Abstract
Objectives
This study aimed to investigate the relationship of serum hemoglobin (HB) level with disease activity and structural damage in Chinese patients with rheumatoid arthritis (RA).
Methods
A total of 890 RA patients and 890 normal subjects were enrolled in the case-control study. A HB threshold of< 110 g/L (women) and < 120 g/L (men) was used to determine anemia. All the patients were divided into three groups: non-anemia group (HB ≥ 120 g/L (male) or 110 g/L (female)), mild anemia group ((90 g/L < HB < lower limit of normal), and medium to severe anemia group (HB ≤ 90 g/L). Serum HB level and anemia prevalence between RA patients and normal subjects were compared. Associations of HB level with disease activity, structural damage, and function of joint in different groups were also investigated.
Results
The average of HB level in RA was (109.08 ± 17.96)g/l, which was lower than that in controls (136.75 ± 14.57)g/l (P < 0.001). Anemia was observed in 47% of the RA patients, while prevalence of anemia in control group was only 4.4%. In RA group, percentages of non-anemia, mild anemia, and medium to severe anemia were 47%, 38%, and 15%. Compared with non-anemia RA patients, RA patients with anemia had higher disease activity, severer structural damage and worse function of joint (P < 0.001). With the increase of anemia, the disease activity, structural damage, and dysfunction of joints increased significantly (P < 0.05–0.001). Linear regression analysis showed that HB level was negatively correlated with disease activity parameters, degree of joint destruction, and function (P < 0.05–0.001). Logistic regression indicated that serum HB level was protective factors for disease activity and structural damage in RA (P < 0.001).
Conclusion
HB level was significantly related to disease activity and structural damage in RA patients.
Key Points • Inflammatory anemia was popular (about a half) in patients with RA. • HB level was related to disease activity and structural damage in RA patients |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease, which could lead to joint swelling, joint tenderness, and progressive joint destruction [1]. RA is also always accompanied with any systemic manifestations including hematologic systemic problem and so on, depending on its severity [2, 3]. According to reports in literature, anemia was one of the most common extra-articular manifestations in RA, affecting 30–70% of the patients [4]. Wilson et al. reviewed 19 articles published since January 1966 on the prevalence of anemia in patients with RA or outcomes for patients with anemia and RA, these studies reported that the prevalence of anemia in RA was from 33 to 60%, and symptoms (including swollen, painful, and tender joints, pain, muscle strength, and energy levels) and quality of life improvement were positively correlated with anemia [5].
Unfortunately, the majority of physicians did not believe that anemia was a serious problem in RA. There were some researches about anemia and its clinical impact in RA patients. Results of some studies suggested that anemia was negatively associated with symptoms and quality of life in RA. Bloxham E et al. [6] reported that anemia was a very common manifestation in RA, which could enhance RA disease activity and reduce the patient’s quality of life. Similarly, Furst et al. [7] regarded that low serum HB level in RA patients was associated with disease severity and the presence of certain comorbidities. There were many researches concerning the association of anemia in RA patients with disease activity. However, relationships between the anemia and structural damage and joint function in RA patients especially in Chinese RA patients were rarely reported in literature.
Therefore, This study investigated the prevalence of anemia in RA and explored relationship between serum HB level with disease activity parameters (swollen joint count (SJC), tenderness joint count (TJC), visual analogue score (VAS), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), anti-cyclic citrullinated peptides (anti-CCP), disease activity score in 28joints (DAS28), clinical disease activity index (CDAI), simplified disease activity index (SDAI), and structural damage and joint function (X-ray staging, Sharp score, health assessment questionnaire (HAQ)) in Chinese patients with RA.
