Abstract
Background
Rheumatoid arthritis (RA) is a chronic systemic autoimmune inflammatory disorder characterized by synovial inflammation that leads to joint damage, bony erosions, and related deformities. Between 30 and 70% of RA patients will experience anemia. Early detection of anemia is of great importance. This study aimed to evaluate the serum level of hepcidin (HEP) in RA patients and to assess its relation to disease activity and anemia. The current cross-sectional study included 44 cases with RA in addition to 44 healthy controls. The disease activity in the RA patient was assessed by using the disease activity score (DAS) 28 score-CRP. The serum levels of HEP and ferritin were assessed in both groups using enzyme-linked immunosorbent assay (ELISA) technique.
Results
Hepcidin level in the RA group was statistically significantly higher as compared to the control group (p = 0.001). The prevalence of Anemia of chronic disease (ACD) was 40.9%, and iron deficiency anemia (IDA) was 27.3% which accounted for 68.2% of the total anemia cases. The HEP level was statistically significantly higher in the RA patients with ACD than those without anemia (P = 0.028), RA patients with IDA (P < 0.001), and control group (P < 0.001). There was a statistically significant positive correlation between HEP level and serum ferritin level (p = 0.005). HEP level was significantly and inversely correlated with hemoglobin (Hb) in patients with ACD. Serum HEP level is higher in RA patients with high disease activity than those with moderate activity, low activity, and patients in remission (p = 0.380). However, the difference was not statistically significant. The best cutoff point of HEP level to identify RA patients from healthy controls was > 355.5 Pg/ml. This point showed moderate sensitivity (70.5%) with moderate specificity (63.6%) with a statistically significant value.
Conclusions
We found the anemia, and particularly ACD, is more common in RA patients. In RA patients with ACD, serum HEP levels were considerably higher. Although serum HEP showed no diagnostic significance when it came to evaluating disease activity, it could be a dependable non-invasive biomarker for the diagnosis of various forms of anemia in RA patients.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is an autoimmune disorder that causes progressive inflammation in the synovial tissue and destruction of joints [1].
A portion of RA patients also experience extra-articular symptoms and consequences, including lung disease, osteoporosis, and anemia, in addition to joint damage [1]. Anemia affects 30–70% of RA patients. Anemia may predict radiographic progression in RA, and RA patients with anemia had a more severe course and physical disability [2, 3].
Diagnosing RA anemia early on is essential. However, iron deficiency anemia (IDA) and anemia of chronic disease (ACD), which are the most prevalent causes of RA, can coexist, making the differential diagnosis challenging. [4].
Hepcidin (HEP) is an antimicrobial liver-derived peptide. This type-II acute-phase protein is produced by inflammatory cytokines, particularly IL-6 [5].
During inflammation, anemia, and hypoxia, HEP expression increases [6]. HEP binds to ferroportin and degrades it, making it an inflammatory mediator and negative iron regulator [7]. HEP is also a key mediator of ACD due to its association with inflammatory cytokines and iron metabolism [8].
Hepcidin is not routinely tested for RA, but it can assess disease activity and diagnose anemia [9]. Nonetheless, there was no correlation between serum HEP levels and ACD or RA activity in certain investigations [10, 11].
Consequently, there was a contradictory relationship between HEP and RA. For this reason, the current study was conducted to evaluate the serum level of HEP in RA patients and to assess its relation to disease activity and anemia.
Patients and methods
Study design and participants
This is a cross-sectional study that was conducted for 1 year. An equal number of age- and sex-matched healthy control volunteers and 44 RA patients were chosen selectively in this study.
Eligibility criteria
Rheumatoid arthritis patients who will participate in the study should fulfill American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) 2010 criteria for diagnosis of RA [12].
The group of RA patients will then be subdivided according to the laboratory results according to anemic or not and type of anemia (ACD or iron deficiency anemia).
Assessment of serum level of HEP was conducted by using an ELISA Kit (Catalogue No.: EH3221) (Wuhan Fine Biotech Co., Ltd.). Assessment of serum level of ferritin was conducted by using of ferritin ELISA Kit (Catalogue No.: FR248T) (The Calbiotech, Inc. (CBI)). Both were conducted according to the manufacturers’ guidelines. The study is conducted in accordance with Helsinki Standards as revised in 2013 [13] and after approval of the local research ethical committee. Informed verbal consent will be obtained from each participant sharing in the study.
