Abstract
In this study, the effect of interesterification (using sodium methoxide) on physicochemical characteristics of fully hydrogenated palm olein (FHPO)/soybean oil blends (10 ratios) was investigated. Interesterification changed free fatty acid content, decreased oil stability index, solid fat content (SFC) and slip melting point (SMP), and does not affected the peroxide value. With the increase of FHPO ratio, oil stability index, SFC and SMP increased in both the interesterified and non-interesterified blends. Fats with higher FHPO ratio had narrower plastic range, as well. Compared to the initial blends, interesterified fats had wider plastic ranges at lower temperatures. Both the non-interesterified and interesterified blends showed monotectic behavior. The Gompertz function could describe SFC curve (as a function of temperature, saturated fatty acid (SFA) content or both) and SMP (as a function of SFA) of the interesterified fats with high R2 and low mean absolute error.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intake of dietary trans fatty acids is associated with several undesirable physiological effects (such as increase of the risk of cardiovascular diseases) [1]. Accordingly, the FDA announced its decision on removal of partially hydrogenated fats (as the main source of trans fatty acids) from GRAS list and provided a three-year mandatory compliance period, which ends by June 18, 2018 [2]. Accordingly, many countries eliminated (or are planning to eliminate) partially hydrogenated fats from their formulations and are producing zero or low trans fats. However, the elimination of partially hydrogenated or trans fats from the product formulation affects the physicochemical properties, dramatically. In this context, interesterification, which modifies fat but does not produce trans fatty acids, have been received much attention.
Chemical interesterification modifies the physicochemical characteristics of fats by re-distribution of fatty acids on the glycerol molecule without altering their fatty acid profile [3]. In this process, blends of hard fats and liquid oils are interesterified to meet the desired physicochemical properties [4]. Oliveira et al. [5] showed that the chemically interesterified blend of palm stearin/patawa oil (50/50) had appropriate melting point and solid fat content (SFC) for production of soft table margarines. Ornla-ied et al. [6] studied the modification of triacylglycerol (TAG) profile and physical and thermal characteristics of palm stearin/rice bran oil blends via lipase-catalyzed interesterification. Slip melting point (SMP) and SFC of the interesterified fats were lower and contained small, mostly β′ needle-shaped crystals. Biswas et al. [7] proposed that the interesterified palm mid-fraction/palm kernel oil/palm stearin blend (14.9/59.6/25.5) could be used in formulation of confectionery fillings.
Due to the decision of FDA regarding the discontinuation of production of partially hydrogenated fats, and the need for production of zero trans fats, the hydrogenation process is now used for production of fully hydrogenated vegetable fats as hard stocks for formulation of trans-free fats. In this regard, full hydrogenation of palm olein (or oil) is the best choice due to the lower hydrogen consumption [8]. Moreover, it has a tendency to β′ crystal formation, leading to a favorable melting and crystallization behavior in the final product [9].
We have studied the effect of interesterification of physicochemical properties of several fat blends including fully hydrogenated soybean oil (FHSBO)/sunflower/canola oil blends [10], palm olein/sunflower/canola oil blends [11], palm olein/canola oil blends [12], FHSBO/canola oil blends [12] and palm stearin/soybean oil blends [13], previously. We have also used sigmoidal functions to describe the melting behavior of some interesterified fat blends [14, 15]. In this work, we report the effect of chemical interesterification on physicochemical characteristics of fully hydrogenated palm olein (FHPO)/soybean oil blends. SFC curve and SMP curve of the blends are also described using the sigmoidal Gompertz function. Results of this study are helpful in developments of trans-free fat products.
Materials and methods
Materials
FHPO and refined and bleached soybean oil were obtained from Parto Daneh Khazar Co (Behshahr-Iran). Sodium methoxide was purchased from Merck (Germany). Bleaching earth was purchased from Tonsil Indonesia Company (Indonesia). Filter-aid earth was purchased from Sofian Tabriz (Iran). Fatty acid methyl ester standards and other chemicals were of analytical grade and purchased from Merck.
Blend preparation
Binary blends of FHPO and soybean oil in mass ratio of 15/85, 25/75, 35/65, 45/55, 55/45, 65/35, 75/25, 85/15, 95/5 and 100/0 were prepared and then interesterified.
