Abstract
This study investigated the microbial inactivation effects of intense pulsed light (IPL) treatment as an alternative to chemical treatment for decontaminating the radish and pak choi seeds. The fR values (which indicate the resistance to IPL treatment) for radish and pak choi seeds were 24.50, 20.81 J/cm2, respectively. This resistance exhibited by seeds to IPL treatment is related to their surface roughness. Their Rq (the root-mean-square roughness), average surface roughness (Ra), and 10-point height roughness (Rz) values indicate that each crevice on a rough surface could shelter microorganisms from IPL. Viability tests of seeds exposed to IPL treatment indicated that the average germination rates of treated seeds exceeded 85% on day 3 of germination, which is considered as an acceptable criterion for germination. Also, on day 5 of germination the average shoot lengths of sprouts exposed to IPL did not differ significantly from those of untreated seeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sprouts such as alfalfa, mung bean, broccoli, mustard, and radish are now widely consumed due to benefits such as abundant proteins, minerals, and vitamins combined with a low calorific value (Kim et al., 2006; Sharma and Demirci, 2003; Waje and Kwon, 2007). They can also be harvested much more rapidly than common leaf vegetables, resulting in higher yields (Kim and Lee, 2010). Seed sprouts are usually consumed in a raw or slightly cooked form, such as in salads and sandwiches, which maintains their nutritional benefits (Kim et al., 2006). However, the consumption of raw sprouts is also a risk factor for food-borne diseases (Fransisca et al., 2011). Radish (Raphanus sativus L.) and pak choi (Brassica campestris L. ssp. chinensis var. communis) seed sprouts are common fresh foods in Korea. During their cultivation, they are exposed to several microbiological contaminants in the soil. So the most probable cause of food-borne diseases from seed sprouts is the contamination of seeds by indigenous pathogenic microorganisms. More than 40 food-borne diseases associated with raw seed sprouts were reported worldwide from 1973 to 2006 (Waje and Kwon, 2007). In Sakai City, Japan, 6000 people were infected with an Escherichia coli O157:H7 outbreak related to contaminated radish sprouts in 1996 (Taormina and Beuchat, 1999). Bacillus cereus and Salmonella spp. have also been associated with food-borne diseases related to contaminated seed sprouts (Fett, 2005). According to the report from the National Advisory Committee on Microbiological Criteria for Food (1999), the most probable cause of food-borne diseases from seed sprouts is the contamination of seeds by pathogenic bacteria in the soil. This prompted the US Food and Drug Administration to recommend treatment with 20,000 ppm free chlorine to decontaminate seeds before sprouting to prevent food-borne diseases (Fransisca et al., 2011; Kim et al., 2006; National Advisory Committee on Microbiological Criteria for Food, 1999; Sharma and Demirci, 2003). However, there are many reports on adverse side effects of chlorine treatment. Combining organic substances with a chlorine solution in water can result in the formation of harmful by-products such as THM (trihalomethanes), which are known to be carcinogens (Dunnick and Melnick, 1993; Fransisca et al., 2012; Li et al., 1996).
Intense pulsed light (IPL) is a nonthermal processing method that represents is an effective alternative inactivation technology to chlorine solutions. This technology involves the treatment samples with light covering a wide spectral range from the ultraviolet (UV) region to the near-infrared region (250–1100 nm) over a very short treatment time (Oms-Oliu et al., 2010). IPL can produce significant microbial reductions of both vegetative cells and spores within only a few seconds (Aron-Maftei et al., 2014), and it does not leave any residual compounds and results in minimal undesirable changes to samples. In recent years there have been many studies of the effects of IPL on solid foods such as vegetables, fruit, powdered foodstuffs, fish, and infant foods, and also semisolid foods (Kramer et al., 2017). It is therefore feasible that IPL treatment could also inactivate pathogenic bacteria contaminating sprout seeds without the loss of viability or other side effects. The objective of this study was to determine the effect of IPL on the inactivation of indigenous microorganisms on the surface of seeds of sprout vegetables commonly eaten in Korea without loss of seed viability such as germination and growth capability.
Materials and methods
Sample preparation
This study investigated radish and pak choi seeds purchased from an online source (Asia Seed Co., Seoul, Korea). The samples were kept at a typical refrigerator temperature (4 °C) and under 80% of relative humidity to maintain their viability and quality.
