Abstract
Introduction
Systemic sclerosis (SSc) is a complex autoimmune disease, characterized by microvascular lesions, autoimmunity, and fibrosis. It is suggested that MIF participates in the amplification of the proinflammatory process in SSc; moreover, the promoter polymorphisms − 794 CATT5–8 (rs5844572) and − 173G>C (rs755622) in the MIF gene have been associated with an increase in MIF serum levels in several autoimmune diseases. The aim of this study was to analyze the relationship of the − 794 CATT5–8 and − 173G>C MIF polymorphisms with mRNA expression, MIF serum levels, and the Th1/Th2/Th17 cytokine profile in SSc.
Materials and methods
A case-control study was carried out that included 50 patients with SSc and 100 control subjects (CS). Both polymorphisms were genotyped by PCR and PCR-RFLP. MIF levels were measured by ELISA kit. The cytokine profile and the MIF mRNA expression were quantified by BioPlex MagPix system and real-time PCR, respectively.
Results
An association between the − 794 CATT7 and − 173*C MIF alleles and the 7C haplotype with SSc susceptibility was found (p < 0.05). Also, the 7C haplotype was associated with increased MIF mRNA expression (p = 0.03) in SSc. In addition, an increase of IL-1β and IL-6 serum levels in SSc patients was found as well as a positive correlation between MIF serum levels and Th1 and Th17 cytokine profiles.
Conclusion
The MIF 7C haplotype is a susceptibility marker for SSc in the southern Mexican population and is associated with MIF mRNA expression. Moreover, there is a positive correlation between MIF serum levels and Th1 and Th17 inflammatory response in SSc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc), also referred to as scleroderma, is an autoimmune disease characterized by three pathological processes: microvascular lesions, impaired immune response, and fibrosis in skin and internal organs [1]. The heterogeneity of the clinical characteristics in SSc allows classifying patients based on their skin condition in limited cutaneous (lcSSc) and diffuse cutaneous SSc (dcSSc), the latter with worse prognosis [2]. Although SSc is a rare disease, it has one of the highest mortality rates among rheumatic diseases [3].
The etiology of SSc remains unknown, but it has been associated with several risk factors such as genetic and environmental factors. The most important genetic factors are the HLA class II genes (HLA-DRB1*01, HLA-DRB1*11, HLA-A*30, and HLA-A*32), which have been associated with disease susceptibility in different populations [4]. Additional other genes outside the HLA region have been identified that contribute to the susceptibility and prognosis of SSc, including the MIF gene, which codes for a protein of the same name [5, 6].
MIF is an immunoregulatory cytokine that contributes to the pathogenesis of autoimmunity, infectious diseases, and cancer [7]. In addition, MIF promotes the survival of different types of cells such as dermal fibroblasts, by inhibiting apoptosis induced by p53 activation and inducing the secretion of proinflammatory cytokines such as TNFα, IL-1β, IL-2, IL-6, IL-8, and IL-12 [8, 9]. The MIF gene is located at the 22q11.23 locus and is made up of three exons and two introns [7]. Several polymorphisms have been described within the MIF promoter region, including the STR (short tandem repeat) − 794 CATT5–8 and the SNP (single nucleotide polymorphism) − 173G>C, which have been associated with different autoimmune diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), systemic lupus erythematosus (SLE), and multiple sclerosis (MS) in the Mexican population [10,11,12,13].
The presence of a higher number of repetitions of STR − 794 CATT5–8 correlates with higher expression of MIF mRNA [14], which is explained by the fact that the transcription factor ICBP90 binds to these repetitions, regulating positively the expression of this gene [15]. With respect to SNP − 173G>C, the C allele also is associated with higher MIF levels in serum and synovial fluid in RA patients [7, 16], most likely due to linkage disequilibrium with the high expression − 794 CATT7 allele.
In 2003, Selvi et al. reported for the first time that serum concentrations of MIF in patients with dcSSc were significantly higher than those in controls. Then, MIF expression was detected in skin biopsies of patients with SSc by immunohistochemical staining [5]. Two independent studies have reported that a functional MIF promoter polymorphism (− 173G<C) was strongly associated with dcSSc [17, 18]. In addition, Becker et al. revealed that MIF can contribute to vasculopathy in the SSc [19]. On the other hand, it was reported that MIF stimulates the process of excessive fibrosis in SSc, increasing the proliferation of fibroblasts and collagen synthesis [20], and decreasing the apoptosis of dermal fibroblasts [9].
Despite the above-mentioned findings, the role of MIF in the pathogenesis of SSc remains poorly understood. The aim of this study was to evaluate the association of the MIF polymorphisms (− 794 CATT5–8 and − 173G>C) with the expression of MIF and its correlation with Th1, Th2, and Th17 cytokine profile in patients with SSc from southern Mexican population.
