaca J, Herrero AM, Herranz B, Álvarez MD, Jiménez-Colmenero F, Cofrades S. Characterization of ethyl cellulose and beeswax oleogels and their suitability as fat replacers in healthier lipid pâtés development. Food Hydrocolloids. 87: 960-969 (2019) https://doi.org/10.1016/j.foodhyd.2018.09.029
Guo S, Song M, Gao X, Dong L, Hou T, Lin X, Lan Y. Assembly pattern of multi-component supramolecular oleogel composed of ceramide and lecithin in sunflower oil: self-assembly or self-sorting? Food Function. 11: 7651-7660 (2020) https://doi.org/10.1039/D0FO00635A
Han C, Wang G, Guo J, Wang J, Yang X. Oral oil release improves lubrication and sensory properties of meat analogs with protein-stabilized oleogel. Food Hydrocolloids. (2023a) https://doi.org/10.1016/j.foodhyd.2023.108788
Han Z, Liu S, Cao J, Yue X, Shao JH. A review of oil and water retention in emulsified meat products: the mechanisms of gelation and emulsification, the application of multi-layer hydrogels. Critical Reviews in Food Science and Nutrition. (2023b) https://doi.org/10.1080/10408398.2023.2199069
Hong X, Zhao Q, Chen J, Ye T, Fan L, Li J. Fabrication and characterization of oleogels and temperature-responsive water-in-oil emulsions based on candelilla (Euphorbia cerifera) wax. Food Chemistry. 397: 133677 (2022) https://doi.org/10.1016/j.foodchem.2022.133677
Hwang HS, Fhaner M, Winkler‐Moser JK, Liu SX. Oxidation of fish oil oleogels formed by natural waxes in comparison with bulk oil. European Journal of Lipid Science and Technology. 120: 1700378 (2018) https://doi.org/10.1002/ejlt.201700378
Hwang HS, Kim S, Winkler‐Moser JK, Lee S, Liu SX. Feasibility of hemp seed oil oleogels structured with natural wax as solid fat replacement in margarine. Journal of the American Oil Chemists’ Society. 99: 1055-1070 (2022) https://doi.org/10.1002/aocs.12619
Igenbayev A, Ospankulova G, Amirkhanov S, Aldiyeva A, Temirova I, Amirkhanov K. Substitution of pork fat with beeswax-structured oleogels in semi-smoked sausages. Applied Sciences. 13: 5312 (2023) https://doi.org/10.3390/app13095312
Jansen-van Vuuren RD, Naficy S, Ramezani M, Cunningham M, Jessop P. CO 2-responsive gels. Chemical Society Reviews. (2023) https://doi.org/10.1039/D2CS00053A
Jiang Y, Liu L, Wang B, Sui X, Zhong Y, Zhang L, Xu H. Cellulose-rich oleogels prepared with an emulsion-templated approach. Food Hydrocolloids. 77: 460-464 (2018) https://doi.org/10.1016/j.foodhyd.2017.10.023
Jung D, Oh I, Lee J, Lee S. Utilization of butter and oleogel blends in sweet pan bread for saturated fat reduction: dough rheology and baking performance. LWT. 125: 109194 (2020) https://doi.org/10.1016/j.lwt.2020.109194
Jung I, Schroeter B, Plazzotta S, De Berardinis L, Smirnova I, Gurikov P, Manzzocco, L. Oleogels from mesoporous whey and potato protein based aerogel microparticles: Influence of microstructural properties on oleogelation ability. Food Hydrocolloids. 108758 (2023) https://doi.org/10.1016/j.foodhyd.2023.108758
Kavimughil M, Leena MM, Moses JA, Anandharamakrishnan C. 3D printed MCT oleogel as a co-delivery carrier for curcumin and resveratrol. Biomaterials. 287: 121616 (2022) https://doi.org/10.1016/j.biomaterials.2022.121616
Kim D, Oh I. The characteristic of insect oil for a potential component of oleogel and its application as a solid fat replacer in cookies. Gels. 8: 355 (2022) https://doi.org/10.3390/gels8060355
Lee S. Utilization of foam structured hydroxypropyl methylcellulose for oleogels and their application as a solid fat replacer in muffins. Food Hydrocolloids. 77: 796-802 (2018) https://doi.org/10.3390/gels8060355