IPL treatment
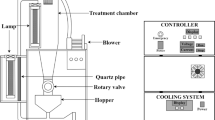
Two main items of equipment are required for applying IPL treatment: a power supply and a treatment chamber containing a lamp. The power supply provides electrical energy that is transferred to and stored in a capacitor. The electrical energy in the capacitor is then momentarily discharged to the lamp, which converts this into light energy with a very large pulse energy. A xenon lamp is used, and the treatment chamber contains a cooling fan that prevents overheating from the IPL treatment. The lamp used in this study (NL9553, Heraeus Noblelight, Cambridge, UK) is filled with xenon gas, and it radiation spans the wavelength range from 250 to 1100 nm.
Seeds were treated with IPL by placing 1 g of each seed type on a polystyrene antistatic weighing dish (square type 100 ml, SIMPORT®, Quebec, Canada) sterilized with 70% alcohol. The dish was placed on the vortex mixer (KMC-1300V, Vision Scientific Co., Gyeonggi, Korea) so entire sides of the seeds were treated with IPL. The lamp was placed 20 cm from the dish. The total fluence for inactivation varied from 0 to 37.80 J/cm2, which was achieved by applying lamp voltages of 1200–2400 V for 0–180 s. The fluence of IPL was measured using a spectroradiometer (ILT-900, International Light Technologies, Peabody, MA, USA).
Microbiological test
Seeds without visible defects were selected for IPL treatments. Sterile peptone water (2 ml) and 1 g of seeds were shaken on a shaking incubator (HB-201SF, Hanbaek Science, Gyeonggi, Korea) at 300 rpm for 5 min to blend the sample evenly. Then, 1 ml of the mixture was serially diluted with the 9 ml of the peptone water, and 0.1 ml of the diluted sample was plated on tryptic soy agar (TSA, Difco, Sparks, MD, USA) and incubated at 37 °C for 48 h to investigate the total aerobic mesophilic bacteria. Then, the number of colony-forming units (CFU/ml) was determined following the standard methods recommended by U.S. Food and Drug Administration “Aerobic Plate Count” method (United States Food and Drug Administration et al., 2016). The survival rate was expressed as the ratio of the initial number of bacteria (N0) to the number of surviving bacteria (N). The native cell counts of radish and pak choi seeds were 3.52 ± 0.05 and 4.11 ± 0.29 (mean ± standard deviation), respectively (80–90% of bacteria and 10–20% of molds and yeasts).
Kinetics of microbial inactivation by IPL
The Weibull equation is used as a general model for enzymatic microbial inactivation kinetics in food (Syamaladevi et al., 2013):
where N is the number of microorganisms surviving after exposure to the fluence of IPL f (this parameter is ‘time’ actually, but since time and fluence are directly proportional, time was substituted for fluence), N0 is the initial number of microorganisms, α is a scale factor, and β is a shape parameter that determines the shape of the Weibull curve. When β > 1 the curve has a convex shape, while β < 1 produces a concave curve that indicates that the microorganism has a higher resistance to IPL treatment. When β = 1, the model becomes a first-order kinetics model (Bialka et al., 2008).
The reliable life fluence (fR) is the fluence of IPL required for a 90% reduction in the number of microorganisms, and is analogous to the decimal reduction time (D value) in a first-order kinetics model. The value of fR is calculated from α and β (Bialka et al., 2008; Syamaladevi et al., 2015):
To compare the resistance to the IPL of radish and pak choi seeds, the fR value was derived using Excel (version 2016, Microsoft, Redmond, WA, USA) was used to analyze the data and parameters. The cutoff for statistical significance was p < 0.05.
Surface morphology of seeds
Surface roughness
The roughness of the seeds was determined using a surface profiler (DektakXT Stylus Profiler, Bruker, Billerica, MA, USA). The surface roughness was quantified using three parameters: Rq (root-mean-square roughness), Ra (average surface roughness), and Rz (10-point height roughness) (Woodling and Moraru, 2005).