Chemical interesterification
Chemical interesterification was done based on the work of Farmani et al. [11]. Briefly, fat blends (300 g) were interesterified using sodium methoxide (1% by weight) at 70 °C and 0.3 bar with vigorous magnet stirring (Heidolph, model MR 3004, Germany) at 300 rpm for 45 min. The reaction was stopped by the addition of 2% (w/w) aqueous citric acid solution and stirring for 15 min at 300 rpm and 110 °C. 2% (w/w) filter-aid was added into the mixture and stirred for another 5 min at 110 °C under vacuum. The catalyst and filter-aid were removed from the fat using Whatman no. 4 filter paper. For bleaching process, 2% bleaching earth was added into the mixture and stirred for 15 min at 110 °C under vacuum by magnet stirring (300 rpm). The bleaching earth was then removed by filtration using Whatman no. 4 filter paper. The filtered oil was used for further analyses.
Free fatty acid content, soap content and peroxide value
Free fatty acids (FFA) content, defined as the percentage (by weight) of free acids existing in fat, was determined as described by the American oil chemists’ society (AOCS) Cd 8-53 method. Soap content and peroxide value (PV) were determined following the instructions of AOCS Cc17-95, AOCS Ca 5a-40 methods, respectively [16].
Iodine value
Iodine value (IV) of samples was determined according to the AOCS Method Cd 1c-85 [16].
Slip melting point determination
SMP was determined by tempering fat samples at 6 °C for 16 h in an open capillary tube as described by AOCS method Cc 3–25 [16].
SFC determination
SFC was measured using a low resolution pulsed nuclear magnetic resonance spectroscope (pNMR, Broker, Minispecmq 20, Germany) according to the AOCS Cd 16b-93 method [16]. After melting fat sample at 100 °C, it was poured into the NMR tube and tempered at 100 °C (10 min), 70 °C (30 min) and 0 °C (60 min). Finally, the sample was conditioned at the desired temperatures (10, 20, 30, 40 or 50 °C) for 45 min. Then the SFC was measured using the pNMR.
Oxidative stability determination
The induction period (IP) of oxidation of fat blends were measured at 110 °C using a Methrom Rancimat Instrument model 743 (Herisau, Switzerland) as described in AOCS method Cd 12b-92 [16].
Fatty acid profile
Preparation and analysis of fatty acid methyl esters were performed as described in AOCS methods Ce 2-66 and Ce 1e-91, respectively [16]. Gas chromatography (Perkin-Elmer, 8420 series) equipped with a CP Sil 88 (100 m, 0.25 mm id and 0.25 mm film thickness) capillary column, followed by flame ionization detection was used. Oven was set at 180 °C and detector and injector at 250 °C. Helium was used as carrier gas and peaks were identified by comparing the retention times with those of authentic standards.
Iso-solid diagrams
The iso-solid diagrams were constructed from SFC, FHPO ratio and SFC measurement temperature data using SigmaPlot software ver. 12 (Sysat software Inc., USA).
Description of SFC curve using the Gompertz function
In this study, the Gompertz function was tested for description of SFC curves of chemically interesterified FHPO/soybean oil blends according to Farmani [14]. The equation was fitted to the experimental data, and model parameters were calculated using SigmaPlot software ver. 12 (Systat software Inc., USA). SFC was described as a function of temperature as described in Eq. 1.
where, α is the upper asymptote, b sets the ordinate axis shift and c sets the growth rate.
SFC was modeled as a function of saturated fatty acid (SFA) content as described in Eq. 2.
The coefficients α, b and c of Gompertz models of SFCf(SFA) curves and SFCf(T) curves were calculated using SigmaPlot software.
The two-variable Gompertz function, which was developed by Farmani [14], was used to describe the SFC of chemically interesterified blends of FHPO and soybean oil as multiple function of temperature and SFA (Eq. 3). The parameters of the function were calculated using SigmaPlot software ver. 12.
Description of SMP as a function of SFA using Gompertz function
The Gompertz function was used to describe the SMP as a function of SFA (Eq. 4), as well. SigmaPlot software ver. 12 was used to calculate model parameters.