Li L, Liu G. Engineering effect of oleogels with different structuring mechanisms on the crystallization behavior of cocoa butter. Food Chemistry. (2023a) https://doi.org/10.1016/j.foodchem.2023.136292
Li L, Liu G, Bogojevic O, Pedersen JN, Guo Z. Edible oleogels as solid fat alternatives: Composition and oleogelation mechanism implications. Comprehensive Reviews in Food Science and Food Safety. 21: 2077-2104 (2022a) https://doi.org/10.1111/1541-4337.12928
Li L, Wan W, Cheng W, Liu G, Han L. Oxidatively stable curcumin‐loaded oleogels structured by β-sitosterol and lecithin: physical characteristics and release behaviour in vitro. International Journal of Food Science & Technology. 54: 2502-2510 (2019) https://doi.org/10.1111/ijfs.14208
Li S, Song Q, Liu K, Zhang Y, Zhao G, Zhou Y. Emulsion-templated oleogels generated through solvent exchange: effects of miscibility of alcohols and oils. LWT. 176: 114545 (2023b) https://doi.org/10.1016/j.lwt.2023.114545
Li S, Wu G, Jin Q, Wang X, Zhang H. Relationship between the microstructure and physical properties of emulsifier based oleogels and cookies quality. Food Chemistry. 377: 131966 (2022b) https://doi.org/10.1016/j.foodchem.2021.131966
Li S, Wu G, Li X, Jin Q, Wang X, Zhang H. Roles of gelator type and gelation technology on texture and sensory properties of cookies prepared with oleogels. Food Chemistry. 356: 129667 (2021) https://doi.org/10.1016/j.foodchem.2021.129667
Li S, Zhu L, Li X, Wu G, Liu T, Qi X, Zhang H. Determination of characteristic evaluation indexes for novel cookies prepared with wax oleogels. Journal of the Science of Food and Agriculture. 102: 5544-5553 (2022c) https://doi.org/10.1002/jsfa.11909
Liu N, Lu Y, Zhang Y, Gao Y, Mao L. Surfactant addition to modify the structures of ethylcellulose oleogels for higher solubility and stability of curcumin. International Journal of Biological Macromolecules. 165: 2286-2294 (2020) https://doi.org/10.1016/j.ijbiomac.2020.10.115
López-Pedrouso M, Lorenzo JM, Gullón B, Campagnol PCB, Franco D. Novel strategy for developing healthy meat products replacing saturated fat with oleogels. Current Opinion in Food Science. 40: 40-45 (2021) https://doi.org/10.1016/j.cofs.2020.06.003
Luo SZ, Hu XF, Jia YJ, Pan LH, Zheng Z, Zhao YY, Jiang ST. Camellia oil-based oleogels structuring with tea polyphenol-palmitate particles and citrus pectin by emulsion-templated method: preparation, characterization, and potential application. Food Hydrocolloids. 95: 76-87 (2019) https://doi.org/10.1016/j.foodhyd.2019.04.016
Ma Y, Ye F, Chen J, Ming J, Zhou C, Zhao G, Lei L. The microstructure and gel properties of linseed oil and soy protein isolate based-oleogel constructed with highland barley β-glucan and its application in luncheon meat. Food Hydrocolloids. 140: 108666 (2023) https://doi.org/10.1016/j.foodhyd.2023.108666
Malvano F, Laudisio M, Albanese D, d’Amore M, Marra F. Olive oil-based oleogel as fat replacer in a sponge cake: a comparative study and optimization. Foods. 11: 2643 (2022) https://doi.org/10.3390/foods11172643
Marangoni AG, Garti N. Edible oleogels: structure and health implications. Elsevier (2018) https://books.google.co.kr/books?hl=zh-CN&lr=&id=kV1gDwAAQBAJ&oi=fnd&pg=PP1&dq=Marangoni,+A.+G.,+%26+Garti,+N.+(Eds.).+(2018).%C2%A0Edible+Oleogels:+Structure+and+Health+Implications.+Elsevier.&ots=TZecFL-SnW&sig=i4l43_4Fx9VwKiybv2zI7B_F3r4&redir_esc=y#v=onepage&q=Marangoni%2C%20A.%20G.%2C%20%26%20Garti%2C%20N.%20(Eds.).%20(2018).%C2%A0Edible%20Oleogels%3A%20Structure%20and%20Health%20Implications.%20Elsevier.&f=false