Material and methods
Subjects
A case-control study was conducted with two study groups: The first group consists of 50 patients with SSc classified according to the 2013 American College of Rheumatology/European League Against Rheumatism classification criteria for SSc [2]. They were enrolled from Rheumatology Department at Hospital General de Chilpancingo “Dr. Raymundo Abarca Alarcón,” Chilpancingo de los Bravo, State of Guerrero, Mexico. The modified Rodnan index Total Skin Score (TSS) and the Spanish version of the health assessment questionnaire disability index (HAQ-DI) were applied to the patients. The second group was comprised of 100 healthy subjects, referred to as control subjects (CS), recruited from the general population. Both groups were unrelated individuals from the same population, and to prevent population heterogeneity, only Mestizo subjects from Southern Mexico were included (specifically from Guerrero state) with at least back three generations of Mexican ancestry.
Quantification of autoantibodies and MIF serum levels
Antibodies against topoisomerase 1 (anti-Scl70, BioSystems Cat. No. COD44863), anti-centromere (CENP-B, BioSystems Cat. No. COD44865), anti-fibrillarin (AFA/snoRNP/U3RNP, CUSABIO Cat. No. CSB-E09697h), and anti-RNA polymerase III (anti-RNA PoIII, CUSABIO Cat. No. CSB-EQ027833HU) were measured with a second-generation enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s recommendations. The sensitivity tests were 0.5 U/mL (anti-Scl70) and 0.3 U/mL (CENP-B). For calculation of the valence AFA and anti-RNA PoIII, we compare the sample well with control (ratio: OD-sample/OD-negative control). Subjects with OD values ≥ 2.1 were regarded as AFA and anti-RNA PoIII positive. Samples were also tested for anti-nuclear antibody (ANA) by indirect IF microscopy using multisport slides with fixed HEp-2 cells (BioSystems, Barcelona, Spain) according to the manufacturer’s recommendations.
MIF serum levels from all individuals were determined by the ELISA method using the Human MIF ELISA Kit Protocol (LEGEND MAX Human Active MIF ELISA Kit, BioLegend) according to the manufacturer’s instructions. The detection limit was 17.4 ± 9.2 pg/mL.
Multiplex serum cytokine immunoassay
The serum levels of Th1 (IFN-γ and TNF-α), Th2 (IL-4 and IL-10), and Th17 (IL-17A, IL-17F, IL-1β, IL-6, IL-21, and IL-23) cytokine profile were measured using the 15-Plex # 171-AA001M assay (Bio-Plex® MAGPIX™ Bio-Rad), with a range assay sensibility of 0.2–0.8 pg/mL; those samples with cytokine concentrations below the lowest point on the standard curve were reported with the lowest value. Serum samples were stored at − 80 °C until the day of the assay and then thawed and processed according to the manufacturer’s instructions. To corroborate our results, the TNF-α cytokine (LEGEND MAX Human Active TNF-α ELISA Kit, BioLegend) was measured additionally in some samples with a conventional ELISA kit. The TNF-α assay sensitivity was 1.7 pg/mL. The cytokine values obtained with the ELISA kit were correlated highly with this bead-based assay (r = 0.91, p < 0.001).
Genotyping of − 794 CATT5–8 and − 173G>C MIF polymorphisms
Total genomic DNA (gDNA) was isolated from peripheral blood leukocytes by the salting out method [21]. The − 794 CATT5–8MIF polymorphism was analyzed by conventional polymerase chain reaction (PCR) and polyacrylamide gel electrophoresis.
The PCR was performed using the primers reported by Radstake et al. [22]. Cycling conditions were initial denaturing 95 °C for 4 min, followed by 35 cycles of 30 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C; and then a final extension of 2 min at 72 °C. Amplification products were further electrophoresed on a 19:1 (7%) polyacrylamide gel at 120 V during 3 h and stained with 0.2% AgNO3 [10, 12].
The − 173G>C MIF polymorphism was genotyped by the PCR-RFLP (restriction fragment length polymorphism) technique. Amplification of the polymorphic fragment was done using the primers reported by Makhija et al. [23]; initial denaturing 95 °C for 4 min followed by 33 cycles of 30 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C; and then a final extension of 2 min at 72 °C. The 366-bp fragment obtained was further digested with the Alu I restriction endonuclease (New England Biolabs, Ipswich, MA, USA) by overnight incubation at 37 °C. Finally, the digestion was resolved on a 29:1 (6%) polyacrylamide gel stained with 0.2% AgNO3. The − 173*G allele resulted in fragments of 268 bp and a 98 bp, while the 173*C allele was represented by 206-bp, 98-bp, and 62-bp fragments [10, 12].
