Abstract
Ultraviolet-C (UV-C 254 nm) light is a possible alternative for chemical disinfection of fresh fruits. However, studies on the influence of surface characteristics on the kinetics of UV-C inactivation of microorganisms on fruits are limited. In this study, UV-C inactivation of generic Escherichia coli (ATCC 23716), a nonpathogenic surrogate strain for E. coli O157:H7, was inoculated onto the skin surface intact pear, pear with surface wounds, and the skin surface of intact peach. Disc shaped (0.057 m diameter × 0.01 m height) fruit surface were exposed at room temperature to UV-C light ranging from 0 to 7.56 ± 0.52 kJ/m2 and microbial inactivation kinetics was determined. Maximum reductions of 3.70 ± 0.125 log CFU/g were achieved for E. coli on intact pear surfaces (P < 0.05), with lesser reduction on wounded pear (3.10 ± 0.329 log CFU/g) and peach surfaces (2.91 ± 0.284 log CFU/g) after 4 min UV-C exposure at 7.56 kJ/m2 UV. The Weibull scale factor (α) values of UV-C inactivation for E. coli on an intact pear surface was 0.001 ± 0.0007 min (0.235 ± 0.001 kJ/m2), wounded pear surface, 0.003 ± 0.001 min (0.240 ± 0.002 kJ/m2) and peach surface, 0.004 ± 0.0004 (0.241 ± 0.0008 kJ/m2). The time required for a 90 % reduction in E. coli cell numbers or the reliable life time (t R) calculated with the Weibull model for intact pear surfaces (0.019 ± 0.009 min, 0.268 ± 0.017 kJ/m2) was smaller than for wounded pear (0.062 ± 0.013 min, 0.348 ± 0.024 kJ/m2) and peach surfaces (0.074 ± 0.012, 0.371 ± 0.012 kJ/m2), suggesting that the wounds on pear surfaces and trichomes (100–1000 μm) on peach surfaces helped to shield and protect microorganisms from UV-C radiation. There was likely a more uniform distribution of bacterial cells onto pear surfaces due to its smaller surface roughness, spreading coefficient, and hydrophobic nature compared to peach. Fourier transform infrared spectroscopy indicate that bacterial membrane damage (phospholipids, protein secondary structures, and polysaccharides) and changes to DNA/RNA in E. coli resulted from UV-C treatment. UV-C can reduce E. coli populations on fresh fruit surfaces, but the efficacy of UV treatment is dependent upon the morphological and surface properties of the fruit and surface integrity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemical sanitizers such as hypochlorite solution can leave a chemical residue (Beuchat et al. 1998) on the fruit surface and may not be effective (Sapers 2001). Alternatives to chemical treatment for surface sanitation of fresh fruits and vegetables have been studied (Novak et al. 2008; Bialka and Demirci 2007) including ultraviolet light (200 and 280 nm (UV-C), more specifically, at 254 nm) which can be effective for microbial inactivation on fruits and vegetable surfaces (Table 1) (Bintsis et al. 2000; González-Aguilar et al. 2001; Cia et al. 2007; Allende and Artés 2003; Erkan et al. 2001). However, kinetic parameters of UV-C inactivation of microorganisms on different food surfaces are not reported, and comparative data for different fruit surfaces subjected to the same treatment are not readily available. UV-C has been approved by FDA for the inactivation of microorganisms on food product surfaces and reduction of microorganisms in juice products (US-FDA 2011).
The efficacy of surface disinfection by UV-C on fruit surfaces is influenced by several factors including: UV-C dose (Joules per square meter), UV-C dose rate (watts per square meter), exposure time (second), surface characteristics, and initial bacterial inoculum level (Otto et al. 2011) and bacterial type. Since UV-C light has limited penetration depth, plant morphological characteristics such as roughness and presence of wounds on fruit surfaces impact microbial inactivation (Wong et al. 1998; Woodling and Moraru, 2005; Schenk et al. 2008); understanding these influences is needed if this technology is to be commercialized. However, little information is available on the influence of fruit surface properties on the efficacy of UV-C for surface decontamination.
The established microbial inactivation mechanism by UV-C exposure is DNA dimer formation (Cutler and Zimmerman 2011). Further, indirect photochemical effects such as free radical formation may also induce ultra-structural changes (Cutler and Zimmerman 2011). Biochemical and morphological changes to bacteria from exposure to continuous UV-C in food matrices is not well understood. However, recent experiments with Fourier transform infrared (FT-IR) spectroscopy examined ultra-structural and chemical changes in microorganisms exposed to continuous UV-C showing changes to microbial cell membrane composition, for example in a study of Cronobacter sakazakii in dry infant formula following treatment with UV radiation (Liu et al. 2012). Infrared spectral features reflect the biochemical compositions of cell wall and cell membranes and can elucidate both the nature and degree of microbial cell injury (Lu et al. 2011a, b, c, d).
The objectives of this study were to determine how surface properties of fruit affect UV-C inactivation of generic E. coli and investigate the type and degree of cell injury using morphological, physical, and spectroscopic methods.
Materials and Methods
Treatment Surfaces and Target Microorganism
Fresh D'Anjou pears and O'Henry peaches were purchased from a local retail store (Dissmore's IGA, Pullman, WA) during July to September 2011, and stored at 4 °C for less than 2 weeks before conducting the experiments.
The National Advisory Committee on the Microbiological Criteria for Foods recommends the use of surrogate microorganisms in place of pathogens in pilot plant studies involving food (Gurtler et al. 2010). Therefore, in the current work, a generic Escherichia coli (ATCC 23716) strain obtained from School of Food Science, Washington State University was selected as a surrogate microorganism due to safety concerns. Since the main purpose of this research was not to design/validate an inactivation process but to understand the influence of fruit surface morphology and physical characteristics on bacterial inactivation by UV-C, use of a surrogate was appropriate. This microbe has been used as a surrogate for E. coli O157:H7 in many thermal and non-thermal process studies (Yuk et al. 2009; Geveke and Brunkhorst 2008; Jin et al. 2008) including UV-C inactivation studies on egg white (Geveke 2008).