Statistical analysis
Triplicate experiments were performed in a completely randomized block design. Data of IV, FFA, PV and IP of oxidation were analyzed by two-way ANOVA (analysis of variances) to evaluate the significant effect of interesterification and FHPO ratio at significance level of p < 0.05. The other experimental data were analyzed by one-way ANOVA and the means were compared at p < 0.05. All the analyses were made using the SPSS software (IBM SPSS Statistics Ver. 21, New York, USA).
Goodness of fit of models was analyzed by evaluation of coefficient of determination (R2), standard error of estimation (SE), mean absolute error (MAE) and correlation coefficient (r) between predicted and experimental values using Statistica ver. 12 (Stat Soft Inc., Tulsa, USA).
Results and discussion
Physicochemical properties
Fatty acid profile of fat blends is shown in Table 1. The mixture containing 85% soybean oil had the highest amount of unsaturated fatty acids, in which the main fatty acids were linoleic and oleic acids. Addition of FHPO into the soybean oil increased the content of SFA and decreased that of polyunsaturated fatty acids. With increase of FHPO in the formula (from 15 to 100%), the content of SFA increased (from 28.5 to 97.4%), and the content of unsaturated fatty acids decreased (from 71.6 to 2.5%). FHPO contained the highest amount of SFA. Palmitic and stearic acids were the main fatty acids of FHPO/soybean oil blends. According to Jeyarani and Reddy [17] higher palmitic acid content increases tendency to the β′ crystal formation which results in a desirable smooth consistency (which is required for purposes such as shortenings and margarines).
As the interesterification has no effect on the fatty acid composition [3], neither interesterification nor blend type (FHPO ratio) affected the IV of fat blends (p < 0.05, Table 2). Karabulut, Turan and Ergin [18] reported similar results.
Table 2 shows FFA level of interesterified and initial fats. FFA content of the initial blends was in the range of 0.24–0.80% that was changed to 0.40–0.50% after interesterification (p < 0.05). Both the chemical interesterification and FHPO ratio had significant effect on the FFA content (as analyzed by two-way ANOVA, p < 0.05). Interesterified blends containing more than 45% FHPO had lower FFA content but those containing 15–35% FHPO had higher FFA level than their non-interesterified blends. The increase of percent FFA by interesterification have been reported, previously [4, 12, 13]. The increase of FFA after interesterification shows a good catalyst activity [4]. It was reported that the decrease of FFA by interesterification can be a result of interaction of catalyst with oil acidity [5]. In fact, in oils with high FFA content, sodium methoxide neutralizes the free acids and consequently, FFA content decreases after the interesterification. A greater amount of catalyst is required for fats with high FFA content (> 0.05%), as it is initially neutralized (poisoned) by FFAs [5].
Soaps are formed as a result of the reaction between the sodium methoxide and fatty acids [12]. The soap content of the initial and interesterified blends was equal to zero. This means that the soaps were removed by post-bleaching (as described in methods section).
Neither interesterification nor FHPO ratio had any effect on PV of blends (p < 0.05, Table 2). However, as reported elsewhere [10–12], chemical interesterification may decrease PV because some peroxides may be adsorbed to the soaps and removed during the post-bleaching [19]. In fact, as the initial blends used in this study contained low PV (0.15–0.25 meq/kg), the change of PV by interesterification was not significant.
Oxidative stability of the initial and interesterified mixtures is listed in Table 2. Both the interesterification and FHPO ratio had significant effect on oxidative stability (p < 0.05). IP of oxidation (at 110 °C) of the initial blends was 7.16–54.46 h. As the portion of FHPO increased in formulations, IP of oxidation increased (p < 0.05). FHPO had the highest oxidative stability. Interesterification decreased the IP of oxidation of blends (p < 0.05) as reported previously [10–13, 20]. Formation of FFA and partial acylglycerols [10, 11, 20, 21], transfer of polyunsaturated fatty acids from the internal sn-2 to the external sn-1 and sn-3 positions of TAGs [20] and the removal of antioxidants of fats by interesterification [11, 20–22] are the main cause of the oxidative stability loss.