Exclusion criteria
The cases with the following criteria were excluded: associated anemia due to other causes (e.g., renal disease, liver disease especially viral hepatitis, heart failure, and autoimmune diseases other than RA) from past history and examination when needed, pregnancy, malnutrition, hematological diseases like thalassemia and sickle cell anemia, medication consumption for anemia, except folic acid in methotrexate or sulfasalazine therapy, malignancy, and acute and chronic infections.
Sociodemographic and clinical assessment
A thorough medical history, physical examination, and local examination were administered to all individuals aged 18 or more who were considered for the study. Disease activity was assessed using disease activity score (DAS) 28-CRP. The DAS28 consists of a 28 tender joint count (range 0–28), and a 28 swollen joint count (range 0–28), in addition to measurement of the CRP and Patient Global Assessment (PGA). Score interpretation is as follows: > 5.1 suggests high disease activity, > 3.2–5.1 suggests moderate disease activity, 2.6–3.2 suggests mild disease activity, and < 2.6 suggests disease remission [14].
Laboratory assessment
All subjects had their venous blood drawn first thing in the morning following a night of fasting to get the following readings: complete blood count (CBC) [15], erythrocyte sedimentation rate (ESR) [16], C-reactive protein (CRP) [17], rheumatoid factor (RF) by ELISA technique [18], anti-cyclic citrullinated peptide (anti- CCP) [19], liver function tests (including serum aspartate transaminase [AST], alanine transaminase [ALT], and serum bilirubin) [20], and kidney functions tests (serum creatinine) [20].
The group of RA patients was subdivided according to the laboratory results whether anemic or not and type of anemia (ACD or IDA) according to ferritin level. Patients were considered anemic if their hemoglobin (Hb) was ≤ 11.5 g/dL for women and ≤ 13.5 g/dL for men [21].
Sample size
Based on data from the literature [10], considering the level of significance of 5%, and power of study of 80%, and based on data from the literature, the sample size can be calculated using the following formula: Sample size = [(Z1-α/2)2.SD2]/d2, where Z1-α/2 = is the standard normal variate, at 5% type 1 error, it is 1.96; SD is standard deviation of variable; and d is absolute error or precision. So, sample size = [(1.96)2.(684.97)2]/(202.4)2 = 43.9. Based on the above formula, the sample size required for the study is 44 in each group.
Statistical analysis
Study data were analyzed using Statistical Package for Social Sciences (SPSS) version 25 for Windows (IBM, SPSS Inc, Chicago, IL, USA). Categorical data were expressed in number and percent. The Chi-Square test (Monte-Carlo test) made the comparison between two or more groups with categorical data. The quantitative data were tested whether normally distributed or not by using the Kolmogorov–Smirnov test and were expressed as median ± SD if parametric or median (range) if non-parametric. Three groups were compared using quantitative variables that followed a normal distribution using the one-way ANOVA test. In the case of abnormally distributed data, the Kruskal–Wallis test was applied. Post hoc Tukey or Bonferroni tests were conducted instead of ANOVA and the Kruskal–Wallis test, respectively. Correlation of numeric data was done by Pearson’s or Spearman correlation (r). The receiver operator characteristic (ROC) curve was used to detect the best cutoff point of the quantitative variable in differentiating two classes of binary categorical outcomes. The dependent and independent risk variables were examined using univariate and multivariate logistic regression analysis. P values < 0.05 are considered significant.
This study followed STROBE guidelines [22].
Results
In this study, 44 individuals with RA and 44 healthy controls (matched for age and sex) were included. RA patients were on one or more drugs. Twenty-three were using methotrexate and hydroxychloroquine, 4 were using leflunomide, 11 were using anti-TNF, 3 were using salazopyrin and 3 were on baricitinib. Table 1 shows the sociodemographic data of RA patients and controls.