Martins AJ, Lorenzo JM, Franco D, Vicente AA, Cunha RL, Pastrana LM, Cerqueira MA. Omega‐3 and polyunsaturated fatty acids‐enriched hamburgers using sterol‐based oleogels. European Journal of Lipid Science and Technology. 121: 1900111 (2019) https://doi.org/10.1002/ejlt.201900111
Meng Z, Guo Y, Wang Y, Liu Y. Oleogels from sodium stearoyl lactylate-based lamellar crystals: structural characterization and bread application. Food Chemistry. 292: 134-142 (2019) https://doi.org/10.1016/j.foodchem.2018.11.042
Moon K, Choi KO, Jeong S, Kim YW, Lee S. Solid fat replacement with canola oil-carnauba wax oleogels for dairy-free imitation cheese low in saturated fat. Foods. 10: 1351 (2021) https://doi.org/10.3390/foods10061351
Oh I, Lee J, Lee HG, Lee S. Feasibility of hydroxypropyl methylcellulose oleogel as an animal fat replacer for meat patties. Food Research International. 122: 566-572 (2019) https://doi.org/10.1016/j.foodres.2019.01.012
Okuro PK, Martins AJ, Vicente AA, Cunha RL. Perspective on oleogelator mixtures, structure design and behaviour towards digestibility of oleogels. Current Opinion in Food Science. 35: 27-35 (2020) https://doi.org/10.1016/j.cofs.2020.01.001
Okuro PK, Santos TP, Cunha RL. Compositional and structural aspects of hydro-and oleogels: similarities and specificities from the perspective of digestibility. Trends in Food Science & Technology. 111: 55-67 (2021) https://doi.org/10.1016/j.tifs.2021.02.053
Omonov TS, Bouzidi L, Narine SS. Quantification of oil binding capacity of structuring fats: a novel method and its application. Chemistry and Physics of Lipids. 163: 728-740 (2010) https://doi.org/10.1016/j.chemphyslip.2010.07.003
Palla CA, Aguilera-Garrido A, Carrín ME, Galisteo-González F, Gálvez-Ruiz MJ. Preparation of highly stable oleogel-based nanoemulsions for encapsulation and controlled release of curcumin. Food Chemistry. 378: 132132 (2022) https://doi.org/10.1016/j.foodchem.2022.132132
Pan H, Xu X, Qian Z, Cheng H, Shen X, Chen S, Ye X. Xanthan gum-assisted fabrication of stable emulsion-based oleogel structured with gelatin and proanthocyanidins. Food Hydrocolloids. 115: 106596 (2021) https://doi.org/10.1016/j.foodhyd.2021.106596
Pang M, Kang S, Liu L, Ma T, Zheng Z, Cao L. Physicochemical properties and cookie-making performance as fat replacer of wax-based rice bran oil oleogels. Gels. 9: 13 (2022) https://doi.org/10.3390/gels9010013
Pascuta MS, Varvara RA, Teleky BE, Szabo K, Plamada D, Nemeş SA, Vodnar DC. Polysaccharide-based edible gels as functional ingredients: Characterization, applicability, and human health benefits. Gels. 8: 524 (2022) https://doi.org/10.3390/gels8080524
Patil U, Patel AR. Polysaccharide-based functional colloids for food applications. In Food, Medical, and Environmental Applications of Polysaccharides. Elsevier pp. 187-229 (2021) https://doi.org/10.1016/B978-0-12-819239-9.00004-X
Pérez-Álvarez JÁ, Roldán-Verdú A, Martínez-Mayoral A, Sayas-Barberá E, Vera CNRD, Viuda-Martos M, Fernández-Lopez J. Chia oleogel as a potential new ingredient for healthy cooked meat sausages. In Proceedings. MDPI. Vol. 70, No. 1, p. 76 (2020, November) https://doi.org/10.3390/foods_2020-07701
Perța-Crișan S, Ursachi CȘ, Chereji BD, Munteanu FD. Oleogels—innovative technological solution for the nutritional improvement of meat products. Foods. 12: 131 (2022) https://doi.org/10.3390/foods12010131
Perța-Crișan S, Ursachi CȘ, Chereji BD, Tolan I, Munteanu FD. Food-grade oleogels: trends in analysis, characterization, and applicability. Gels. 9: 386 (2023) https://doi.org/10.3390/gels9050386