Materials and methods
Patients and normal subjects
In this cross-sectional and case-control study, 890 RA patients who fulfilled the 1987 revised criteria of the ACR [8, 9] or ACR/EULAR 2010 criteria for RA [10] were recruited from the Department of Rheumatology and Immunology, the first affiliated hospital of Anhui Medical University from March 2015 to June 2018. Eight hundred and ninety age- and gender-matched Han Chinese normal subjects were enrolled from the physical examination center of the first affiliated hospital of Anhui Medical University. The exclusion criteria included patients with acute or chronic infectious disease such as thyroid disease or parathyroid disease, other endocrinal diseases, severe liver, kidney disease, or primary hematological disorders. If estrogen, androgen, anticonvulsant, anticoagulant, or any kinds of anti-anemia drugs, for example, chalybeates or folic acid, were used at the same time, the patient was also excluded. Other exclusion criteria were alcoholics, smokers, and HIV subjects. Patients who were pregnant or breastfeeding, patients with a history of other inflammatory or non-inflammatory arthritis, neoplasms, infectious and inflammatory diseases, and other decompensated diseases were excluded from the study. This study was performed according to the principles of the Declaration of Helsinki, and each participant provided informed consent before entering the study. And this study was approved by the ethical committee of Anhui Medical University.
Clinical or laboratory examinations and projects evaluation
General characteristics of all the participants in our study were recorded in detail including age, gender, height, weight, and disease course. Body mass index (BMI) was calculated by dividing body weight by the square of height in meters (kg/m2). All the RA patients underwent full rheumatologic clinical and laboratory examinations with a special emphasis on disease activity parameters, structural damage indicators, and functional variables. Disease activity was assessed using DAS28, CDAI, and SDAI score according to the standard formulas and clinical disease activity indexes including SJC, TJC, and VAS. DAS28 values ≤ 5.1was regarded as low disease activity and DAS28 values > 5.1 as high disease activity [11, 12]. Joint function of RA patients was judged with reference to ACR 1991 revised criteria for the classification of global functional status (from grade I to grade IV) [13]. Health assessment questionnaire (HAQ) was used for assessing whole functional status. HAQ score ranged from 0 to 3, where a higher score indicated a higher degree of disability [14]. Serum hematology parameters including peripheral blood HB levels, ESR, CRP, RF, and anti-CCP levels were measured by standard laboratory techniques in RA patients. Patients were divided into two groups (anemia group and non-anemia group), based on their serum HB concentration. These criteria used a threshold HB< 110 g/L for women and< 120 g/L for men [15]. Patients also were divided into another three groups according to grades of anemia, serum HB ≥ 120 g/L (male) or 110 g/L (female) as non-anemia group, 90 g/L < HB < lower limit of normal as mild anemia group, and HB ≤ 90 g/L as medium to severe anemia group.
Assessment for hand X-rays and Sharp score
The MECALL castor-50-hf model X-ray scanner (Rome, Italy) was used for two-hand (including wrist and fingers)images to evaluate the X-ray period (a total of four periods, from grade I to grade IV) [16]. Bone erosion and joint space in both hands were evaluated according to Sharp and van der Heijde method [17]. Sharp scores and X-ray staging were based on the observations of two radiologists with double-blind method.
Statistical analysis
Categorical variables are presented with frequencies and percentages. Quantitative variables are presented as the median and 25th–75th percentiles (interquartile range) or as the mean ± standard deviation (SD). Furthermore, The difference between two groups was assessed by the t test or non-parametric tests. The statistical tests used for count data were the chi-square test. Group comparisons were performed using Spearman’s correlation test. Risk factors were analyzed using logistic multiple regression. All statistical analyses were performed by SPSS version 22.0 (SPSS Inc., Chicago, IL). The term “significant” in this report was used to denote statistical significance (P < 0.05).
Results
Demographic characteristics between RA and controls
Eight hundred and ninety RA patients (cases) and 890 normal Han Chinese persons (controls) from the first affiliated hospital of Anhui Medical University were recruited in our study. There were no significant differences concerning age, gender, and BMI (P > 0.05). Distribution of 890 RA patients and 890 normal subjects were investigated, Demographics characteristics between cases and controls were listed in Table 1. The average serum HB level in RA was 109.08 ± 17.96 g/l, which was significantly lower than that in controls (136.75 ± 14.57 g/l, t = 50.051, P < 0.001). Prevalence of anemia in RA patients was 47% (418/890), which was higher than that in normal subjects (4.4%, 39/890) (x2 = 36.121, P < 0.001). In case group, percentages of non-anemia, mild anemia, and medium to severe anemia were 46.97% (418/890), 37.75% (336/890), and 15.28% (136/890), while percentages of non-anemia, mild anemia, and medium to severe anemia in control were 91.35% (813/890), 7.3% (65/890), and 1.35% (12/890) (x2 = 176.545, P < 0.001) (sFig. 1). And the percentage of methotrexate use in non-anemia, mild anemia, and medium to severe anemia were 28.7% (120/418), 21.1% (71/336), and 18.4% (25/136), (x2 = 8.846, P = 0.012) and folic acid use in non-anemia, mild anemia, and medium to severe anemia were 25.1% (105/418), 18.5% (62/336), and 17.6% (24/136), (x2 = 10.340, P = 0.006), while the use of glucocorticoids and NSAIDs was not related to anemia (P > 0.05) (Table 1).