The laboratory analysis in the two study groups is shown in Table 2; RBC (red blood cell) count, MCV (mean corpuscular volume), MCHC (mean corpuscular hemoglobin concentration), and platelets (PLTs) count were statistically significantly lower in the RA cases compared to the control group (P < 0.001, 0.002, < 0.001 and < 0.001 respectively). On the other hand, RDW (red cell distribution width), AST, ESR, and CRP were statistically significantly higher in the RA cases than in the control group (P < 0.001 < 0.024, < 0.001, and < 0.001 respectively). Rheumatoid factor was positive in 77.3% of the cases in the RA group, while it was positive in 18.3% in the control group, with a high statistically significant difference between the two groups (p < 0.001). Anti-cyclic citrullinated peptides (CCP) antibodies were positive in 90.9% of the RA cases, while it was negative in all the cases in the control group, with a high statistically significant difference between the two groups (p < 0.001). The mean HEP level in the RA group was 409.51 ± 136.19 pg/ml which was statistically significantly higher as compared to the control group (337.16 ± 41.79 pg/ml) (p = 0.001).
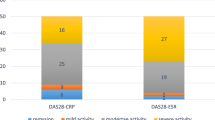
In the RA group, there were 14 non-anemic patients (31.8%), 18 patients had ACD (40.9%), and 12 patients had IDA (27.3%). The mean DAS-28 (CRP) score in the RA cases was 4.08 ± 1.095. According to this score, there were 6 cases (13.6%) in remission, 6 cases (13.6%) with low disease activity, 22 cases (50%) with moderate activity, and 10 cases (22.7%) were highly active.
There was a statistically significant positive correlation between HEP level and serum ferritin level (r = 0.476, p = 0.005). Other clinical and laboratory variables did not show any significant correlation with serum HEP levels in RA patients (Table 3).
There was no statistically significant difference in HEP level in the cases with RA between male and female patients. Similarly, no statistically significant difference was found between RA patients with positive or negative RF and positive or negative Anti- CCP antibodies (Table 4).
Serum HEP level is higher in RA patients with high disease activity than those with moderate activity, low activity, and patients in remission. However, the difference was not statistically significant (p = 0.380) (Table 5).
The HEP level was statistically significantly higher in the RA patients with ACD (493.94 ± 105.72 pg/ml) than those without anemia (413.29 ± 106.27 pg/ml) (P = 0.028), RA patients with IDA (277.17 ± 105.34 pg/ml) (P < 0.001), and control group (337.16 ± 41.79 pg/ml) (P < 0.001). The HEP level was statistically significantly higher in RA patients without anemia (413.29 ± 106.27 pg/ml) as compared to both RA patients with IDA (277.17 ± 105.34 pg/ml) (P < 0.001) and control group (337.16 ± 41.79 pg/ml) (P = 0.013). The level of HEP in the control group (337.16 ± 41.79 pg/ml) was higher as compared to the RA patients with IDA (277.17 ± 105.34 pg/ml), but it did not reach a statistically significant value (P = 0.104) (Table 5).
Table 6 shows that the best cutoff point of HEP level to identify RA patients from healthy controls was > 355.5 Pg/ml. This point showed moderate sensitivity (70.5%) with moderate specificity (63.6%) with a statistically significant value (p < 0.001*) and AUC (0.700) (Fig. 1).
Discussion
In our study, we combined the evidence of HEP status in RA with the correlation between serum HEP level and RA activity as well as anemia. Several studies have investigated this relationship; however, the results were inconsistent with controversial results. In order to better understand the relationship between disease activity, anemia, and HEP serum levels in RA patients, the present study set out to do just that.
The current study included 44 cases with RA in addition to 44 age and sex-matched subjects as a control group. In the RA group, there were 14 NA patients (31.8%), 18 patients had ACD (40.9%) and 12 patients had IDA (27.3%).
This result agreed with that of Kadu et al. [23] who included 60 patients with RA; anemia was present in 60% (36/60) of patients, ACD in 55.5% (20/60) and IDA in 44.4% (16/60).
The current results also came in agreement with Borah and Iqbal [24] which included 31 patients with RA. Anemia was observed in 20 patients (64.5%). Out of the 20 anemic patients, 12 patients (60%) were found to be having ACD and 8 patients (40%) were found to be having IDA.
In the current study, ACD was observed in 60% (18/30) of anemic patients. This supports Borah and Iqbal [24] and Sabău et al.’s [25] findings that ACD is present in 60% and 52.6% of anemic RA patients, since ACD is the most common anemia in RA.
While comparing the RA group to the control group, we found that the RA group had a mean HEP level that was statistically significantly higher.