SMP is the temperature at which a fat column in a capillary tube starts to move up. It changes with the chain length of fatty acids, degree of unsaturation, trans fatty acids content and the position of the fatty acids in the glycerol backbone [18]. SMP of blends before and after chemical interesterification is presented in Table 3. The SMP of the interesterified fats was lower than their initial ones (p < 0.05). This can be explained by the conversion of some parts of the higher-melting trisaturated (SSS) TAGs into the lower-melting monosaturated–diunsaturated (SUU) and disaturated–monounsaturated (SUS) TAGs by the interesterification process [9, 23]. The SMP of interesterified fats increased with the increase of FHPO ratio (p < 0.05), which may be due to the increase of tri-saturated TAGs content. Similar results were observed previously [10, 12, 13, 18, 23, 24]. Interesterified blends containing 15–45% FHPO in their formulations had SMP ranging from 27.5 to 44 °C, similar to those found for most margarines and shortenings [25].
SFC is a measure of the percentage of fat in crystalline (solid) phase to total fat across a temperature range. SFC of the fat blends is presented in Table 3. At all temperatures, with an increase in the ratio of FHPO in blends, the SFC increased (p < 0.05), which is due to the increase of saturated TAG level. Interesterification caused a decrease in SFC of fat blends (p < 0.05). In fact, in blends made from fully hydrogenated fats and liquid vegetable oils, interesterification decreases the level of trisaturated TAGs and consequently fat SFC [23]. Another reason for SFC reduction by interesterification is conversion of β crystals (coarse crystals) into the β′ form (tiny crystals). Smaller crystals melt more quickly under the influence of temperature [26]. According to Norizzah et al. [24], only β′ crystals were detectable in chemically interesterified palm stearin/palm kernel olein blends. They attributed this to the diversification of glycerides by interesterification [24]. Generally, SFC difference between the interesterified and non-interesterified fats was greater at higher SFC measurement temperatures, for a given blend. In other word, interesterification increased the slope of the SFC curve. In fact, interesterification decreases the content of high-melting point TAGs and consequently that of SFC at higher temperatures [3].
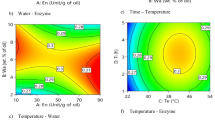
Iso-solid curves provide valuable information about the state of a blend at a given temperature and compatibility of fat mixtures [27]. According to Fig. 1, both the non-interesterified and interesterified blends showed monotectic behavior. That is, SFC increased continuously (with no local maximum or minimum) as the ratio of FHPO increased. This reflects good compatibility (ideal solubility) of FHPO with soybean oil either before or after interesterification. However, for interesterified fats, the iso-solid lines were steeper and became closer to each other with the increase of temperature and FHPO ratio. Therefore, the diluting effect was smaller when FHPO ratio increased. Monotectic behavior was also reported for palm mid-fraction (20–40%)/palm kernel oil blends (at 10–15 °C), palm stearin (40–80%)/palm kernel oil blends, palm mid-fraction/palm stearin blends (at < 30 °C) [7], blends of FHSBO and soybean oil [23], and non-interesterified and enzymatically interesterified blends of FHPO and soybean oil [8].
Modeling studies
In this study, the SFC curve of the interesterified fats were described as a function of temperature, SFA or both of them. The SFCf(T) curves of the interesterified fat blends are presented in Fig. 2(A). Generally, the SFCf(T) curve starts from a maximum value at low temperatures, declines at intermediate temperatures, and finally reaches to 0% at high temperatures. Parameters of the Gompertz function, which was used for description of SFCf(T) curves, together with their goodness of fit are shown in Table 4. With increase of FHPO ratio in blends, a general increase in parameters α, b and c was observed. This means that with increase of FHPO ratio, the SFCf(T) curve of interesterified fats starts from a higher SFC, shifts right in the X axis and is more steep. R2 and p value of models were between 0.98 and 0.99 and < 0.0001, respectively. The correlation coefficient (r) between experimental and predicted SFC and mean absolute error (MAE, an average of the absolute errors (the difference between experimental and predicted value)) of the prediction are indicators of the precision of the model. Goodness of fit of the models indicated that the Gompertz model could make predictions with low MAE (0.21–1.66%) and high r value (r > 0.98, Table 4). The potential of the Gompertz model in description of SFCf(T) curve of chemically interesterified FHSBO/canola oil blends [14], enzymatically interesterified FHPO/soybean oil blends [15] and some other fat blends [28] has been reported, previously.