Pintado T, Cofrades S. Quality characteristics of healthy dry fermented sausages formulated with a mixture of olive and chia oil structured in oleogel or emulsion gel as animal fat replacer. Foods. 9: 830 (2020) https://doi.org/10.3390/foods9060830
Pinto TC, Martins AJ, Pastrana L, Pereira MC, Cerqueira MA. Oleogel-based systems for the delivery of bioactive compounds in foods. Gels 7: 86 (2021) https://doi.org/10.3390/gels7030086
Pugh RJ, Hamlett CAE, Fairhurst DJA. Short overview of bubble in foods and chocolate containing bubbles. Advances in Colloid and Interface Science (2023) https://doi.org/10.1016/j.cis.2023.102835
Puşcaş A, Mureşan V. The feasibility of shellac wax emulsion oleogels as low-fat spreads analyzed by means of multidimensional statistical analysis. Gels. 8: 749 (2022a) https://doi.org/10.3390/gels8110749
Pușcaș A, Mureșan V, Muste S. Application of analytical methods for the comprehensive analysis of oleogels—a review. Polymers. 13: 1934 (2021) https://doi.org/10.3390/polym13121934
Pușcaș A, Mureșan V, Socaciu C, Muste S. Oleogels in food: A review of current and potential applications. Foods. 9: 010070 (2020) https://doi.org/10.3390/foods9010070
Pușcaș A, Tanislav AE, Mureșan AE, Fărcaș AC, Mureșan V. Walnut oil oleogels as milk fat replacing system for commercially available chocolate butter. Gels. 8: 613 (2022b) https://doi.org/10.3390/gels8100613
Qiu HT, Qiu ZZ, Chen ZY, Liu LL, Wang JB, Jiang HY, Liu GQ. Antioxidant properties of blueberry extract in different oleogel systems. LWT. 137: 110364 (2021) https://doi.org/10.1016/j.lwt.2020.110364
Qiu HT, Qu KX, Eun JB, Zhang H. Analysis of thermal oxidation of different multi-element oleogels based on carnauba wax, β-sitosterol/lecithin, and ethyl cellulose by classical oxidation determination method combined with the electronic nose. Food Chemistry. 405: 134970 (2023a) https://doi.org/10.1016/j.foodchem.2022.134970
Qiu HT, Qu KX, Zhang H, Eun JB. Thermal oxidation stability of different multi-element oleogels via 1H NMR spectroscopy. Food Chemistry. 394: 133492 (2022) https://doi.org/10.1016/j.foodchem.2022.133492
Qiu HT, Qu KX, Zhang H, Eun JB. Characterization and comparison of physical properties and in vitro simulated digestion of multi-component oleogels with different molecular weights prepared by the direct method. Food Hydrocolloids. 108850 (2023b) https://doi.org/10.1016/j.foodhyd.2023.108850
Qu KX, Qiu HT, Zhang H, Yin XF. Characterization of physically stable oleogels transporting active substances rich in resveratrol. Food Bioscience. (2022) https://doi.org/10.1016/j.fbio.2022.101830
Quilaqueo M, Iturra N, Contardo I, Millao S, Morales E, Rubilar M. Food-grade bigels with potential to replace saturated and trans fats in cookies. Gels. 8: 445 (2022) https://doi.org/10.3390/gels8070445
Salama HH, Hashim AF. A functional spreadable canola and milk proteins oleogels as a healthy system for candy gummies. Scientific Reports. 12: 12619 (2022) https://doi.org/10.1038/s41598-022-16809-9
Scholten E. Edible oleogels: how suitable are proteins as a structurant?. Current Opinion in Food Science. 27: 36-42 (2019) https://doi.org/10.1016/j.cofs.2019.05.001
Shakeel A, Farooq U, Gabriele D, Marangoni AG, Lupi FR. Bigels and multi-component organogels: an overview from rheological perspective. Food Hydrocolloids. 111: 106190 (2021) https://doi.org/10.1016/j.foodhyd.2020.106190
Silva RCD, Ferdaus MJ, Foguel A, da Silva TLT. Oleogels as a fat substitute in food: a current review. Gels. 9: 180 (2023) https://doi.org/10.3390/gels9030180