Disease activity among different groups in RA
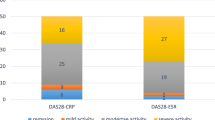
Clinical and laboratory parameters in RA patients were described in Tables 1 and 2, compared with RA patients without anemia, RA patients in anemia group had higher disease activity including higher VAS scores, more swelling joint count and tenderness joint count, higher peripheral blood ESR, CRP, and RF level, higher DSA28, CDAI, and SDAI (P < 0.05–0.001). And as shown in Table 2, there were still significantly different about many disease activity parameters including VAS, SJC, TJC, ESR, CRP, RF, DAS28, CDAI, and SDAI among non-anemia group, mild anemia group, and medium to severe anemia group (P < 0.05–0.001). Furthermore, there were clearly increasing trends concerning above parameters along with degree of anemia (P < 0.001) (Fig 1).
Structural damage among different groups in RA
Our results indicated that component ratio of X-ray stage (grade I to grade IV) were significant differences among non-anemia group, mild anemia group, and medium to severe anemia group (P < 0.05). There was a clear increasing tendency about percentages of grade I to grade IV along with severity of anemia in RA (Fig. 2). The median Sharp score was significantly different among non-anemia group, mild anemia group, and medium to severe anemia group (P < 0.001), there was also a clear increasing trend on mean Sharp score along with severity of anemia in RA. The highest median Sharp score was found in medium to severe anemia group (Tables 1 and 2, Fig. 2).
Joint function among different groups in RA
Component ratio of joint function grading (grade I to grade IV) were significant differences among non-anemia group, mild anemia group, and medium to severe anemia group (P < 0.05). The percentage of grade III and IV in group with medium to severe anemia was the highest (44.78% and 8.96%), which was much higher than that in group with mild anemia (38.72% and 5.49%) or group without anemia (26.76% and 3.41%) (P < 0.001). HAQ was a widely used self-reported functional ability measurement method. The median HAQ scores were significantly different among non-anemia group, mild anemia group, and medium to severe anemia group (P < 0.001), there was also a clear increasing trend on HAQ score along with severity of anemia in RA (P < 0.001). The highest median HAQ score was found in medium to severe anemia group (Tables 1 and 2, sFig. 2).
Linear regression between serum HB level and relevant parameters in RA
Linear regression by univariate analysis and multivariate analysis about serum HB level and clinical or laboratory parameters in RA were shown in Table 3. And multivariate analysis models were adjusted for potential confounders including age, gender, and BMI, which also shown in Table 3. There were statistically significant negative correlations between serum HB level and disease activity parameters (VAS, SJC, ESR, CRP, RF, DAS28, CDAI, and SDAI) and degree of joint destruction and function (X-ray staging, Sharp score, and HAQ) (P < 0.001).