Scanning electron microscopy
The surface morphology of the radish and pak choi was examined at the Korea Institute of Science and Technology using an environmental scanning electron microscopy (FEI XL-30FEG, FEI, Burlington, VT, USA). A sample preparation process such as coating or drying is not needed with this type of microscope. The surfaces were observed at a magnification of 1000 × under 40.00–93.33 Pa at 25 °C.
Seed germination and growth rate
To figure out the viability of seeds treated with IPL, germination and growth rate test were conducted. Twenty seeds were placed on 90 mm diameter filter paper (NO. 1, Whatman Ltd., Maidstone, England) in sterile petri dishes and the plates were cultured in the dark incubator at 25 °C for 5 days. The 5 ml of sterile water was added every 24 h for 5 days to provide sufficient humidity during germination.
In order to evaluate germination rate, the number of germinated seeds which is protruding at least 2 mm were counted every 24 h during 5 days (Kim et al., 2006). The germination rate was calculated by followed equation (Jiafeng et al., 2014):
where N is number of germinated seeds, Nt is the total number of seeds used for this study. So, Nt value was fixed at 20. And for evaluating the growth rate of seeds treated with IPL, the shoot and root length of seed sprouts which are protruding at least 2 mm was measured every 24 h, from 2 to 5 days after germination (Kim et al., 2006).
Statistical analyses
Each experiment was performed three times per sample. For statistical analysis, the SPSS program (version 22, IBM, Chicago, IL, USA) was used to determine significant differences with Duncan’s test.
Results and discussion
Inactivation kinetics of microorganisms on seed surfaces
The microbial inactivation effects of IPL treatment on the surface of radish and pak choi seeds were investigated as a function of IPL fluence for lamp voltages of 1200–2400 V and treatment times of 0–180 s, corresponding to pulse intensities from 0 to 37.80 J/cm2. The reductions of microorganisms on the surface of radish and pak choi are presented in Fig. 1. The microbial inactivation effect was greater for longer treatment times and higher energy intensities of IPL treatment. Also, the rate of the microbial inactivation was highest at the voltage of 2400 V. The maximum IPL fluence of 37.80 J/cm2 resulted in 1.41 and 1.78 log reductions of the microorganisms on radish and pak choi, respectively.
This study used the Weibull model to determine the sterilization tendency of seeds treated with IPL. According to previous researches (Bialka et al., 2008; Syamaladevi et al., 2015), the Weibull model provides a better fit than a first-order kinetics model to microbial inactivation. As shown in Fig. 2, it seemed that there is no significant difference between the log reduction level of radish and pak col. The obtained reliable life fluence values (fR) for radish and pak choi were 24.50 and 20.81 J/cm2, respectively. Although this study showed a similar value, higher fR values indicate that a higher IPL energy dose is required to decontaminate the surface of seeds of bacteria (Syamaladevi et al., 2015).
Effects of surface roughness on microbial inactivation of seeds by IPL treatment
Since IPL has a low penetrative ability, the surface characteristics of seed could be one of the most important factors affecting the microbial inactivation of seeds by IPL treatment. The surface morphologies of radish and pak choi were examined using environmental scanning electron microscopy at the Korea Institute of Science and Technology at a magnification of 1000 ×. As shown in Fig. 3, radish and pak choi seeds have surfaces those are quite rough and irregular, with many pores and crevices larger than 10 µm. Since many microorganisms are smaller 5 µm, these pores on the seed surface are large enough to shield bacteria from IPL. Previous studies (Syamaladevi et al., 2013; Wang et al., 2009) found that a washing solution could not reach microorganisms located inside surface crevices, and it was likely in the present study that the properties of the seed surfaces sheltered some of the bacteria from IPL treatment. Therefore, while IPL can decontaminate seeds of their microbial population, its effectiveness is likely to be highly dependent on the seed surface morphology (Syamaladevi et al., 2013).