This finding is consistent with the meta-analysis of eleven trials that found significantly higher serum HEP levels in RA patients, which included 664 RA patients and 319 healthy controls [9].
The latest results matched Stefanova et al.’s [26] 114 RA patients and 42 healthy controls. RA patients had higher pro-hepcidin levels than healthy controls.
The results also supported Dagli et al.’s [27] study that indicated RA patients had greater serum pro-hepcidin and HEP levels than healthy controls.
Abdel-Khalek et al. [28] found that anemic RA patients had higher serum HEP than controls. HEP levels may rise due to high IL-6 levels, which induce HEP and cause RA.
However, the current results differed from Teke et al. [29] which included 15 RA patients and 31 controls. They found no significant difference in HEP levels between groups.
The current results contradict Jayaranee et al. [11], who collected blood from 20 healthy and 60 RA patients. HEP levels were not statistically different between cases and controls.
Despite inflammation, hypoxia may explain the latter studies’ lack of significant difference from control groups. Method of test, circadian variations in HEP values, and individual variations due to age, disease duration, and treatment may affect results. Clinical research HEP tests are also difficult because HEP aggregates and sticks to surfaces [30].
In the current study, the HEP level was statistically significantly higher in the RA patients with ACD than those without anemia, RA patients with IDA, and control group.
This supported Khalaf et al.’s [31] finding that ACD had the highest serum HEP level compared to ACD/IDA, IDA non-anemic (NA), and control, which was statistically significant.
RA patients had high HEP levels, higher in the ACD subgroup than in the ACD + IDA subgroup and higher than in controls, according to Abdel-Khalek et al. [28].
Van Santen et al. [32] found that the IDA RA group had the lowest HEP median level, which suppresses HEP and induces gut iron absorption, making it a potential biomarker for iron deficiency in inflammatory conditions.
Although there is no correlation between anemia and serum pro-hepcidin, there is a correlation between the major disease process and its activity as revealed by Kim et al. [33]. The concentration of pro-hepcidin was not different in patients with ACD compared to those without. The fact that serum pro-hepcidin may not provide an accurate picture of iron status in a diseased human population may account for this. Another theory is that RA’s inflammatory response causes an overproduction of HEP, which, if expressed at an unhealthy level, could disrupt the body's usual physiological response to iron metabolism.
The present investigation confirms previous findings that serum HEP level is a strong predictor of Hb level. Consistent with the findings of Sabău et al. [30], we found that in ACD patients, there was a strong inverse relationship between HEP concentration and Hb.
Demirag et al. [34] included 40 RA patients, 19 of whom had anemia and 21 of whom did not, in line with our findings. Only 10 out of 19 patients with anemia had iron deficiency as the cause of their condition. Compared to other groups, the authors found that the RA group with non-IDA had higher serum HEP [34].
According to research conducted by Swellam et al. [35], there is a strong correlation between HEP and laboratory anemia profiles. This suggests that HEP binds to ferroprotein, which in turn decreases intestinal iron absorption and prevents iron mobilization from macrophages and hepatocytes. As a result, low serum iron levels and anemia are caused by decreased iron reutilization [35].
Inflammation-induced HEP production may be balanced by iron deficiency in these patients. In that case, low CRP and low HEP indicate iron deficiency, high CRP and high HEP indicate inflammation, and high CRP and low HEP indicate both [36].
The current study differed from Sahebari et al. [10], which enrolled 80 RA patients (36 with ACD and 44 without ACD). They found no significant difference in median (interquartile range) HEP levels between RA with and without ACD (1207 [985.2] vs. 923.8 [677.3] ng/mL; P = 0.57) [10].
The variations in the results could be due to different distribution and prevalence of anemia in the included studies [10, 30].
A statistically significant positive correlation was found between HEP and serum ferritin in this study. Other RA patients’ clinical and laboratory variables did not correlate with serum HEP.
Khalaf et al. [31] found high correlations between ferritin, hs-CRP, DAS28, and ESR with HEP (P < 0.001), while Hb, serum iron, and TIBC were negatively correlated (P values of 0.016, 0.022, and < 0.001).
High-activity RA patients had higher serum HEP levels than moderate-activity, low-activity, and remission patients in this study. The difference was not statistically significant.