Logistic regression analysis of disease activity or structural damage in RA
Binary logistic regression (backward: likelihood ratio) analyses were performed to identify potential risk factors for disease activity or structural damage in RA patients. A new indicator accounting for whether a RA patient had severe disease activity (DAS28 > 5.1) or not (DAS28 ≤ 5.1) was defined as dependent variable (0 = not, 1 = yes). Gender, age, BMI, VAS, HB, CRP, and RF were defined as independent variables. Logistic regression results indicated that serum HB level (OR = 0.976, 95%CI 0.958–0.993, P = 0.008) was the protective factor for disease activity, while VAS (OR = 1.769, 95%CI 1.465–2.135, P < 0.001), CRP (OR = 1.012, 95%CI 1.004–1.021, P = 0.004), RF (OR = 1.004, 95%CI1.001–1.006, P = 0.001) were risk factors for disease activity (Fig. 3). According to X-ray stage, another new variable accounting for whether a RA patient had structural damage (X-ray stage III–IV) or not (X-ray stage I–II) was defined as the dependent variable (0 = not,1 = yes). Disease duration, VAS, HB, CRP, RF, DSA28, and HAQ were defined as independent variables. Results showed that serum HB level (OR = 0.988, 95%CI 0.977–0.998, P = 0.023) was also the protective factor of structural damage, while disease duration (OR = 1.340, 95%CI 1.278–1.405, P < 0.001) and HAQ (OR = 1.316, 95%CI 1.010–1.714, P = 0.042) were risk factors for structural damage (Fig. 3).
Discussion
RA is a chronic systemic inflammatory disease that primarily involves the joints and sometimes multiple systems, including the hematological system. Anemia is one of the most common extra-articular manifestations of RA. According to results of our study, anemia was not only associated with disease activity and active symptoms of patients but also might be an indicator for predicting structural damage of the disease.
Prevalence of anemia in RA was reported from 30 to 70%. In 2004, Wilson et al. [5] reviewed 19 articles published since January 1966 and estimated the prevalence of anemia in RA at between 33% and 60%, and anemia was positive correlated with symptoms of RA. Similarly, in 2006, Wolfe F et al. [18] recruited 2120 RA patients, and they found that anemia occurred in 31.5% of RA patients, which was 3 times the prevalence in the general population, but severe chronic anemia was 3.4%. Furst et al. [7] investigated 10,397 RA patients in the CORRONA registry, results showed 1734 (16.7%) of RA patients met the WHO definition of anemia, and 37 (0.4%) were severe anemia patients. In our study, we found anemia in normal subjects was only 4.4%, while anemia in RA patients was 47%, which was consistent with previous studies. And the frequency of non-anemia, mild anemia, medium to severe anemia were 47%, 38%, and 15%, the incidence of medium to severe anemia was slightly higher than previous studies, which might be due to disease activity and disease duration.
There was a significant correlation between anemia and disease severity in patients with chronic disease [19]. The well-established relationship between inflammation and anemia has been confirmed by several studies concerning significant associations between lower HB concentrations and DAS28. It has been reported that CDAI (17.4 ± 14.0 vs 13.9 ± 12.4) and DAS28 (5.9 ± 6.5 vs 4.7 ± 5.7) (P < 0.05) in the low HB group were significantly higher than those in the normal HB group, indicating a greater degree of disease activity [7]. Acute phase reactants (CRP and ESR) in RA were more strongly related to HB than other predictors, the research showed that a 10-unit change in ESR was associated with a 0.28 (95% CI 0.24 to 0.33) unit change in hemoglobin, and a 1-unit change in CRP was associated with a 0.15 (95% CI 0.13 to 0.17) unit change [18]. Song et al. [3] and Pereira et al. [20] suggested that anemia might be more strongly correlated with other markers of disease activity, such as RF or CRP levels, pain, and disability, among others. In papers from China, there were also important associations between anemia and disease activity of RA, Yao et al. analyzed 258 RA patients, the results indicated that RA patients with anemia had the tendency of having a higher DAS28 score (6.87 ± 0.74 vs 6.36 ± 0.64, P < 0.01), moderate to high DSA28 score was risk factor for RA with anemia (r = 0.375, P < 0.001) [21]. It was similar to these studies, results from our study showed that SJC and TJC, VAS, ESR, CRP and RF, DSA28, and CDAI and SDAI score were commonly elevated in RA patients with anemia, suggesting a greater degree of disease activity in the low HB group. There were statistically significant negative correlations between HB level and disease activity. Logistic regression results also indicated that serum HB level was the protective factor for disease activity. From all these results, we could draw a conclusion that anemia in patients with RA might be another manifestation of inflammation. The term of inflammatory anemia should be more appropriate for this kind of anemia than traditional chronic anemia.