To clarify the comparison of surface properties between radish and pak choi, the values of the root-mean-square roughness (Rq), average surface roughness (Ra), and 10-point height roughness (Rz) for radish and pak choi seeds are shown in Table 1. These all three values were higher for radish seeds. These results suggest that there is a positive correlation between the fR and the sample roughness. Syamaladevi et al. (2013) also found the fR of Escherichia coli inoculated on surface of pear and peach. When the pear and peach were UV-C treated, the fR value for intact pear surfaces (0.268 ± 0.017 kJ/m2) was smaller than for wounded pear (0.348 ± 0.024 kJ/m2) and peach surfaces (0.371 ± 0.012 kJ/m2). They mentioned that the wounds on pear and trichomes on peach surfaces affect to shield microorganisms from UV-C radiation. Adhikari et al. (2015) also found that the Rq and Ra values of the strawberries were higher than (296 μm and 287 μm, respectively) that of apples (30.3 μm and 25.4 μm) and pears (40.2 μm and 32.8 μm). And this attributed the microbial reduction level of strawberries, apples, and pears (0.9 ± 0.0 log, 2.9 ± 0.2 log, and 2.1 ± 0.1 log, respectively). Our findings are similar to those of them, and it means that there is a possibility that the roughness of the surface can be applied not only to the fruits but also to tiny seeds. To conclude, while IPL can decontaminate seeds of their microbial population, it is necessary to consider the surface morphology of seeds such as the surface roughness when applying IPL treatments to seeds.
Viability of seeds treated by IPL
Germination rate
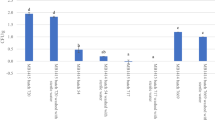
The germination rates of radish and pak choi seeds receiving IPL treatment were presented in Table 2. After 3 days of germination, the germination rate was 95–100% for radish seeds and 97–100% for pak choi seeds for total IPL fluences from 0 to 37.80 J/cm2. These results demonstrate that the germination capability of seeds was not significantly affected by the IPL fluence, and the percentage of germinated seeds under all treatment conditions exceeded 85%, which is considered as an appropriate standard for seed germination (Kim et al., 2006). A previous study found that the germination rate of wheat seeds was reduced by 14–15% after pulsed-light treatment at 1.0–32.0 J/cm2 (Aron-Maftei et al., 2014). Another study involving pulsed UV light (Sharma and Demirci, 2003) found that a longer treatment time and shorter distance between the lamp and sample could significantly reduce the germination rate and loss of viability of alfalfa seeds, and the authors attributed this to an excessive heating effect. In this study, the distance between the sample and the lamp was 20 cm and there was a cooling fan to prevent overheating, so there was no heating effect of the sample during the IPL process, which did not negatively affect the germination rate.
Growth rate of sprouts
As shown in Table 3, the shoot lengths of radish and pak choi seed sprouts which on day 5 of germination were 5.57–6.42 and 1.96–2.05 cm, respectively, for total fluences from 0 to 37.80 J/cm2. These results indicate that the growth rate of the seed sprouts was not adversely affected by IPL treatment, with no significant differences in the growth rates of radish and pak choi seed sprouts observed after IPL treatment (p < 0.05).
In conclusion, after applying IPL treatment to radish and pak choi seeds, the percentage of germinated seeds under all treatment conditions exceeded 85%, which is considered as an appropriate standard for seed germination. Moreover, the shoot lengths of radish and pak choi sprouts exposed to IPL did not differ significantly from those of untreated seeds. The germination and growth rates of seeds and their sprouts were not affected by IPL treatment that resulted in up to 2 log reductions of indigenous microorganisms on the seed surface. These findings indicate that IPL treatment is a promising inactivation technology for securing microbial safety while maintaining the viability of sprout seeds.