Inoculum Preparation
The E. coli culture was stored in 30 % (w/w) glycerol (20 % water v/v) at −80 °C in tryptic soy broth (TSB, Hardy Diagnostics, Santa Maria, CA). Frozen cultures were activated by two successive passages, first inoculating 0.1 ml in 9 ml of TSB and incubated at 37 °C for 18 to 24 h. Then, 1 ml of the inoculum was added to 100 ml of TSB and incubated at 37 °C for 18 to 24 h. This stationary phase culture served as a stock culture for inoculation of fruit surfaces. The average initial inoculum level in all the experiments was 4.5 ± 1.2 × 109 CFU/ml.
Fruit Surface Preparation
Fresh whole pears or peaches were washed with distilled water. The fruits were then air dried inside a biological safety cabinet for 0.5–1 h at room temperature to remove surface moisture. A sharpened, ethanol-sterilized stainless steel cutting disc and knives were used to slice axial section of the pears and peaches into 0.057 m diameter and approximately 0.01 m thick discs (approx. 30 g) leaving the peel on. Each fruit disc was kept on the sterile Petri dishes with the peel surface facing up. A sterile needle was used to wound surfaces of pear slices (one wound per fruit disc, in the equatorial zone; 2 mm diameter and 1 mm depth. The stock culture was mixed vigorously by hand 25 times in a 30-cm arc, then 0.5 ml of the E. coli ATCC 23716 culture was aseptically and uniformly inoculated onto the peel surfaces of pear and peach fruits and wounded region of pear slices. UV-C treatments were carried out approximately 10 min after inoculation.
Ultraviolet-C Treatment
The ultraviolet-C treatment of the fruit discs were carried out inside a UVC Emitter™ Table-top System (Reyco Systems, Meridian ID) at a wavelength of 254 nm at room temperature. This equipment consists of a motorized roller conveyor (base) placed below an array of four 110 V 16-inch Steril-Aire™ UVC Emitters™ mounted in a stainless steel hood (0.45 × 0.30 m). The height of these arrays above the base was adjustable from 0.05 to 0.2 m height above the base. The UVC array was comprised of four Steril-Aire™ 16SE food-grade, shatter resistant, sleeved UVC Emitters™ mounted in bulk head fittings. For the current experiments, the UVC emitters were adjusted to 0.1 m above the fruit disks during irradiation treatments. The UV power was measured using a UV radiometer (EIT UVICURE PLUS II, EIT, Inc., Sterling, VA, USA). Based upon the preliminary experiments, specific UV doses of 0.59 ± 0.07, 1.14 ± 0.08, 2.16 ± 0.16, 4.00 ± 0.33, 5.71 ± 0.26, and 7.56 ± 0.52 kJ/m2 corresponding to 0.25, 0.5, 1, 2, 3, and 4 min were selected to treat the inoculated pear and peach discs at the center of the UV-C chamber. A digital timer was used to control the UV exposure times. The temperature of the chamber was 23 °C as monitored using a digital thermometer, and no change in the temperature was observed during the time of UV-C exposure used in this study. Inoculated and non-irradiated fruit discs were used as control. Sample preparation and UV-C treatment was conducted inside a Class II laminar hood to avoid post-irradiation contamination.
Microbial Cell Enumeration
After the UV-C treatments, the fruit discs were aseptically transferred into separate sterilized stomacher bags containing 100 ml of sterile 0.1 % peptone water (Becton, Dickinson and Co., Cockeysville, MD). The samples were blended (Stomacher® 400 CIRCULATOR, Seward Laboratory Systems Inc. Port Saint Lucie, FL, USA) for 3 min. A 1-ml portion of the supernatant was aseptically transferred into 9 ml 0.1 % peptone water and serial dilutions prepared, with 0.1 ml sample spread plated on tryptic soy agar (TSA, Hardy Diagnostics, Santa Maria, CA) in triplicate. Agar plates were incubated for 24 ± 2 h at 37 °C and colony forming units (CFU) counted. Each experiment was repeated at least three times. The initial level of colony forming units on pear and peach surfaces were approximately 2.05 ± 1.07 × 108 CFU/g for fruit slices.
UV-C Inactivation Kinetics
The Weibull equation has a shape factor, and hence it is more flexible in describing microbial inactivation kinetics (Cunha et al. 1998) and is used to model microbial, enzymatic, and other degradation reactions in foods (Odriozola-Serrano et al. 2009; Cunha et al. 1998). The Weibull equation is
where N is the number of surviving bacteria after time t, N o is the initial concentration of the microorganism, α is the scale factor, and γ is the shape parameter determining the shape of the curve. The value of γ > 1 yields a survival curve with a convex shape; while a value of γ < 1 yields a curve with a concave shape which indicates higher microbial resistance. When γ = 1, the Weibull model is equivalent to the first order model. The values of α and γ are determined by non-linear optimization. The reliable life time (t R), estimated from Weibull parameters (α and γ), is the time required for 90 % reduction in the number of target microorganism (Van Boekel 2002) and is similar to decimal reduction time (D value). The value of t R can be estimated from
where α is the scale factor and γ is the shape parameter of the Weibull equation.
Characterization of Fruit Surfaces
Microscopy Techniques
Environmental Scanning Electron Microscopy
The fresh pear and peach fruit samples were cut from their outside surfaces into 2–3 mm slices with a stainless steel razor and analyzed using an environmental scanning electron microscope (ESEM) (Quanta 200 ESEM, FEI Co. [Field Emission Instrument], Hillsboro, OR) with magnifications from 100 to 800. At least two to three slices from different parts of each fruit sample were taken for surface morphology analysis (N = 3) and micrographs generated (Leica Microsystems Inc., Buffalo Grove, IL, USA).
Atomic Force Microscopy
To determine surface roughness, external fruit surfaces of 1 cm2 were mounted to atomic force microscopy (AFM) sample disks (Hershko et al. 1998; Yang et al. 2005), and measurements taken using a Veeco Multimode Picoforce coupled with NanoScope IIIa controller, a 3 × 3 μm2 J-scanner and a silicon cantilever. The resonance frequency was 200–300 kHz, and nominal spring constant was 40 N/m, respectively, with a scan rate of 1.5 Hz. The integral and proportional gains were 0.3 and 0.5, respectively. At least three different locations on each fruit peel surface were imaged (N = 2).