Various evidences indicated that the relationship between anemia and disease activity in RA patients. However, the relationship between anemia and structural damage and joint function in RA patients was rarely reported before. Steenbergen et al. [22] believed that anemia could be used as a tool for evaluating disease activity in RA patients and as an indicator for predicting the risk of bone destruction in RA patients, 676 RA patients were enrolled in this study, the results showed that patients with anemia had higher SJC (8 (4–13) vs 10 (5–17)), ESR (27 (16–45) vs 54(4–0.8–77.0)), and CRP (13.0 (6.0–28.0) vs 41.0 (18.0–70.0)) (p < 0.05) than patients without anemia, and with the aggravation of anemia, the higher SJC, ESR, and CRP (P < 0.05). In addition, patients with anemia had more severe joint damage progression (β = 1.03, P = 0.012). Möller et al. showing that RA patients with anemia have more severe radiological progression, the study presumed that anemia in RA captures disease processes that are unmeasured by established disease activity markers and that evaluation of the HB level may help identify patients with rapid radiological progression [23]. And Möller et al. also found that anemia was a common phenomenon in RA patients with early aggressive, lower HB levels had a stronger association than DAS28-CRP with later progression of joint damage, anemia is most likely related to inflammation, HB levels and anemia are valuable DAS28-CRP-independent indicators of joint damage progression in patients with RA [24]. Results in our study indicated that there was a clear increasing trend in about percentages of grade III and grade IV along with severity of anemia in RA. And there was a clear increasing trend on mean Sharp score along with severity of anemia in RA. The highest median Sharp score was found in medium to severe anemia group. Associations between anemia and HAQ were reported in some literature. In previous study, Wolfe et al. analyzed 2120 RA patients and 7124 patients without inflammatory disease, results showed that the average HAQ score of patients with HB level < 10 g/dl was 0.18 (95% CI 0.16 to 0.21) units which was higher than that in patients without anemia [14]. Han et al. studied 2495 RA patients from three placebo-controlled trials treated with infliximab (with methotrexate), they found that higher HAQ score (indicating greater impairment) correlated with lower HB levels (P < 0.05), and an at least 1-g increase in HB after 22 weeks was independently associated with HAQ score improvement (OR = 1.43, 95% CI 1.10–1.86) [25]. In our study, the median HAQ scores were significantly different between non-anemia group and anemia group, and there was a clear increasing trend in the mean HAQ score along with severity of anemia. The highest median HAQ score was found in medium to severe anemia group. Moreover, the correlations analysis showed significant relationship between HB level and degree of joint destruction and function (X-ray staging, Sharp score, and HAQ). Logistic regression results confirmed that serum HB level was the protective factor for structural damage in RA. Briefly,RA patients with low HB level had more joint involvement and severer bone destruction also worse function than patients without anemia.
Anemia developed as a result of long-term disease, and several mechanisms had been proposed, including abnormalities of iron absorption, macrophages release, and cytokine networks dysfunction, all of which might lead to inadequate erythropoiesis. It had also been suggested that the production of cytokine in RA led to a decrease in iron availability [26, 27], and increased absorption and retention within reticulo-endothelial cells. Many RA patients had long-term treatment with glucocorticoids, non-steroidal anti-inflammatory drugs, and disease-modifying antirheumatic drugs, which could often result in gastrointestinal mucosal damage or even ulcer, leading to the absorption of iron and vitamin B12, which was also an important factor for anemia in RA patients. In addition, cytokines also played a direct toxic effect on erythropoietin. During the establishment and progression of disease in RA, the activation of inflammatory cells increased, resulting in the excessive production of cytokines such as TNFα, IL-1β, and IL-6 [28], these cytokines, in turn, acted on erythropoietin progenitor cells, promoting hemolysis and subsequently reducing the number of circulating red blood cells [29]. Recent evidence suggested that treatment of inflammation could improve anemia in RA. Kullich et al. [30] found that the level of TNF-α was significantly higher in RA patients who had anemia, compared to patients without anemia. Likewise, Sakthiswary et al. [31] demonstrated that RA patients treated with adalimumab exhibited improved levels of HB. Inflammatory factors could also regulate the secretion of hepcidin, while hepcidin was also involved in the immune regulation process associated with chronic inflammation and had other immune functions that regulated iron metabolism [32]. Inflammation arised from various etiologies and could easily developed into anemia. Inflammatory anemia was usually normocytic and normochromic and with mild clinical manifestations. Characteristic changes in systemic iron handling, erythrocyte production, and erythrocyte life span all contribute to inflammatory anemia. In particular, a large number of researches suggest that anemia may be a stronger correlation with high activity and severity of the disease. Most of RA-related anemias are consistent with anemia of chronic disease, which occurs in patients with acute or chronic immune activation [33]. It was found that after treatment of RA, the level of HB increased after inflammation was controlled. In summary, anemia in RA was caused by multiple factors, it was predominantly associated with inflammation [29]. The anemia caused by chronic inflammation, also known as inflammatory anemia.