References
Adhikari A, Syamaladevi RM, Killinger K, Sablani SS. Ultraviolet-C light inactivation of Escherichia coli O157: H7 and Listeria monocytogenes on organic fruit surfaces. Int. J. Food Microbiol. 210: 136–142 (2015)
Aron-Maftei N, Ramos-Villarroe AY, Nicolau AI, Martín-Belloso O, Soliva-Fortuny R. Pulsed light inactivation of naturally occurring moulds on wheat grain. J. Sci. Food Agric. 94: 721–726 (2014)
Bialka KL, Demirci A, Puri VM. Modeling the inactivation of Escherichia coli O157: H7 and Salmonella enterica on raspberries and strawberries resulting from exposure to ozone or pulsed UV-light. J. Food Eng. 85: 444–449 (2008)
Dunnick JK, Melnick RL. Assessment of the carcinogenic potential of chlorinated water: experimental studies of chlorine, chloramine, and trihalomethanes. J. Natl. Cancer Inst. 85: 817–822 (1993)
Fett WF. Interventions to ensure the microbial safety of sprouts. pp. 187–209. In: Microbiology of fruit and vegetables. Gorny JR, Yousef AE, Sapers GM (eds). CRC Press, Inc., Boca Raton, FL, USA (2005)
Fransisca L, Zhou B, Park H, Feng H. The effect of calcinated calcium and chlorine treatments on Escherichia coli O157: H7 87–23 population reduction in radish sprouts. J. Food Sci. 76: M404–M412 (2011)
Fransisca L, Park HK, Feng H. E. coli O157: H7 population reduction from alfalfa seeds with malic acid and thiamine dilauryl sulfate and quality evaluation of the resulting sprouts. J. Food Sci. 77: M121–M126 (2012)
Jiafeng J, Xin H, Ling LI, Jiangang L, Hanliang S, Qilai X,…, Yuanhua D. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci. Technol. 16: 54–58 (2014)
Kim DS, Lee KB. Physiological characteristics and manufacturing of the processing products of sprout vegetables. Korean J. Food Cook. Sci. 26: 238–245 (2010)
Kim HJ, Feng H, Kushad MM, Fan X. Effects of ultrasound, irradiation, and acidic electrolyzed water on germination of alfalfa and broccoli seeds and Escherichia coli O157: H7. J. Food Sci. 71: M168–M173 (2006)
Kramer B, Wunderlich J, Muranyi P. Recent findings in pulsed light disinfection. J. Appl. Microbiol. 122: 830–856 (2017)
Li JW, Yu Z, Cai X, Gao M, Chao F. Trihalomethanes formation in water treated with chlorine dioxide. Water Res. 30: 2371–2376 (1996)
National Advisory Committee on Microbiological Criteria for Foods. Microbiological safety evaluations and recommendations on sprouted seeds. Int. J. Food Microbiol. 52: 123–153 (1999)
Oms-Oliu G, Martín-Belloso O, Soliva-Fortuny R. Pulsed light treatments for food preservation. A review. Food Bioprocess Technol. 3: 13–23 (2010)
Sharma RR, Demirci A. Inactivation of Escherichia coli O157: H7 on inoculated alfalfa seeds with pulsed ultraviolet light and response surface modeling. J. Food Sci. 68: 1448–1453 (2003)
Syamaladevi RM, Lu X, Sablani SS, Insan SK, Adhikari A, Killinger K, …, Annapure U. Inactivation of Escherichia coli population on fruit surfaces using ultraviolet-C light: influence of fruit surface characteristics. Food Bioprocess Technol. 6: 2959–2973 (2013)
Syamaladevi RM, Adhikari A, Lupien SL, Dugan F, Bhunia K, Dhingra A, Sablani SS. Ultraviolet-C light inactivation of Penicillium expansum on fruit surfaces. Food Control, 50: 297–303 (2015)
Taormina PJ, Beuchat LR. Behavior of enterohemorrhagic Escherichia coli O157: H7 on alfalfa sprouts during the sprouting process as influenced by treatments with various chemicals. J. Food Prot. 62: 850–856 (1999)
United States Food and Drug Administration, Maturin L, Peeler JT. Bacteriological Analytical Manual. Chapter 3: Aerobic Plate Count. Available from: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm063346.htm. Accessed 1 Sept 2016 (2016)
Waje C, Kwon JH. Improving the food safety of seed sprouts through irradiation treatment. Food Sci. Biotechnol. 16: 1–6 (2007)
Wang H, Feng H, Liang W, Luo Y, Malyarchuk V. Effect of surface roughness on retention and removal of Escherichia coli O157: H7 on surfaces of selected fruits. J. Food Sci. 74: E8–E15 (2009)
Woodling SE, Moraru CI. Influence of surface topography on the effectiveness of pulsed light treatment for the inactivation of Listeria innocua on stainless steel surfaces. J. Food Sci. 70: M345–M351 (2005)
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affais (MAFRA) (317030-3) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017079924).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SM., Hwang, HJ., Cheigh, CI. et al. Bactericidal effect of intense pulsed light on seeds without loss of viability. Food Sci Biotechnol 28, 281–287 (2019). https://doi.org/10.1007/s10068-018-0456-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0456-4