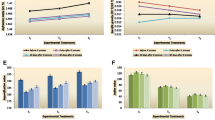
Predicted vs experimental melting curves of chemically interesterified binary blends of fully hydrogenated palm olein (FHPO) and soybean oil (SBO); (A) solid fat content (SFC) as a function of temperature (SFC f(T)); (B) SFC as a function of saturated fatty acid (SFA) content (SFC f(SFA)); (C) SFC as multiple function of temperature and SFA (SFC f(T,SFA)); (D) slip melting point as a function of SFA (SMP f(SFA). Suffixes exp and prd, show experimental and predicted data, respectively. Markers and solid lines represent experimental and predicted curves, respectively
As an application of SFCf(T) models, they were used for calculation of plastic range. Plastic range is a range of temperature within which the fats have SFC between 15 and 25% [13]. It was obtained by substitution of the defied SFC value in the model and calculation of the temperatures. Table 3 shows the plastic range of different fat blends. Except the blend 25/75 (FHPO/soybean oil), plastic range of the non-interesterified fats did not fall within the SFC measurement temperature. Compared to the starting blends, interesterified fats had wider plastic range at lower temperatures. Interesterified fats having 25–75% FHPO in their formulation had plastic ranges within the SFC measurement temperature. Increase of FHPO ratio, shifted the plastic range to higher temperatures and made it narrower (Table 3). An improvement in plasticity of palm fraction/mahua fat (1:1 and 1:2) and palm fraction/mango fat (1:1) blends by interesterification was also reported by Khatoon et al. [1]. Improvement in plasticity is related to alterations in molecular structure of oil as the saturated-type TAGs reduced and TAGs with polyunsaturated fatty acids increased after interesterification [1]. It is reported that smaller fine crystals appeared after interesterification will result in a better plasticity and overall texture characteristics [29].
Farmani [14] and Ebrahimi et al. [15] reported the effect of SFA on SFC of interesterified fats using the sigmoidal functions. Here, SFC of chemically interesterified FHPO/soybean oil blends is described using the Gompertz function. According to Fig. 2(B), contrary to the effects of temperature, SFC increased as SFA increased. These curves have a sigmoid shape, as well. Accordingly, we used the sigmoidal Gompertz functions for description of SFCf(SFA) curves. The SFCf(SFA) curves starts from zero and tends to the highest SFC (100%).The coefficients α, b, c and goodness of fit of Gompertz models are shown in Table 4. For all the curves, R2 was higher than 0.99 and p value was lower than 0.0001. The correlation coefficients between predicted and experimental SFC values were more than 0.99. The MAE of models was between 0.62 and 0.97%. These means that the models could predict the SFC of interesterified fats with high accuracy. The results were similar to the results of the Farmani [14] and Ebrahimi et al. [15] who described the SFC of the interesterified fats as a function of SFA content.
To describe the multiple effects of SFA and temperature on SFC, we used the two-variable Gompertz function developed elsewhere [14]. Equation 5 shows the function with its coefficients (fitted by non-linear regression analysis using SigmaPlot).
The model had a p value lower than 0.0001, R2 = 0.99 and standard error of 2.23. Predicted SFC values of blends vs their experimental values are shown in Fig. 2(C) as a surface plot. The surface plot of the SFC starts from the value of the 0 at the lowest SFA content and the highest SFC measurement temperature. It grows with SFA increase or temperature decrease. Analysis of goodness of fit of model (by comparing the experimental and predicted values) showed an r value greater than 0.99 and MAE of 1.72% that were very good for Eq. 5. In other word, the model could fit the experimental data, in the best way. We have previously used the two-variable Gompertz model (Eq. 3) for description of the SFC curve of the chemically interesterified FHSBO/canola oil blends [14]. Standard error, r value and MAE of that model were 1.41, 0.98 and 1.00% [14] which are close to that of the Eq. 5.
Naeli et al. [30] evaluated the suitability of different models in description of SMP of chemically interesterified palm stearin/soybean oil blends. They found that the Gompertz model could describe the SMP as a function of SFA. In this study, we described the SMP of the interesterified blends as a function of SFA, as well. The SMPf(SFA) curve was also sigmoidal (Fig. 2(D)). Generally, when the SFA increased, the SMP of the blends increased, too. However, the rate of SMP increase was lower at low or high SFA contents. Equation 6 describes the SMPf(SFA) curve using the Gompertz function.