The study had some limitations. First, the number of male RA patients recruited in this study was far less than that of female patients, so the conclusions might not be appropriate for male population. Second, this study was a single-center cross-sectional study. Third, we did not observe changes on HB before and after treatment in RA patients, so a prospective study about HB after any kind of treatment might us more valuable results.
Despite these limitations, these results indicated that inflammatory anemia might occur commonly in RA, as a predictor of worse outcome in RA patients. Our data suggested that inflammatory anemia was associated with disease activity and structural damage. This study might broaden the clinical basis of recent discoveries in the link of inflammation, hematopoiesis and highlighted the clinical implications of anemia in RA.
References
Zvaifler NJ (1973) The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol 16:265–336
Koch AE (2007) The pathogenesis of rheumatoid arthritis. Am J Orthop 36(7 Suppl):5–8
Choy EH, Panayi GS (2001) Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 344(12):907–916
Song SN, Iwahashi M, Tomosugi N, Uno K, Yamana J, Yamana S, Isobe T, Ito H, Kawabata H, Yoshizaki K (2013) Comparative evaluation of the effects of treatment with tocilizumab and TNF-alpha inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther 15(5):R141
Wilson A, Yu HT, Goodnough LT, Nissenson AR (2004) Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med 116(Suppl 7A):50s–57s
Bloxham E, Vagadia V, Scott K, Francis G, Saravanan V, Heycock C, Rynne M, Hamilton J, Kelly CA (2011) Anaemia in rheumatoid arthritis: can we afford to ignore it? Postgrad Med J 87(1031):596–600
Furst DE, Chang H, Greenberg JD, Ranganath VK, Reed G, Ozturk ZE et al (2009) Prevalence of low hemoglobin levels and associations with other disease parameters in rheumatoid arthritis patients: evidence from the CORRONA registry. Clin Exp Rheumatol 27(4):560–566
de Launay D, van de Sande MG, de Hair MJ, Grabiec AM, van de Sande GP, Lehmann KA et al (2012) Selective involvement of ERK and JNK mitogen-activated protein kinases in early rheumatoid arthritis (1987 ACR criteria compared to 2010 ACR/EULAR criteria): a prospective study aimed at identification of diagnostic and prognostic biomarkers as well as therapeutic targets. Ann Rheum Dis 71(3):415–423
Scott DL, Symmons DP, Coulton BL, Popert AJ (1987) Long-term outcome of treating rheumatoid arthritis: results after 20 years. Lancet (London, England) 1(8542):1108–1111
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69(9):1580–1588
Fransen J, Creemers MC, Van Riel PL (2004) Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford) 43(10):1252–1255
Terao C, Hashimoto M, Yamamoto K, Murakami K, Ohmura K, Nakashima R et al (2013) Three groups in the 28 joints for rheumatoid arthritis synovitis--analysis using more than 17,000 assessments in the KURAMA database. PLoS One 8(3):e59341
Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F (1992) The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 35(5):498–502
Kirwan JR, Reeback JS (1986) Stanford Health Assessment Questionnaire modified to assess disability in British patients with rheumatoid arthritis. Br J Rheumatol 25(2):206–209
Ge J-B, XY-J. Anemia. Internal Medicine 8st ed. 2013
Kosta PE, Voulgari PV, Zikou AK, Drosos AA, Argyropoulou MI (2011) The usefulness of magnetic resonance imaging of the hand and wrist in very early rheumatoid arthritis. Arthritis Res Ther 13(3):R84
van der Heijde D (2000) How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 27(1):261–263