Contact Angle and Surface Energy Determination
Contact angle is a measure of surface hydrophobicity and was conducted using a sessile drop method using a face contact angle set-up equipped with a camera (VCA Optima, AST Products Inc., MA, USA) (Bernard et al. 2011). External skin sections from pear and peach of approximately 2 × 2 cm2 and 1 mm thickness were cut with a sharp knife. Small drops (0.5–1.0 μl) of a polar liquid (double-distilled water) or a nonpolar liquid (diiodomethane (99 % purity; Sigma-Aldrich) were deposited onto the fruit surfaces using a microliter syringe and a 0.5-mm diameter needle at room temperature (23 °C). Twenty data points were taken for each fruit sample (N = 20). Side-view images were captured using a camera and the contact angles between the drops and the surfaces were calculated.
Surface Energy Calculation
The surface energy of the fruit peel surfaces was determined from contact angle measurements using Fowkes' equation (Ribeiro et al. 2007; Bernard et al., 2011):
- W a :
-
Reversible work of adhesion milliNewton per meter (mN/m)
- γ L :
-
Surface energy of the liquid (mN/m)
- θ :
-
Contact angle between solid and liquid
- γ L d :
-
Dispersion component of the surface energy of the liquid (mN/m)
- γ S d :
-
Dispersion component of the surface energy of the solid (mN/m)
- γ L p :
-
Polar component of the surface energy of the liquid (mN/m)
- γ S p :
-
Polar component of the surface energy of the solid (mN/m)
- W c :
-
Cohesion coefficient (mN/m)
- W s :
-
Spreading coefficient (mN/m)
- W a :
-
is related to spreading or adhesion of the liquid on the solid surface while W c is the cohesion of liquid molecules causing contraction (Ribeiro et al. 2007). The spreading coefficient (W s), also known as wettability, is related to spreading of liquid on the solid surface (Ribeiro et al. 2007).
Microscopy for E. coli ATCC 23716
Environmental Scanning Electron Microscopy
Following UV treatment (0, 2.16, and 7.56 kJ/m2 UV doses), the fruit discs were aseptically transferred into separate sterilized stomacher bags containing 100 ml of sterile 0.1 % peptone water. The samples were blended for 3 min. Bacterial samples were fixed with glutaraldehyde and osmium tetroxide, then rinsed using 0.1 M phosphate buffer and dehydrated with ethanol/water in increasing concentrations of ethanol (30 %, 70 %, 95 %, 100 %). After dehydration, the bacteria samples were sputter coated with gold and examined (Machado et al. 2010) using an environmental scanning electron microscope (Quanta 200 ESEM, FEI Co [Field Emission Instruments], Hillsboro, OR).
FT-IR Spectroscopy
After UV-C treatment (0 and 7.56 kJ/m2 UV doses) and homogenization by stomacher, FT-IR spectra were taken on the supernatant (10 mL). Spectral interference from food matrices is the challenge with this technique, and filtration as previously described (Liu et al. 2012, 2011a) was used in the current study. Supernatant was filtered through a 10.0-μm pore size polycarbonate membrane filter (K99CP04700; GE Water & Process Technologies, Trevose, PA) and then through a 0.2-μm pore size aluminum oxide membrane filter (25 mm diameter, Anodisc, Whatman Inc., Clifton, NJ) under vacuum to harvest bacterial cells. The anodisc membrane filter was removed from the Whatman vacuum filtration apparatus (Whatman Catalog number 1960–032) and air dried under laminar flow at room temperature for 30 min yielding a homogeneous film of bacterial cells (Lin et al. 2004).
A Nicolet 380 FT-IR spectrometer (Thermo Electron Inc., San Jose, California) was used to collect spectral features of recovered bacteria. The aluminum oxide membrane filter coated with a layer of bacterial cells was placed in direct contact with the diamond crystal cell of attenuated total reflectance (ATR) detector. FT-IR spectral features were recorded at the wavenumbers of 4,002 to 399 cm−1 with a spectral resolution of 8 cm−1, and each spectrum was added together by 32 interferograms. Eight spectra were acquired for untreated and UV-C treated E. coli at different locations on the aluminum oxide membrane filter for a total of 24 spectra for each group of bacterial cells (N = 3).
Spectroscopic-Based Chemometric Analyses
FT-IR spectra were automatic baseline corrected following a smooth of Gaussian function of 9.463 cm−1. The processed spectra were read by Matlab (Math Works Inc., Natick, MA). The spectral reproducibility was determined by calculating D y1y2 according to the procedure of Liu et al. (2012). Second derivative transformations (with a gap value of 12 cm−1) were conducted to magnify the visualization of minor differences among raw spectra (Lu et al. 2011c). Two different types of chemometric models were established to segregate untreated and UV-C-treated samples based upon the spectral features between 1,800 to 900 cm−1 (“fingerprint” region). Principal component analysis (PCA), an unsupervised chemometric method, was used to generate a two-dimensional model for segregation of different samples into distinct clusters (Lu et al. 2010). Hierarchical cluster analysis is a supervised chemometric method using prior knowledge (i.e., sample name) to create a dendrogram for category differentiation (Lu et al. 2011a, b, c, d).
Statistical Analysis
The data for inactivation of E. coli by UV-C were analyzed for statistical significance using SAS 9.1 (SAS Institute, Inc., Cary, NC). A value of P < 0.05 was selected as statistically significant using the two-way ANOVA by Fisher's least significant difference (LSD) method. UV-C treatment time and type of surface were the two factors considered for the two-way ANOVA analysis. Further, one-way ANOVA by Fisher's least significant difference (LSD) method was also performed when no interaction between UV-C treatment time and type of surface was found E. coli inactivation rate. Further, we conducted contrast test to determine the statistical significance in log reductions between surfaces at each UV-C exposure time individually.
Results and Discussion
UV Inactivation Kinetics of E. coli on Fruit Surface
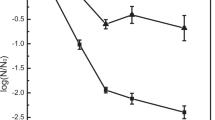
The average population of E. coli on fruit surfaces prior to UV-C treatment was 2.1 ± 1.1 × 108 CFU/g. UV-C treatment significantly reduced the number of E. coli on intact pear skin, wounded pear skin, and peach skin surfaces (P < 0.05) (Fig. 1). No significant interaction between treatment surface and time was observed (P ≥ 0.05). Cell numbers decreased significantly (P < 0.05) during the first 2 min of treatment by 3.59 ± 0.096 log CFU/g for intact pear surfaces and 2.60 ± 0.069 to 2.50 ± 0.151 log CFU/g for E. coli cells on the wounded pear skin and intact peach skin. UV-C was relatively ineffective beyond 2 min treatment time, and no significant difference in E. coli inactivation was observed between 2 min (dose 4.00 ± 0.33 kJ/m2) (P ≥ 0.05) and 4 min (dose 7.56 ± 0.52 kJ/m2) treatments for all three surfaces. The difference in log reductions in E. coli population between intact pear and peach surfaces was not significant (P ≥ 0.05) after 15 and 30 s of UV exposure. The log reduction of E. coli on pear surface was significantly higher than that of peach and wounded pear surface at 1.0 min (P < 0.05) and beyond reflecting the poor UV-C penetration within the damaged pear tissue and the protective effect of the hair-like projections (trichomes) on the surface of the peach. Inactivation of E. coli was lower for wounded pear and peach surfaces compared to intact pear surfaces. No significant difference in E. coli inactivation by UV-C was found for wounded pear and peach surfaces (P ≥ 0.05).