Within the same line, the current study agreed with Sahebari et al. [10] that showed that HEP level is higher in RA patients with high disease activity (DAS28-ESR > 5.1) than in those inactive to moderately active (DAS28-ESR > 5.1). Active RA and inactive to moderately active RA groups did not differ significantly [10].
Chen et al. [9] found that serum HEP positively correlated with RA disease activity measured by RF, DAS28, and ESR, but the current results disagreed.
Further, Emerah et al. [37] found a correlation between prohepcidin, RF, CRP, ESR, and DAS28 in Egypt. The HEP of active RA patients was significantly higher than that of inactive-to-moderate patients [37].
Results may vary because people from different regions have different genetics, physical conditions, and environmental factors. HEP levels can also be affected by ELISA kits, anemia criteria, and disease activity.
Hepcidin, ferritin, and haptoglobin are produced in the liver as acute-phase reactants, and their expression is dependent on interleukin-6 (IL-6) signaling. Tocilizumab is a humanized monoclonal antibody that inhibits IL-6 binding to its receptor. The interrelationships of interleukin (IL)-6 receptor inhibition with iron metabolism markers including hepcidin have been determined in patients with RA and hepcidin levels fell alongside increases in hemoglobin after treatment with tocilizumab [38].
Similarly, Song et al. [39] noted that hepcidin-mediated iron metabolism may contribute to the pathogenesis of RA-related anemia. Moreover, tocilizumab was more effective than TNF-α inhibitors for improving anemia and normalizing iron metabolism in RA patients by inhibiting hepcidin production [39].
In the current study, the best cutoff point of HEP level to identify RA from healthy control was > 355.5 Pg/ml. This point showed moderate sensitivity (70.5%) with moderate specificity (63.6%) with a statistically significant value (p < 0.001) and AUC (0.700).
No previous studies have reported the diagnostic cutoff point of HEP in the diagnosis of RA. It could provide a simple test for early detection of RA, but its validation is still needed for further studies.
Limitations
The current study has some limitations such as the small sample size of the included cases. Also, the cases were recruited from a single center that could not reflect the effect of the geographic factors on the results.
At last, the cross-sectional nature of the study did not give a conclusion on the effect of treatment on anemia and its relation to the marker level.
Conclusion
Anemia, particularly ACD and IDA, is highly prevalent in rheumatoid arthritis. In patients with RA, serum HEP may be a useful non-invasive biomarker for the diagnosis of various forms of anemia. However, the marker has no significant correlation with RA disease activity.
Recommendations
Further prospective interventional studies should be conducted to determine the effect of therapy on anemia in cases with RA and determine the value of hepcidin in the assessment of anemia response for treatment. Moreover, further studies should be performed to validate the use of HEP as a diagnostic biomarker of anemia in other rheumatological diseases. Further studies are necessary to explain how HEP is directly involved in RA pathogenesis. In addition, future studies to determine serum levels of HEP at different time points during the clinical course of RA patients will be needed to confirm our results.
Availability of data and materials
All data availability for this work are available upon request to the corresponding author.
Abbreviations
- ACD:
-
Anemia of chronic disease
- ALT:
-
Alanine transaminase
- Anti-CCP:
-
Anti-cyclic citrullinated peptide
- AST:
-
Aspartate transaminase
- CRP:
-
C- reactive protein
- DAS:
-
Disease activity score
- DAS:
-
Disease activity score
- ELISA:
-
Enzyme-linked immunosorbent assay
- ESR:
-
Erythrocyte sedimentation rate
- HB:
-
Hemoglobin
- HEP:
-
Hepcidin
- IDA:
-
Iron deficiency anemia
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- TIBC:
-
Total iron binding capacity
References
Peng X, Wang Q, Li W, Ge G, Peng J, Xu Y et al (2023) Comprehensive overview of microRNA function in rheumatoid arthritis. Bone Research 11(1):8
Chen YF, Xu SQ, Xu YC, Li WJ, Chen KM, Cai J, Li M (2020) Inflammatory anemia may be an indicator for predicting disease activity and structural damage in Chinese patients with rheumatoid arthritis. Clin Rheumatol 39:1737–1745
Shadick N, Hagino O, Praestgaard A, Fiore S, Weinblatt M, Burmester G (2023) Association of hemoglobin levels with radiographic progression in patients with rheumatoid arthritis: an analysis from the BRASS registry. Arthritis Res Ther 25(1):88
Al-Rubaie H A, Al-Bayaa I M and Al-Amiri Y A. (2019) The value of soluble transferrin receptor and soluble transferrin receptor-ferritin index in discriminating iron deficiency anaemia from anaemia of chronic disease in patients with rheumatoid arthritis. Open Rheumatol J 13(1):9–14.