To confirm the results, genotyping of both polymorphisms was done in duplicate in all cases and confirmed by automatized sequencing of a randomly selected subset of − 794 CATT5–8 and − 173G>C MIF genotypes (Applied Biosystems, USA).
MIF mRNA expression analysis
The peripheral blood sample was collected in EDTA blood collection tubes (BD Vacutainer NJ, USA). The total leukocyte was isolated using dextran reagent (5%) (Sigma-Aldrich Co.), and the total RNA was obtained using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) conforming to the Chomczynski and Sacchi method [24]. The RNA concentration and purity were measured by spectrophotometry (ratio A260/A280) (NanoDrop 2000, Thermo Scientific). After, the cDNA was synthesized from 1 μg of total RNA, and the reverse transcription was performed using primer oligodT (Promega Corporation, USA) as indicated by the manufacturer.
We conducted the quantification of MIF mRNA by real-time PCR, using UPL hydrolysis probes (Roche Applied Science, Penzberg, Germany). The primers and probes for quantification were obtained with a design program by Roche Applied Science (Universal Probe Library Assay Design Center), using the sequence of MIF mRNA with the NCBI ID number NM_002415.1 (40 number test, Cat. No. 04687990001); the following nucleotide sequences were used as primers: 5′-ACCGCTCCACAGCAAGC-3′ (forward) and 5′-CGCGTTCATGTCGTAATAGTTG-3′ (reverse). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference gene (Cat. No. 05190541001). The PCR reaction was performed on a LightCycler Nano System (Roche Applied Science, Germany). All samples were run in triplicate using the conditions indicated in the UPL Gene Expression Assay protocol in a LightCycler Nano System (Roche Applied Science).
After validation of the reaction efficiency for the target gene (MIF) and the reference gene (GAPDH), the mRNA expression analysis was performed through 2−ΔΔCq and 2−ΔCq methods. Despite that both two methods are comparable, we chose to use both methods because the 2−ΔΔCq method reports only the global (per group) relative changes in gene expression, whereas 2−ΔCq method reports individual expression values for each patient, so statistical tests can be used to determine differences between the study groups [25].
Statistical analysis
Categorical variables were expressed as percentages and absolute frequency. The distributions of all continuous variables were examined using the Shapiro–Wilk normality test. Continuous variables distributed normally were expressed as the mean ± standard deviation (S.D.), and those non-normally distributed were expressed as median and 5–95th centiles. Mann–Whitney U test was used to evaluate differences between two groups. Kruskal–Wallis test was used to analyze differences between three or more groups (for variables distributed non-normally) followed by Dunn’s adjustment for multiple comparisons. Linear correlation coefficients were examined using the Spearman’s correlation test. For the genetic analysis, the Hardy-Weinberg equilibrium and comparisons of allele and genotype distributions between groups were evaluated with the χ2 or Fisher exact test, as appropriate. Haplotypes were reconstructed using the SHEsis software [26]. The odds ratio (OR) and 95% confidence interval (95% CI) were estimated to analyze the risk of MIF genotypes and haplotypes associated with SSc. Statistical analysis was performed using STATA version 11.1 and GraphPad Prism version 6.0 Software. A probability (p) value of less than 0.05 was considered significant.
In order to assess the robustness of the results, the power of each statistical test was conducted according to the suggestion by Hong and Park [27] for genetic association studies. This analysis was performed using the G power 3.1. program [28] and the web browser program “Genetic Power Calculator” [29].
Results
Clinical and demographic characteristics
The study group consisted of 50 patients diagnosed with SSc (43 females and 7 males; aged 48 ± 15.5 years), and the control group consisted of 100 subjects matched for gender and age with the SSc group. The most common type of systemic sclerosis was lcSSc (86%), the median of the evolution of the disease was 5.5 years, and the average age of onset of this disease was 40 years. The ANAs (78%) and CENP-B (28%) were the most frequent autoantibodies in the patients. At the time of inclusion, 62% of the patients were being treated with anti-rheumatic drugs modifying the disease (DMARD), mainly methotrexate (46%), and 39% of the patients did not have treatment. The demographic and clinical characteristics of the SSc patients are shown in Table 1.