A number of earlier studies have been conducted to determine the effectiveness of UV irradiation on microbial control on food surfaces (Table 1). In general, UV can be effective and potentially more effective than chemical sanitizers. Inactivation of E. coli in foods appears to be predominantly from a non-thermal effect (Geveke 2008). At equivalent UV-C intensities, the log reduction in the population of E. coli in egg white increased from 1.63 to 2.48 log CFU/g when the temperature increased from 30 °C to 50 °C (Geveke 2008). However, the effect of temperature was negligible as only 0.13 log CFU/g reduction in the E. coli population was observed at 50 °C without UV energy (Geveke 2008). Yaun et al. (2004) used UV light to reduce the population of Salmonella spp. and E. coli O157:H7 on leaf lettuce, tomato, and apples surfaces and found that UV-C was more effective against these foodborne pathogens than 20–320 ppm chlorine (Yaun et al. 2004). Schenk et al. (2008) reported inactivation (2.6 to 3.4 log CFU/g reduction) in the populations of Listeria innocua, Listeria monocytogenes, E. coli, and Zygosaccharomyces bailii on pear slices without peel with lower reduction ranging from 1.8 to 2.5 log CFU/g on pear slices with the peel attached. In studies with other food products, UV has been found to provide greater inactivation of microbes on the surface rather than in the underlying tissue. UV light at 20 mW/cm2 reduced the population of E. coli O157:H7 between 1.53–2.14 log CFU/g on blueberry calyx and 3.11–5.53 log CFU/g on blueberry skin following 1–10 min treatments (Kim and Hung, 2012) and was more effective than electrolyzed water and ozone inactivating E. coli O157:H7 (Kim and Hung, 2012) (Table 1). Manzocco et al. (2011) reported reductions in Enterobacteriaceae between 1.65–2.14 log CFU/g on fresh-cut melon cubes exposed to UV-C light. However, a direct comparison between the inactivation rates of similar/different microorganisms on different/similar surfaces respectively may not be possible, as the survival of microorganisms depends upon several other factors such as type of strain, initial inoculums level, surface characteristics, and growth conditions (Guerrero-Beltran and Barbosa-Canovas 2004).
The E. coli inactivation kinetics by UV-C treatment on intact pear, wounded pear, and peach surfaces (Fig. 1) fitted a non-linear Weibull model (R 2 = 0.99). The α (0.003 ± 0.001 min, 0.240 ± 0.002 kJ/m2) values and reliable life time (t R = 0.062 ± 0.013 min, 0.348 ± 0.024 kJ/m2) of UV-C inactivation kinetics of E. coli on the wounded pear surface were significantly greater than (P < 0.05) those of intact pear surface (α = 0.001 ± 0.0007 min, 0.235 ± 0.001 kJ/m2), t R = 0.019 ± 0.009 min (0.269 ± 0.017 kJ/m2) (Table 2). Further, no significant difference in the values of α (0.004 ± 0.0004 min, 0.241 ± 0.0008 kJ/m2) and reliable life time (t R = 0.074 ± 0.012 min, 0.371 ± 0.022 kJ/m2) for UV-C inactivation kinetics of E. coli were observed between peach and wounded pear surfaces (P ≥ 0.05). Further, the α values and reliable life time (t R) of UV-C inactivation kinetics of E. coli on peach surface were significantly greater than that of intact pear surface (P < 0.05). This may indicate that the population of E. coli may have differing susceptibility to UV-C exposure over time (Van Boekel 2002) but more likely indicates that the penetration of UV radiation may not be sufficient to target cells entrained within the interstitial spaces of plant tissue. UV effectiveness is matrix dependent. Chun et al. (2009) reported Weibull scale factor (α) values for the UV inactivation of L. monocytogenes, S. enterica Typhimurium, and Campylobacter jejuni on agar plates were 0.78, 0.82, 0.78 J/m2, respectively, where the reliable life time (t R) values were 2.48, 2.39, and 2.18 J/m2, respectively.
Surface Characteristics of Fruits
The surface characteristics of fruits may influence the effectiveness of UV-C inactivation of E. coli. Environmental scanning electron microscopy (ESEM) shows surface characteristics of intact pear (Fig. 2a), wounded pear (Fig. 2b), and peach (Fig. 2c). The greater survival of E. coli on wounded pear and peach surfaces could be attributed to the shielding of microbes by the wounds on pear surfaces and the trichomes on peach surfaces (Fig. 3). Trichomes are approximately 100–1,000 μm, being 10–100 times larger than the E. coli cells.
Food surface properties such as hydrophobicity, electric charge, and roughness may influence the adhesion and distribution of bacterial cells on food surface (Araujo et al. 2010). Contact angle is related to the hydrophobicity of the surface and spreadability of liquid on the surface. The contact angles (θ) for water on intact pear and peach surfaces were 96.8 ± 7.7 and 138.7 ± 4.7°, and for diiodomethane, they were 38.7 ± 5.0 and 56.1 ± V9.1°, respectively (Fig. 4 and Table 3). Velasquez et al. (2011) reported contact angles for selected test liquids on 16 fruit surfaces where the determined contact angle for water on pear surface was 89.7°. In general, surfaces with water contact angle value > 65 are considered to be hydrophobic where θ < 65 are considered to be hydrophilic (Vogler 1998). Also, contact angle θ = 0 indicate complete wetting, 0 < θ < 90 indicates surface spreading of the liquid and θ > 90 indicates a surface upon which the liquid forms a bead (Woodling and Moraru 2005). Contact angle measurements indicated that pear and peach surfaces were hydrophobic in nature; however, pear surfaces were less hydrophobic than peach surfaces; hence a broader spatial distribution of bacterial cells could be achieved (Choi et al. 2002). Furthermore, the peach surface and trichomes possess a cuticular covering, containing high concentrations of the hydrophobic component cutan (Fernandez et al. 2011). The higher effectiveness of UV-C inactivation of E. coli cells on pear surfaces could also be attributed to the lower hydrophobicity of pear surfaces compared to peach. It is likely that there was greater adherence of bacteria along with a more uniform distribution of cells onto pear surfaces compared to peach surfaces. The greater effectiveness of the UV treatment for smooth pear surface may have been due to the lack of protective features such as trichomes, since based on physical properties alone, pear surface properties were more amenable to surface attachment of E coli cells.