Vp P, Sethu G, Ganapathy D (2020) Review on iron regulatory hormone hepcidin. PalArch’s J Archaeology Egypt/Egyptology 17(7):1414–1421
Malyszko J, Malyszko JS, Matuszkiewicz-Rowinska J (2019) Hepcidin as a therapeutic target for anemia and inflammation associated with chronic kidney disease. Expert Opin Ther Targets 23(5):407–421
Lin F, Tuffour A, Hao G, Peprah FA, Huang A, Zhou Y, Zhang H (2023) Distinctive modulation of hepcidin in cancer and its therapeutic relevance. Front Oncol 13:1141603
Yacoub MF, Ferwiz HF, Said F (2020) Effect of interleukin and hepcidin in anemia of chronic diseases. Anemia 2020:3041738
Chen Y, Xu W, Yang H, Shao M, Xu S, Deng J., ... & Pan F (2021) Serum levels of hepcidin in rheumatoid arthritis and its correlation with disease activity and anemia: a meta-analysis. ImmunolInvestig 50(2–3);243–258
Sahebari M, Rezaieyazdi Z, Hashemy SI, Khorasani S, Shahgordi S, Alizadeh MK., ... & Khodashahi M (2019) Serum hepcidin level and rheumatoid arthritis disease activity. European Journal of Rheumatology, 6(2):76
Jayaranee S, Sthaneshwar P, Sokkalingam S (2009) Serum prohepcidinconcentrations in rheumatoid arthritis. Pathology 41(2):178–182
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham Iii CO et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581
World Medical Association W M (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Fransen J, Van Riel PLCM (2009) The disease activity score and the EULAR response criteria. Rheum Dis Clin 35(4):745–757
Bauer JD (1984) Hemoglobin, porphyrin, and iron metabolism. Clinical Chemistry, theory, analysis, and correlation. Mosby Company, ST. Louis, pp 611–655
Bull B, Caswell M, Ernst E, Jou J, Kallner A, Koepke J et al (1993) ICSH recommendations for measurement of erythrocyte sedimentation-rate. J Clin Pathol 46(3):198–203
Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J et al (2001) Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem 47(3):418–425
Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
Kumari B, Kumar P, Chaudhary RKP (2017) Evaluation of anti-cyclic citrullinated peptide autoantibodies and c-reactive protein in common autoimmune skin diseases with and without arthritis. J Clin Diagn Res 11(7):BC06
Rigby WF, Lampl K, Low JM, Furst DE (2017) Review of routine laboratory monitoring for patients with rheumatoid arthritis receiving biologic or nonbiologic DMARDs. Int J Rheumat 2017:9614241
Nita E, Bairaktari E, Kolios G, Migkos MP, Somarakis GP, Markatseli T., ... & Voulgari PV (2021) Role of hepcidin in anemia of chronic disease in rheumatoid arthritis. J Lab Phys 13(04), 317–322
Cuschieri S (2019) The STROBE guidelines. Saudi J Anaesth 13(Suppl 1):S31–S34. https://doi.org/10.4103/sja.SJA_543_18. PMID:30930717;PMCID:PMC6398292
Kadu KP, Kadu SK and Bhore SA (2021) Prevalence of anaemia in patients of rheumatoid arthritis 8(3):2454-2237
Borah DJ, Iqbal F (2007) Anemia in recent onset rheumatoid arthritis. JK Sci 9(3):120–122
Sabau A, Craciun A, Gabriela C, Bolosiu H D, Rednic S, Damian L and et al. (2011) Association between acute phase reactant levels, and disease activity score (DAS28), in patients with rheumatoid arthritis and anemia. Romanian J Rheumatol 20(4):225
Stefanova KI, Delcheva GT, Maneva AI, Batalov AZ, Geneva-Popova MG, Karalilova RV, Simitchiev KK (2016) Pathobiochemical mechanisms relating iron homeostasis to parameters of inflammatory activity and autoimmune disorders in rheumatoid arthritis. Folia Medica 58(4):257 and Autoimmune Disorders in Rheumatoid Arthritis. Folia Medica, 58(4), 257
Dagli M, Sivrikaya A, Yilmaz S (2013) Serum prohepcidin and hepcidin levels in patients with rheumatoid arthritis: a prospective study/Romatoid Artritli Hastalarda Serum Hepsidin ve Prohepsidin Düzeyleri: Prospektif Çalisma. Türkiye Klinikleri Tip Bilimleri Dergisi 33(4):946