R2 and p value of the model were 0.99 and < 0.0001, respectively. This means that this model can describe the SMP as a function of SFA, very well. A comparison of the experimental vs predicted SMP values is shown in Fig. 2 (D). As can be seen, the predicted SMP curve fits the experimental data, very well. The correlation coefficient between the predicted and experimental SMP values was > 0.99 and MAE of the prediction was 0.4 °C. Naeli et al. [30], reported a MAE of 0.21 °C and an r value of 0.99 for the Gompertz model describing the SMPf(SFA) curve of chemically interesterified palm stearin/soybean oil blends. Ebrahimi et al. [15] reported that the SMP f(SFA) of enzymatically interesterified FHPO/soybean oil blends had a S shape and could be better described by sigmoidal (Gompertz) models rather than linear models. MAE and r value of the Gompertz model developed by Ebrahimi et al. [15] were also close to our results (0.65 °C and 0.99, respectively).
As indicated in this study, chemical interesterification can be used as a technique for modification of physicochemical properties of FHPO/soybean oil blends. Chemically interesterified FHPO/soybean oil mixtures had lower SFC and melting point than the starting mixtures. The interesterified blends, especially those containing lower FHPO ratio may be used directly for production of trans-free fat products. The other blends (having high melting point and high SFA content) can be used as a hard stock for formulation of trans-free fats. The obtained results indicated that the Gompertz model described the SFCf(T), SFCf(SFA) and SMPf(SFA) curves, in the best way. Findings of this study may be helpful in better understanding and description of physicochemical properties of chemically interesterified fats.
References
Khatoon S, Reddy S, Reddy Y. Plastic fats with zero trans fatty acids by interesterification of mango, mahua and palm oils. Eur. J. Lipid Sci. Technol. 107: 786–791 (2005)
Wang FC, Gravelle AJ, Blake A, Marangoni AG. Novel trans-fat replacement strategies. Curr. Opin. Food Sci. 7: 27–34 (2016)
Rousseau D, Marangoni AG. Chemical interesterification of food lipids: theory and practice. pp. 267–298. In: Food Lipids, Chemistry, Nutrition, and Biotechnology. Akoh CC, Min DB Food Lipids (eds). CRC Press, Inc., Boca Raton, FL, USA (2008)
Petrauskaite V, De Greyt W, Kellens M, Huyghebaert A. Physical and chemical properties of trans-free fats produced by chemical interesterification of vegetable oil blends. J. Am. Oil Chem. Soc. 75: 489–493 (1998)
Oliveira PD, Rodrigues AMC, Bezerra CV, Silva LHM. Chemical interesterification of blends with palm stearin and patawa oil. Food Chem. 215: 369–376 (2017)
Ornla-ied P, Sonwai S, Lertthirasuntorn S. Trans-free margarine fat produced using enzymatic interesterification of rice bran oil and hard palm stearin. Food Sci. Biotechnol. 25: 673–680 (2016)
Biswas N, Cheow YL, Tan CP, Kanagaratnam S, Siow LF. Cocoa butter substitute (CBS) produced from palm mid-fraction/palm kernel oil/palm stearin for confectionery fillings. J. Am. Oil Chem. Soc. 1–11 (2017)
Ebrahimi L, Farmani J, Bahmaei M. Physicochemical and rheological characterization of lipase-interesterified trans-free blends of fully hydrogenated palm olein and soybean oil. Adv. Food Sci. 37: 111–118 (2015)
Masuchi M, Gandra K, Marangoni A, de Sa´Perenha C, Chiu M, Grimaldi R. Fats from chemically interesterified high-oleic sunflower oil and fully hydrogenated palm oil. J. Am. Oil Chem. Soc. 91: 859–866 (2014)
Farmani J, Hamedi M, Safari M, Madadlou A. Trans-free Iranian vanaspati through enzymatic and chemical transesterification of triple blends of fully hydrogenated soybean, rapeseed and sunflower oils. Food Chem. 102: 827–833 (2007)
Farmani J, Hamedi M, Safari M. Production of zero trans Iranian vanaspati using chemical transesterification and blending techniques from palm olein, rapeseed and sunflower oil. Int. J. Food Sci. Technol. 43, 393–399 (2008).