Bacterial adhesion and surface distribution are dependent upon hydrophobicity. E. coli K12 is moderately hydrophilic group with θ < 65° (Mitik-Dineva et al. 2009; Burks et al. 2003). Thermodynamically, hydrophilic cells tend to adhere onto hydrophilic substrates (Bos et al. 1999), and hydrophobic interactions play an important role in the adhesion of hydrophilic cells to hydrophobic substrates (Ong et al. 1999). Ong et al. (1999) reported that E. coli D21 adhered more strongly to hydrophobic OTS-treated glass than hydrophilic glass. In the current study, hydrophilic E. coli cells may be adhered less strongly onto hydrophobic intact peach surfaces and this reduced attachment may have counteracted some of the protective effect of trichomes.
The surface energy values of the intact pear and peach surfaces were determined using Eqs. 3, 4, and 5. Surface free energy components for test liquids are presented in Table 4. Since surface energy of the solid (γ s) values were less than 100 mN/m, pear and peach surfaces are low energy surfaces with surface interactions with liquids being through apolar weak dispersive forces such as van der Waals forces (Zisman 1964; Velazquez et al. 2011). Surface free energy of more hydrophobic peach surfaces (36.6 ± 6.4 mN/m) was less than that of pear surfaces (40.6 ± 2.9 mN/m) (Table 3). Velazquez et al. (2011) reported surface energy values of 16 fruits including a pear, between 37 and 44 mN/m. Pear surfaces exhibited greater work of adhesion (W a = 64.3 ± 9.7 mN/m) and spreading coefficient/wettability (W s = −81.5 ± 9.5 mN/m) in comparison to the peach surfaces (where W a = 18.3 ± 3.9 mN/m; W s = 127.5 ± 3.9 mN/m), and this would support finding for better wetting, spreading, and distribution of E. coli on pear surfaces based upon surface angle measurements. Hydrophobic/hydrophilic interactions between substrates and bacterial surfaces play a major role in the adhesion/attachment of bacterial cells. Ong et al. (1999) reported that adhesion of more hydrophilic or high surface energy E. coli D21 cells was better on high energy substrate such as glass and mica. However, a clear understanding on the effect of surface adhesion of bacteria cells and UV-C inactivation is yet to be achieved, it can be presumed from these physical properties' results that washing with water would be ineffective in removing Gram-negative surface microflora because surface energy and surface features would encourage surface adhesion of bacteria to fruit surfaces.
The root mean square surface roughness (R q) was 2,136 ± 7 nm and the average surface roughness (R a) values (1,859 ± 12 nm) for the intact pear surface. Surface roughness analysis by AFM could not be conducted on peach surfaces due to their higher surface roughness and the presence of trichomes. The reported R a values of uncoated onion skin and shaved peach surface were 78 and 6.5 nm, respectively, while the R q value of shaved peach surface was 8.1 nm (Hershko et al. 1998; Yang et al. 2005). Greater surface roughness may result in increased surface adhesion of microorganisms due to increased surface area and potential shielding of bacteria which may result in protecting microbes from shear forces associated with washing steps (Scheuerman et al. 1998).
Inactivation Mechanism
Environmental Scanning Electron Microscopy
ESEM analysis revealed few readily discernible structural changes to E. coli following 1 and 4 min UV-C treatments (Fig. 5). Others have observed no structural disruption or surface irregularities from pulsed UV-treated Bacillus subtillis and Aspergillus niger using scanning electron microscopy (Levy et al. 2012).
Fourier Transform Infrared Spectroscopy
Spectroscopic methods, such as infrared spectroscopy, can determine the degree and chemical nature of bacterial injury caused by various antimicrobial treatments such as UV as shown here and in recent work (Liu et al. 2012); sonication (Lin et al. 2004), cold and freezing (Lu et al. 2011a), sanitizer treatments (Al-Qadiri et al., 2008a), heat (Al-Qadiri et al., 2008b), and exposure to bioactive compounds derived from vegetables (Lu et al. 2011a, b). Second derivative transformations and other chemometric models (i.e., PCA, HCA, and partial least squares regression, PLSR) are employed to either magnify minor biochemical compositional variations from raw spectral features or to segregate samples based upon treatment levels. Microbial cell injury detected spectroscopically has been verified by studies of bacterial survival, leakage of cellular contents, and ultrastructural changes resulting from physical and chemical treatments (Lu et al. 2011b, c, d).
FT-IR spectral features of intact pear surface following inoculation and after 4 min treatment with UV-C radiation at 7.56 kJ/m2 UV dose are shown in Fig. 6. Because of irregularities in food surfaces, to ensure reliable measurements, spectral reproducibility was determined (D y1y2 values ranged from 13.56 ± 2.15 to 17.89 ± 3.94), showing good reproducibility for each treatment. The intragroup variation of spectral features was significantly (P < 0.05) smaller than the intergroup variation of spectral features. Thus, spectral subtraction between groups was feasible (Liu et al. 2012). Spectral subtraction (Figs. 6b–a and c–a) was separately employed to remove spectral interference from the fruit surface allowing for examination of only the spectral features from the bacteria.