Abdel-Khalek MA, El-Barbary AM, Essa SA-M, Ghobashi AS (2011) Serum hepcidin: a direct link between anemia of inflammation and coronary artery atherosclerosis in patients with rheumatoid arthritis. J Rheumatol 38(10):2153–2159
Teke HU, Cansu DU, Yıldız P, Temiz G, Bal C (2017) Clinical significance of serum IL-6, TNF-α, hepcidin, and EPO levels in anaemia of chronic disease and iron deficiency anaemia: The laboratory indicators for anaemia. Biomed Res 28:2704–2710
Sabău A, Văleanu M, Boloşiu HD, Crăciun AM (2013) Evaluation of serum hepcidin variation in patients with rheumatoid arthritis according to anemia profile and its correlation with disease activity. Revista Română de Medicină de Laborator Vol 21:1-4
Khalaf W, Al-Rubaie HA, Shihab S (2019) Studying anemia of chronic disease and iron deficiency in patients with rheumatoid arthritis by iron status and circulating hepcidin. Hematol Rep 11(1):7708
van Santen S, van Dongen-Lases EC, de Vegt F, Laarakkers CMM, van Riel PLCM, van Ede AE et al (2011) Hepcidin and hemoglobin content parameters in the diagnosis of iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum 63(12):3672–3680
Kim H-R, Kim K-W, Yoon S-Y, Kim S-H, Lee S-H (2010) Serum pro-hepcidin could reflect disease activity in patients with rheumatoid arthritis. J Korean Med Sci 25(3):348–352
Demirag MD, Haznedaroglu S, Sancak B, Konca C, Gulbahar O, Ozturk MA, Goker B (2009) Circulating hepcidin in the crossroads of anemia and inflammation associated with rheumatoid arthritis. Intern Med 48(6):421–426
Swellam M, Gabal KMA, Youssef SS (2013) Interleukin-1 receptor antagonist gene polymorphism and hepcidin in rheumatoid arthritis: Correlations with clinical and laboratory indices of disease activity. IUBMB Life 65(10):883–888
Sasu BJ, Li H, Rose MJ, Arvedson TL, Doellgast G, Molineux G (2010) Serum hepcidin but not prohepcidin may be an effective marker for anemia of inflammation (AI). Blood Cells Mol Dis 45(3):238–245
Emerah A, Abbas SF, Pasha HF (2014) Serum prohepcidin concentrations in rheumatoid arthritis and its relation to disease activity. Egyptian Rheumatology and Rehabilitation 41(3):130–134. https://doi.org/10.4103/1110-161X.140530
Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C (2013) Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther 15:1–6
Song, S. N. J., Iwahashi, M., Tomosugi, N., Uno, K., Yamana, J., Yamana, S., ... & Yoshizaki, K. (2013). Comparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Research & Therapy, 15, 1-10.
Acknowledgements
All authors wish to thank and appreciate all cooperative patients who participated in this study.
Informed consent
Written informed consent has been obtained from all participants.
Funding
We have no funding sources that supported our work.
Author information
Authors and Affiliations
Contributions
All authors contributed in the study conception and design. M.M.G. participated in data acquisition and analysis, did the laboratory investigations statistical analysis, and methodology, and wrote and revised the manuscript. S.A.R.A.K.B., A.A.E.H.S., and A.A.M.E. were responsible for the supervision, analyzing data, and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Our institutional IRB Proposal Code is MS.22.03.1917.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Genedy, M.M., Shabana, A.A.E.H., Elghzaly, A.A.M. et al. Assessment of hepcidin in Egyptian patients with rheumatoid arthritis and its relation to anemia: a single-center study. Egypt Rheumatol Rehabil 51, 44 (2024). https://doi.org/10.1186/s43166-024-00276-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-024-00276-3