Silva TJ, Barrera‐Arellano D, Ribeiro APB. Oleogel‐based emulsions: concepts, structuring agents, and applications in food. Journal of Food Science. 86: 2785-2801 (2021) https://doi.org/10.1111/1750-3841.15788
Sivakanthan S, Fawzia S, Madhujith T, Karim A. Synergistic effects of oleogelators in tailoring the properties of oleogels: a review. Comprehensive Reviews in Food Science and Food Safety (2022) https://doi.org/10.1111/1541-4337.12966
Sobolev R, Frolova Y, Sarkisyan V, Makarenko M, Kochetkova A. Effect of beeswax and combinations of its fractions on the oxidative stability of oleogels. Food Biosciences. 48: 101744 (2022) https://doi.org/10.1016/j.fbio.2022.101744
Sun P, Xia B, Ni ZJ, Wang Y, Elam E, Thakur K, Wei ZJ. Characterization of functional chocolate formulated using oleogels derived from β-sitosterol with γ-oryzanol/lecithin/stearic acid. Food Chemistry. 360: 130017 (2021) https://doi.org/10.1016/j.foodchem.2021.130017
Tarté R, Paulus JS, Acevedo NC, Prusa KJ, Lee SL. High-oleic and conventional soybean oil oleogels structured with rice bran wax as alternatives to pork fat in mechanically separated chicken-based bologna sausage. LWT. 131: 109659 (2020) https://doi.org/10.1016/j.lwt.2020.109659
Tirgarian B, Yadegari H, Bagheri A, Neshagaran E, Mardani M, Farmani J. Reduced-fat chocolate spreads developed by water-in-oleogel emulsions. Journal of Food Engineering. 337: 111233 (2023) https://doi.org/10.1016/j.jfoodeng.2022.111233
Wang Q, Decker EA, Rao J, Chen B. A combination of monoacylglycerol crystalline network and hydrophilic antioxidants synergistically enhances the oxidative stability of gelled algae oil. Food & Function. 10: 315-324 (2019) https://doi.org/10.1039/C8FO00997J
Wang Q, Rao Z, Chen Y, Lei X, Zhao J, Li F, Ming J. Characterization of responsive zein-based oleogels with tunable properties fabricated from emulsion-templated approach. Food Hydrocolloids. 133: 107972 (2022a) https://doi.org/10.1016/j.foodhyd.2022.107972
Wang S, Chen K, Liu G. Monoglyceride oleogels for lipophilic bioactive delivery–Influence of self-assembled structures on stability and in vitro bioaccessibility of astaxanthin. Food Chemistry. 375: 131880 (2022b) https://doi.org/10.1016/j.foodchem.2021.131880
Wang Z, Chandrapala J, Truong T, Farahnaky A. Oleogels prepared with low molecular weight gelators:texture, rheology and sensory properties, a review. Critical Reviews in Food Science and Nutrition (2022c) https://doi.org/10.1080/10408398.2022.2027339
Wang Z, Gao Y, Wei Z, Xue C. Ovalbumin fibril-stabilized oleogel-based Pickering emulsions improve astaxanthin bioaccessibility. Food Research International. 161: 111790 (2022d) https://doi.org/10.1016/j.foodres.2022.111790
Wei F, Lu M, Li J, Xiao J, Rogers MA, Cao Y, Lan Y. Construction of foam-templated oleogels based on rice bran protein. Food Hydrocolloids. 124: 107245 (2022) https://doi.org/10.1016/j.foodhyd.2021.107245
Whitby CP. Structuring edible oils with fumed silica particles. Frontiers in Sustainable Food Systems. 4: 585160 (2020) https://doi.org/10.3389/fsufs.2020.585160
Willett SA, Akoh CC. Encapsulation of menhaden oil structured lipid oleogels in alginate microparticles. LWT. 116: 108566 (2019) https://doi.org/10.1016/j.lwt.2019.108566
Wojtunik-Kulesza K, Oniszczuk A, Oniszczuk T, Combrzyński M, Nowakowska, D., & Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—a non-systematic review. Nutrients. 12: 1401 (2020) https://doi.org/10.3390/nu12051401