Second derivative transformations were performed to more easily examine the chemical compositional variations between E. coli cells before and after 4 min treatment of UV-C radiation at 7.56 kJ/m2 (Fig. 7). The band at 1,018 cm−1 is related to υ(CO), υ(CC), δ(OCH), and ring structure of polysaccharides and/or pectin (Movasaghi et al. 2008). The band at 1,105 cm−1 is assigned to carbohydrates (Lu et al. 2011a). The band shift from 1,240 to 1,224 cm−1 indicates DNA variations in bacterial cells before and after UV-C treatment. The band at 1,224 cm−1 is assigned to asymmetric stretching of phosphate groups of phosphodiester linkages in DNA and RNA (Naumann 2001), while the band at 1,240 cm−1 is assigned to PO2 asymmetric vibrations of nucleic acids (Naumann 2001). The band at 1,444 cm−1 is due to δ(CH2) of lipids and/or fatty acids (Lu et al. 2011a, b, c, d). The bands at 1,545 and 1,647 cm−1 are assigned to amide II and amide I (Lu et al. 2011a, b, c, d), respectively, both of which are secondary protein structures. Collectively, the variations of phospholipids, protein secondary structures, and polysaccharides are related to the bacterial cell membrane damage by UV-C treatment. Further, the DNA/RNA structural variations can be observed from second derivative transformed FT-IR spectra and have been validated in earlier studies that show that UV-C radiation distorts the DNA helix, which blocks microbial replication and subsequently causes E. coli death (Cutler and Zimmerman 2011).
Unsaturated organic compounds, which are the building structures of DNA and RNA and are important for cell maintenance including, pyramidines, purines and flavin are susceptible to UV-C radiation (Cutler and Zimmerman, 2011). Absorption of UV-C by these unsaturated organic compounds resulted in hydration of the nucleic acid base or base dimerization, i.e., DNA dimers (thymine and cytosine) and RNA dimers (uracil and cytosine) (Jagger, 1967; Cutler and Zimmerman, 2011). The most common photoproducts of nucleic acids by exposure to UV-C are cyclobutyl pyrimidine dimers (Guerrero-Beltran and Barbosa-Canovas, 2004). Cutler and Zimmerman (2011) reported that sugars and phosphates of nucleic acids do not absorb radiation above 210 nm; however, these FT-IR results show variation in phosphate and polysaccharides in the nucleic acids resulting in cell membrane damage.
Two types of chemometric models, namely PCA and HCA, were established and validated for segregation of untreated E. coli samples from UV-C treated E. coli samples (Fig. 8). The wavenumber regions between 1,800 and 900 cm−1 were selected for model analysis. The tight clusters (Fig. 8a) demonstrated significant differences (P < 0.05) between untreated and UV-C treated samples. In addition, the interclass distances based upon Mahalanobis distance measurement ranged from 10.29 to 13.42. Clusters with interclass distance values higher than 3 are believed to be significantly different from each other (Lu et al. 2011a, b, c, d). The composite dendrogram derived from hierarchical cluster analysis was well established and sorted on the basis of different groups (Fig. 8b). Taken together, both types of segregation chemometric models show that cell injury occurred since untreated and UV-C-treated bacterial samples could be clearly differentiated.
For scaling up of the UV-C process to use in the industrial level, it is important to identify the efficacy of UV-C light for the inactivation of pathogenic bacteria and other microorganisms on whole fruits including pear, and this research is progressing. Further, it is important to understand the energy required for surface disinfection of fruits by UV-C.
Conclusions
Physical and morphological characteristic of fruit surface have a great impact on the inactivation kinetics of E. coli by UV-C. UV-C treatment can reduce E. coli 23716 on discs of surfaces of intact pear >3 log CFU/g following a 2-min treatment at 4.24 kJ/m2. The presence of wounds on pear surfaces and trichomes on peach surfaces shielded the E. coli against UV-C, resulting in its reduced effectiveness. UV-C inactivation kinetics of E. coli fitted Weibull equation. Further, the surface roughness of peach and the relatively lower hydrophobicity of pear explain in part the lower effectiveness of UV-C treatment for peach relative to pear surfaces. Bacterial cell membranes (phospholipids, protein secondary structures, and polysaccharides) were damaged by UV-C radiation treatment and DNA/RNA structural variations were observed by FT-IR, suggesting that these were the major causes of E. coli injury and inactivation. The results of this study indicate that the surface characteristics influence the efficacy of UV-C to achieve specific levels of reduction in E. coli population, which is an important consideration for the design of UV-C systems for sanitization of fruit surfaces.
References
Allende, A., & Artés, F. (2003). UV-C radiation as a novel technique for keeping quality of fresh processed ‘Lollo Rosso’ lettuce. Food Research International, 36, 739–746.
Al-Qadiri, H. M., Al-Alami, N. I., Al-Holy, M. A., & Rasco, B. A. (2008). Using Fourier transform infrared (FT-IR) absorbance spectroscopy and multivariate analysis to study the effect of chlorine-induced bacterial injury in water. Journal of Agricultural and Food Chemistry, 56, 8992–8997.
Al-Qadiri, H. M., Lin, M., Al-Holy, M., Cavinato, A. G., & Rasco, B. A. (2008). Detection of sublethal thermal injury in Salmonella enterica serotype Typhimurium and Listeria monocytogenes using Fourier transform infrared (FT-IR) spectroscopy (4000 to 600 cm−1). Journal of Food Science, 73(2), M54–M61.
Araujo, E. A., Andrade, N. J., Silva, L. H. M., Carvalho, A. F., Silva, C. A., & Ramos, A. M. (2010). Control of microbial adhesion as a strategy for food and bioprocess technology. Food and Bioprocess Technology, 3, 321–332.
Bernard, S. A., Balla, V. K., Davies, N. M., Bose, S., & Bandyopadhyay, A. (2011). Bone cell-materials interactions and Ni ion release of anodized equiatomic NiTi alloy. Acta Biomaterialia, 7(4), 1902–1912.
Beuchat, L. R., Nail, B. V., Adler, B. B., & Clavero, M. R. S. (1998). Efficacy of spray application of chlorinated water in killing pathogenic bacteria on raw apples, tomatoes and lettuce. Journal of Food Protection, 61(10), 1305–1311.
Bialka, K. L., Demirci, A. (2007). Decontamination of Escherichia coli O157 : H7 and Salmonella enterica on blueberries using ozone and pulsed UV-Light. Journal of Food Science, 72(9), M391–M396.
Bintsis, T., Litopoulou-Tzanetaki, E., & Robinson, R. K. (2000). Existing and potential applications of ultraviolet light in the food industry—a critical review. Journal of the Science of Food and Agriculture, 80, 637–645.
Bos, R., van der Mei, H. C., & Busscher, H. J. (1999). Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiology Reviews, 23, 179–230.