Wolfe F, Michaud K (2006) Anemia and renal function in patients with rheumatoid arthritis. J Rheumatol 33(8):1516–1522
Strippoli GF, Manno C, Schena FP, Craig JC(2003) Haemoglobin and haematocrit targets for the anaemia of chronic renal disease. The Cochrane database of Syst Rev(1):CD003967. https://doi.org/10.1002/14651858.CD003967
Pereira ICP, Sousa NCF, Pereira DMS, Mendes SJF, Muniz TF, Colares VLP et al (2018) Treatment with either leflunomide or adalimumab reduces anaemia in patients with rheumatoid arthritis. An Acad Bras Cienc 90(2 suppl 1):2161–2166
Yao Xueming MW, Ying H, Fang T, Yang A, Qin Z (2014) Study on anemia of patients with rheumatoid arthritis and its effects on their diseases. Rheum Arthritis 11(3):20–22
van Steenbergen HW, van Nies JA, van der Helm-van Mil AH (2013) Anaemia to predict radiographic progression in rheumatoid arthritis. Ann Rheum Dis 72(7):e16
Moller B, Scherer A, Forger F, Villiger PM, Finckh A (2014) Anaemia may add information to standardised disease activity assessment to predict radiographic damage in rheumatoid arthritis: a prospective cohort study. Ann Rheum Dis 73(4):691–696
Moller B, Everts-Graber J, Florentinus S, Li Y, Kupper H, Finckh A (2018) Low hemoglobin and radiographic damage progression in early rheumatoid arthritis: secondary analysis from a phase III trial. Arthritis Care Res 70(6):861–868
Han C, Rahman MU, Doyle MK, Bathon JM, Smolen J, Kavanaugh A, Westhovens R, St Clair EW, Baker D, Bala M (2007) Association of anemia and physical disability among patients with rheumatoid arthritis. J Rheumatol 34(11):2177–2182
Spivak JL (2000) The blood in systemic disorders. Lancet 355(9216):1707–1712
Masson C (2011) Rheumatoid anemia. Joint Bone Spine 78(2):131–137
Moreland LW, Curtis JR (2009) Systemic nonarticular manifestations of rheumatoid arthritis: focus on inflammatory mechanisms. Semin Arthritis Rheum 39(2):132–143
Papadaki HA, Kritikos HD, Valatas V, Boumpas DT, Eliopoulos GD (2002) Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood 100(2):474–482
Kullich W, Niksic F, Burmucic K, Pollmann G, Klein G (2002) Effects of the chemokine MIP-1alpha on anemia and inflammation in rheumatoid arthritis. Z Rheumatol 61(5):568–576
Sakthiswary R, Syahrul Sazliyana S, Mohd Shahrir MS, Shahril NS, Hussein H (2012) Beyond the joints in rheumatoid arthritis: effects of adalimumab on hematologic and lipid indices. EXCLI J 11:142–149
Singh B, Arora S, Agrawal P, Gupta SK (2011) Hepcidin: a novel peptide hormone regulating iron metabolism. Clin Chim Acta (11–12):412, 823–430
Weiss G, Goodnough LT (2005) Anemia of chronic disease. N Engl J Med 352(10):1011–1023
Acknowledgments
We thank all the patients for their enthusiastic participation in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig S1
the distribution of anemia in RA patients. (A)Non-anemia group versus anemia group. (B)Non-anemia group versus mild anemia group versus medium to severe anemia group (JPG 52 kb)
Supplementary Fig. S2
Joint function in different RA patients. (A) Grade of joint function classification in different groups of anemia (P < 0.001), (B) Comparison of HAQ in different groups of anemia (P < 0.001) (JPG 150 kb)
Rights and permissions
About this article
Cite this article
Chen, Yf., Xu, Sq., Xu, Yc. et al. Inflammatory anemia may be an indicator for predicting disease activity and structural damage in Chinese patients with rheumatoid arthritis. Clin Rheumatol 39, 1737–1745 (2020). https://doi.org/10.1007/s10067-019-04873-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04873-y