Xia T, Gao Y, Liu Y, Wei Z, Xue C. Lactoferrin particles assembled via transglutaminase-induced crosslinking: Utilization in oleogel-based pickering emulsions with improved curcumin bioaccessibility. Food Chemistry. 374: 131779 (2022) https://doi.org/10.1016/j.foodchem.2021.131779
Yao Y, Zhou H, Liu W, Li C, Wang S. The effect of cooling rate on the microstructure and macroscopic properties of rice bran wax oleogels. Journal of Oleo Science. 70: 135-143 (2021) https://doi.org/10.5650/jos.ess20112
Ye L, Liu CL, Zhu HR, Luo JX. Experimental investigation on effect of cross-flow Reynolds number on film cooling effectiveness. AIAA Journal. 57: 4804-4818 (2019) https://doi.org/10.2514/1.J057943
Yılmaz E. Sensory properties and aromatics profile of edible oleogels. In Development of Trans-free Lipid Systems and their Use in Food Products. Royal Society of Chemistry. pp. 315-349 (2022) https://books.google.co.kr/books?hl=zh-CN&lr=&id=G3VdEAAAQBAJ&oi=fnd&pg=PT264&dq=Sensory+properties+and+aromatics+profile+of+edible+oleogels.&ots=-azKeg_0mz&sig=ATEAC-_iFjg9UgkVvHVPkVGBu3s&redir_esc=y#v=onepage&q=Sensory%20properties%20and%20aromatics%20profile%20of%20edible%20oleogels.&f=false
Yu Y, Wang T, Gong Y, Wang W, Wang X, Yu D, Wang L. Effect of ultrasound on the structural characteristics and oxidative stability of walnut oil oleogel coated with soy protein isolate-phosphatidylserine. Ultrasonics Sonochemistry. 83: 105945 (2022) https://doi.org/10.1016/j.ultsonch.2022.105945
Zhang H, Yang X, Zhong R, Huo Y, Zhu Y, Liang P. Antioxidative properties of fish roe peptides combined with polyphenol on the fish oil oleogel. Journal of the Science of Food and Agriculture. 103: 1714-1726 (2023a) https://doi.org/10.1002/jsfa.12336
Zhang P, Wang Y, Liu Y, Wu Y, Ouyang J. Improved stability of β-carotene by encapsulation in SHMP-corn starch aerogels. Food Chemistry. 406: 135040 (2023b) https://doi.org/10.1016/j.foodchem.2022.135040
Zhang R, Cui M, Ye J, Yuan D, Mao L. Physicochemical stability of oleogel-in-water emulsions loaded with β-carotene against environmental stresses. LWT. 155: 112965 (2022) https://doi.org/10.1016/j.lwt.2021.112965
Zhao W, Wei Z, Xue C. Recent advances on food-grade oleogels: fabrication, application and research trends. Critical Reviews in Food Science and Nutrition. 62: 7659-7676 (2022) https://doi.org/10.1080/10408398.2021.1922354
Zhao W, Wei Z, Xue C, Meng Y. Development of food-grade oleogel via the aerogel-templated method: oxidation stability, astaxanthin delivery and emulsifying application. Food Hydrocolloids. 134: 108058 (2023) https://doi.org/10.1016/j.foodhyd.2022.108058
Zheng S, Lu M, Xiao J, Zhang X, Li J, Zhang H, Lan Y. A novel strategy for preparation of rice bran protein oleogels based on high internal phase emulsion template. Journal of the Science of Food and Agriculture (2023) https://doi.org/10.1002/jsfa.12672
Acknowledgements
The authors acknowledge the support of the “Key R&D Program of the Northeast Cold Region Beef Cattle Science and Technology Innovation Engineering Research Center” through Project 111 (Project no. D20034).
Author information
Authors and Affiliations
Contributions
The authors confirm that this manuscript has not been published elsewhere and is not under consideration by any other journal. The authors have no potential conflicts of interest to disclose.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, H., Zhang, H. & Eun, JB. Oleogel classification, physicochemical characterization methods, and typical cases of application in food: a review. Food Sci Biotechnol 33, 1273–1293 (2024). https://doi.org/10.1007/s10068-023-01501-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-023-01501-z