Burks, G. A., Velegol, S. B., Paramonova, E., Lindenmuth, B. E., Feick, J. D., & Logan, B. E. (2003). Macroscopic and nanoscale measurements of the adhesion of bacteria with varying outer layer surface composition. Langmuir, 19, 2366–2371.
Cia, P., Pascholati, S. F., Benato, E. A., Camili, E. C., & Santos, C. A. (2007). Effects of gamma and UV-C irradiation on the postharvest control of papaya anthracnose. Postharvest Biology and Technology, 43, 366–373.
Choi, W. Y., Park, H. J., Ahn, D. J., Lee, J., & Lee, C. Y. (2002). Wettability of chitosan coating solution on 'Fuji' apple skin. Journal of Food Science, 67(7), 2668–2672.
Chun, H., Kim, J., Chung, K., Won, M., & Song, K. B. (2009). Inactivation kinetics of Listeria monocytogenes, Salmonella enterica serovar typhimurium and Campylobacter jejuni in ready to eat sliced ham using UV-C irradiation. Meat Science, 83, 599–603.
Cunha, L. M., Oliveira, F. A. R., & Oliveira, J. C. (1998). Optimal experimental design for estimating the kinetic parameters of processes described by the Weibull probability distribution function. Journal of Food Engineering, 37(2), 175–191.
Cutler, T. D., & Zimmerman, J. J. (2011). Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Animal Health Research Reviews, 12(1), 15–23.
Erkan, M., Wang, C. Y., & Krizek, D. T. (2001). UV-C radiation reduces microbial populations and deterioration in Cucurbita pepo fruit tissue. Environmental and Experimental Botany, 45, 1–9.
Fernandez, V., Khayet, M., Montero-Prado, P., Heredia-Guerrero, A., Liakopoulos, G., Karabourniotis, G., et al. (2011). New insights into the properties of pubescent surfaces: peach fruit as a model. Plant Physiology, 156, 2098–2108.
Geveke, D. J. (2008). UV inactivation of E. coli in liquid egg white. Food and Bioprocess Technology, 1, 201–206.
Geveke, D. J., & Brunkhorst, C. (2008). Radio frequency electric fields inactivation of Escherichia coli in apple cider. Journal of Food Engineering, 85, 215–221.
Gomez, P. L., Alzamora, S. M., Castro, M. A., & Salvatori, D. M. (2010). Effect of ultraviolet-C light dose on quality of cut-apple: microorganism, color and compression behavior. Journal of Food Engineering, 98(1), 60–70.
González-Aguilar, G. A., Wang, C. Y., Buta, J. G., & Krizek, D. T. (2001). Use of UV-C irradiation to prevent decay and maintain postharvest quality of ripe ‘Tommy Atkins’ mangoes. International Journal of Food Science and Technology, 36, 767–773.
Guerrero-Beltran, J. A., & Barbosa-Canovas, G. V. (2004). Review: advantages and limitations on processing foods by UV light. Food Science and Technology International, 10(3), 137–147.
Gurtler, J. B., Rivera, R. B., Zhang, H. Q., & Geveke, D. J. (2010). Selection of surrogate bacteria in place of E. coli O157:H7 and Salmonella Typhimurium for pulsed electric field treatment of orange juice. International Journal of Food Microbiology, 139, 1–8.
Hershko, V., Weisman, D., & Nussinovitch, A. (1998). Method for studying surface tomography and roughness of onion and garlic skins for coating purposes. Journal of Food Science, 63, 317–321.
Jagger, J. (1967). Introduction to Research in Ultraviolet Photobiology. Englewood Cliffs, NJ: Prentice-Hall Inc.
Jin, T., Zhang, H., Boyd, G., & Tang, J. (2008). Thermal resistance of Salmonella enteritidis and Escherichia coli K12 in liquid egg determined by thermal-death-time disks. Journal of Food Engineering, 84, 608–614.
Kim, C., & Hung, Y. (2012). Inactivation of E. coli O157:H7 on blueberries by electrolyzed water, ultraviolet light, and ozone. Journal of Food Science, 77(4), M206–M211.
Koutchma, T., Brian, P., & Eduardo, P. (2007). Validation of UV coiled tube reactor for fresh juices. Journal of Environmental Engineering and Science, 6(3), 319–328.
Levy, C., Aubert, X., Lacour, B., & Carlin, F. (2012). Relevant factors affecting microbial surface decontamination by pulsed light. International Journal of Food Microbiology, 152(3), 168–174.
Lin, M., Al-Holy, M., Al-Qadiri, H., Kang, D., Cavinato, A. G., Huang, Y., et al. (2004). Discrimination of intact and injured Listeria monocytogenes by Fourier transform infrared spectroscopy and principal component analysis. Journal of Agricultural and Food Chemistry, 52(19), 5769–5772.
Liu, Q., Lu, X., Swanson, B. G., Rasco, B. A., & Kang, D. (2012). Monitoring ultraviolet (UV) radiation inactivation of Cronobacter sakazakii in dry infant formula using Fourier transform infrared spectroscopy. Journal of Food Science, 77(1), M86–M93.
Lu, X., Webb, M., Talbott, M., Van Eenennaam, J., Palumbo, A., Linares-Casenave, J., et al. (2010). Distinguishing ovarian maturity of farmed white sturgeon (Acipenser transmontanus) by Fourier transform infrared spectroscopy: a potential tool for caviar production management. Journal of Agricultural and Food Chemistry, 58, 4056–4064.
Lu, X., Liu, Q., Wu, D., Al-Qadiri, H. M., Al-Alami, N. I., Kang, D.-H., et al. (2011a). Using of infrared spectroscopy to study the survival and injury of Escherichia coli O157:H7, Campylobacter jejuni and Pseudomonas aeruginosa under cold stress in low nutrient media. Food Microbiology, 28, 537–546.
Lu, X., Rasco, B. A., Jabal, J. M. F., Aston, D. E., Lin, M., & Konkel, M. E. (2011b). Investigating antibacterial mechanisms of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni using FT-IR spectroscopy, Raman spectroscopy and electron microscope. Applied and Environmental Microbiology, 77, 5257–5269.
Lu, X., Rasco, B. A., Kang, D.-H., Jabal, J. M. F., Aston, D. E., & Konkel, M. E. (2011c). Infrared and Raman spectroscopic studies of the antimicrobial mechanisms of garlic concentrates and diallyl constituents on foodborne pathogens. Analytical Chemistry, 83, 4137–4146.