Farmani J, Safari M, Hamedi M. Trans-free fats through interesterification of canola oil/palm olein or fully hydrogenated soybean oil blends. Eur. J. Lipid Sci. Technol. 111: 1212–1220 (2009)
Naeli MH, Farmani J, Zargaran A. Rheological and physicochemical modification of trans-free blends of palm stearin and soybean oil by chemical interesterification. J. Food Process Eng. 1–12 (2016)
Farmani J. Modeling of solid fat content of chemically interesterified fully hydrogenated soybean oil and canola oil blends as a function of temperature and saturated fatty acids. Food Measure. 9: 281–289 (2015)
Ebrahimi L, Farmani J, Bahmaei M. Description of melting curves of enzymatically interesterified blends of fully hydrogenated palm olein and soybean oil by sigmoidal functions. Food Biosci. 17: 29–34 (2017)
AOCS. Official methods and recommended practices of American oil chemists’ society.4th ed. Champaign, American Oil Chemists’ Society Press (1996)
Jeyarani T, Reddy SY. Preparation of plastic fats with zero trans FA from palm oil. J. Am. Oil Chem. Soc. 80: 1107–1113 (2003)
Karabulut I, Turan S, Ergin G. Effects of chemical interesterification on solid fat content and slip melting point of fat/oil blends. Eur. Food Res. Technol. 218: 224–229 (2004)
Kellens M. Oil modification processes. pp. 153–195. In: Edible Oil Processing. Hamm W, Hamilton RJ, Calliauw G (eds.). Wiley-blackwell. UK (2013)
Ledochowska E, Wilczynska E. Comparison of the oxidative stability of chemically and enzymatically interesterified fats. Fett/Lipid. 100: 343–348 (1998)
Kowalski B, Tarnowska K, Gruczynska E, Bekas W. Chemical and enzymatic interesterification of tallow and rapeseed oil equal-weight blend. Eur. J. Lipid Sci. Technol. 106: 655– 664 (2004)
Lee JH, Akoh CC, Lee KT. Physical properties of trans free bakery shortening produced by lipase-catalyzed interesterification. J. Am. Oil Chem. Soc.85: 1–11 (2008)
Ribeiro A, Gioielli L, Gonçalves A, Grimaldi, R. Zero trans fats from soybean oil and fully hydrogenated soybean oil: Physico-chemical properties and food applications. Food Res. Int.42: 401–410 (2009)
Norizzah AR, Chong CL, Cheow CS, Zaliha O. Effects of chemical interesterification on physicochemical properties of palm stearin and palm kernel olein blends. Food Chem. 86: 229– 235 (2004)
Guedes AMM, Ming CC, Ribeiro APB, Da Silva RC, Gioielli LA, Gonçalves LAG. Physicochemical properties of interesterified blends of fully hydrogenated Crambe abyssinica Oil and soybean Oil. J. Am. Oil Chem. Soc. 91:111–123 (2014)
Fauzi SHM, Rashid NA, Omar Z. Effects of chemical interesterification on the physicochemical, microstructural and thermal properties of palm stearin, palm kernel oil and soybean oil blends. Food Chem. 137: 8–17 (2013)
Kadivar S, De Clercq N, Mokbul M, Dewettinck K. Influence of enzymatically produced sunflower oil based cocoa butter equivalents on the phase behavior of cocoa butter and quality of dark chocolate. LWT-Food Sci. Technol. 66: 48-55 (2016)
Augusto PED, Soares BMC, Chiu MC, Goncalves LAG. Modeling the effect of temperature on lipid solid fat content (SFC). Food Res. Int. 45: 132–135 (2012)
Marangoni AG, Rousseau D. Engineering triacylglycerols: the role of interesterification. Trends Food Sci. Technol. 6:329–335 (1995)
Naeli M. H, Farmani J, Zargaran A. Modeling of slip melting point of chemically interesterified fats as a function of fatty acid composition. Iran. J. Nutr. Sci. Food Technol.1175–76 (2016). [In Persian]
Acknowledgments
Authors are thankful to the managers of Parto Daneh Khazar (Behshahr, Iran) for their assistances.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mahjoob, R., Mohammadi Nafchi, A., Omidbakhsh Amiri, E. et al. An investigation on the physicochemical characterization of interesterified blends of fully hydrogenated palm olein and soybean oil. Food Sci Biotechnol 27, 343–352 (2018). https://doi.org/10.1007/s10068-017-0262-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0262-4