Lu, X., Al-Qadiri, H. M., Lin, M., & Rasco, B. A. (2011d). Application of mid-infrared and Raman spectroscopy to the study of bacteria. Food and Bioprocess Technology, 4, 919–935.
Machado, L. F., Pereira, R. N., Martins, R. C., Teixeira, J. A., & Vicente, A. A. (2010). Moderate electric fields can inactivate Escherichia coli at room temperature. Journal of Food Engineering, 96, 520–527.
Manzocco, L., Da Pieve, S., & Maifreni, M. (2011). Impact of UV-C light on safety and quality of fresh-cut melon. Innovative Food Science and Emerging Technologies, 12, 13–17.
McDonald, K. F., Curry, R. D., Clevenger, T. E., Unklesbay, K., Eisenstark, A., Golden, J., et al. (2000). IEEE Transactions on Plasma Science, 28(5), 1581–1587.
Mitik-Dineva, N., Wang, J., Truong, V. K., Stoddart, P., Malherbe, F., Crawford, R. J., et al. (2009). Current Microbiology, 58, 268–273.
Movasaghi, Z., Rehman, S., & Rehman, I. (2008). Fourier transform infrared (FTIR) spectroscopy of biological tissues. Applied Spectroscopy Reviews, 43, 134–179.
Naumann, D. (2001). FT-infrared and FT-Raman spectroscopy in biomedical research. Applied Spectroscopy Reviews, 36, 239–298.
Novak, J., Demirci, A., Han, Y., Han, Y. (2008). Novel chemical processes: Ozone, supercritical CO2, electrolyzed oxidizing water, and chlorine dioxide gas. Food Science and Technology International, 14(5), 437–441.
Ong, Y., Razatos, A., Georgiou, G., & Sharma, M. M. (1999). Adhesion forces between E. coli bacteria and biomaterial surfaces. Langmuir, 15, 2719–2725.
Otto, C., Zahn, S., Rost, F., Zahn, P., Jaros, D., & Rohm, H. (2011). Physical methods for cleaning and disinfection of surfaces. Food Engineering Reviews, 3(3–4), 171–188.
Odriozola-Serrano, I., Soliva-Fortuny, R., & Martin-Belloso, O. (2009). Influence of storage temperature on the kinetics of the changes in anthocyanins, vitamin C, and antioxidant capacity in fresh-cut strawberries stored under high oxygen atmospheres. Journal of Food Science, 74(2), C184–C191.
Ribeiro, C., Vicente, A. A., Teixeira, J. A., & Miranda, C. (2007). Optimization and edible coating composition to retard strawberry fruit senescence. Postharvest Biology and Technology, 44, 63–70.
Sapers, G. M. (2001). Efficacy of washing and sanitizing methods. Food Technology and Biotechnology, 39(4), 305–311.
Schenk, M., Guerrero, S., & Alzamora, S. M. (2008). Response of some microorganisms to ultraviolet treatment on fresh-cut pear. Food and Bioprocess Technology, 1, 384–392.
Scheuerman, T. R., Camper, A. K., & Hamilton, M. A. (1998). Effects of substratum topography on bacterial adhesion. Journal of Colloid and Interface Science, 208(1), 23–33.
Sommers, C. H., Sites, J. E., & Musgrove, M. (2010). Ultraviolet light (254 nm) inactivation of pathogens on foods and stainless steel surfaces. Journal of Food Safety, 30, 470–479.
US-FDA (United States Food and Drug Administration). (2011). Ultraviolet radiation for the processing and treatment of food. Code of Federal Regulations, 179.
van Boekel, M. A. J. S. (2002). On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. International Journal of Food Microbiology, 74(1–2), 139–159.
Velazquez, P., Skurtys, O., Enrione, J., & Osorio, F. (2011). Evaluation of surface free energy of various fruit epicarps using acid–base and Zisman approaches. Food Biophysics, 6, 349–358.
Vogler, E. A. (1998). Structure and reactivity of water at biomaterial surfaces. Advances in Colloid and Interface Science, 74, 69–117.
Woodling, S. E., & Moraru, C. I. (2005). Influence of surface topography o the effectiveness of pulsed light treatment for the inactivation of Listeria innocua on stainless-steel surfaces. Journal of Food Science, 70(7), M345–MM351.
Wong, E., Linton, R. H., & Gerrard, D. E. (1998). Reduction in Escherichia coli and Salmonella senftenberg on pork skin and pork muscle using ultraviolet light. Food Microbiology, 15, 415–423.
Yang, H., An, H., Feng, G., & Li, Y. (2005). Visualization and quantitative roughness analysis of peach skin by atomic force microscopy under storage. LWT - Food Science and Technology, 38, 571–577.
Yaun, B. R., Sumner, S. S., Eifert, J. D., & Marcy, J. E. (2004). Inhibition of pathogens on fresh produce by ultraviolet energy. International Journal of Food Microbiology, 90(1), 1–8.
Yaun, B. R., Sumner, S. S., Eifert, J. D., & Marcy, J. E. (2003). Response of Salmonella and Escherichia coli O157: H7 to UV energy. Journal of Food Protection, 66(6), 1071–1073.
Yuk, H., Geveke, D. J., & Zhang, H. Q. (2009). Non-thermal inactivation of Escherichia coli K12 in buffered peptone water using a pilot-plant scale supercritical carbon dioxide system with a gas–liquid porous metal contactor. Food Control, 20, 847–851.
Zisman, W. A. (1964). Relation of the equilibrium contact angle to liquid and solid constitution. Advances in Chemistry, 43, 1–51.
Acknowledgements
This research work was funded, in part, with a grant from Pear Bureau Northwest. Technical assistance from Mr. Pavlo Rudenko and Dr. Elena Ten, School of Mechanical and Materials Engineering, Dr. Valerie Lynch-Holm, Franceschi Microscopy and Imaging Center, and Mr. Ofero Caparino, Biological Systems Engineering, WSU are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial Support
This research work was funded, in part, with a grant from Pear Bureau Northwest.
Rights and permissions
About this article
Cite this article
Syamaladevi, R.M., Lu, X., Sablani, S.S. et al. Inactivation of Escherichia coli Population on Fruit Surfaces Using Ultraviolet-C Light: Influence of Fruit Surface Characteristics. Food Bioprocess Technol 6, 2959–2973 (2013). https://doi.org/10.1007/s11947-012-0989-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-0989-0