Distribution of the MIF − 794 CATT5–8 and − 173G>C polymorphisms and haplotype analysis
The distribution of the genotypic and allelic frequencies of the polymorphisms − 794 CATT5–8 and − 173G>C of the MIF gene in SSc patients and CS is summarized in Table 2. The two polymorphisms of the MIF gene were in Hardy-Weinberg equilibrium in the CS group (− 794 CATT5–8MIF, χ2 = 4.5, p = 0.55; − 173G>C MIF, χ2 = 2.4, p = 0.14). We found significant differences in the genotypic and allelic frequencies between SSc patients and CS for each of the two polymorphisms evaluated. Mainly, allele 7 of STR − 794 CATT5–8 (OR 2.09, 95% CI 1.16–3.77, p = 0.01) and allele C of − 173G>C SNP (OR 2.00, 95% CI 1.17–3.43, p = 0.01) were associated with an increased SSc risk in our study population.
In addition, a strong linkage disequilibrium was identified between both two MIF gene polymorphisms (D′ value = 0.89, r2 = 0.489, p < 0.001, Fig. 1). Based on the finding of a strong LD between the two studied polymorphisms, we performed a haplotype analysis in patients with SSc and CS (Table 2). Five different haplotypes were identified in our population, where the G6 (− 173G/− 794 CATT6) and C7 haplotypes (− 173C/− 794 CATT7) were the most frequent, representing 81% and 80% in SSc patients and CS, respectively. The C7 haplotype was found more frequently in patients with SSc compared to CS, confirming its association as a susceptible haplotype in our population (OR 2.08, 95% CI 1.17–3.7, p < 0.01) (Table 2). The demographic and clinical characteristics were not associated with the genotypes or haplotypes of MIF (− 794 CATT5–8 and − 173G>C) (data not shown).
Pairwise linkage disequilibrium relationships between the MIF variants. a) The Lewontin’s coefficient D′ and b) the correlation coefficient r2 were calculated using SHEsis software. Higher values and darker squares indicate stronger linkage disequilibrium between the polymorphisms (MIF − 794 CATT5–8 and MIF − 173G>C)
MIF mRNA expression
MIF mRNA expression was evaluated in SSc patients and CS (Fig. 2a, b). This analysis by the 2−ΔΔCq method showed that MIF mRNA expression in SSc was 2.7-fold less compared to CS; this difference was significant when assessed by the 2−ΔCq method (p < 0.01).
Relative expression of mRNA. MIF mRNA expression in CS and SSc (a, b). MIF mRNA expression in susceptibility (7C/7C) and nonsusceptibility (6G/6G) haplotype carriers. The analysis was performed in CS (c, d) and SSc (e, f). Relative expression analysis was performed using 2−ΔCq and 2−ΔΔCq methods, and GAPDH was the reference gene. Statistical comparisons between groups were made using the Mann-Whitney U test
To investigate the impact of the MIF polymorphisms on the mRNA expression, we only considered homozygosity haplotypes (HH), which are haplotypes, formed by only homozygous patients for each SNPs. The MIF mRNA expression according to the susceptibility (7C/7C) and non-susceptibility (6G/6G) haplotypes was evaluated in SSc patients and CS. In CS, MIF mRNA expression was similar in carriers of both haplotypes (p = 0.86; Fig. 2c, d). However, in SSc patients, we found that carriers of the 7C/7C haplotype had 2.3-folds more MIF expression than carriers of the 6G/6G haplotype (Fig. 2e, f, p = 0.03).
Analysis of MIF serum levels according to MIF promoter polymorphisms
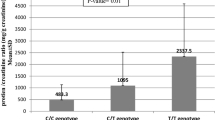
MIF serum levels were compared in SSc patients and CS, but we did not find significant differences between both groups (p = 0.51, data not shown). To determine whether the MIF promoter polymorphisms were associated with serum protein levels, the genotypes were grouped according to the dominant genetic model proposed for each polymorphism in both study groups. Nevertheless, the genotypes of two polymorphisms of the MIF gene did not show an association with MIF levels. MIF serum levels were also tested for association with MIF haplotypes, but did not find significant differences in SSc patients (p = 0.54, data not shown).
Serum levels of Th1/Th2/Th17 cytokines
The comparison between serum levels of Th1/Th2/Th17 cytokines in SSc patients and CS is shown in Table 3. In the Th1 cytokine profile (IFN-γ and TNF-α) did not observe significant differences between SSc patients and controls (p = 0.137 and p = 0.372, respectively). Concerning the Th2 cytokine profile (IL-4 and IL-10) also did not find significant differences when comparing both study groups (p = 0.212 and p = 0.733, respectively). Nevertheless, differences between both study groups were found concerning Th17 cytokines levels: The serum levels of IL-17A show a median of 5.91 pg/mL in CS vs. 3.87 pg/mL in SSc patients (p = 0.013), whereas for IL-1β, the median observed was 0.65 pg/mL in SSc patients versus 0.24 pg/mL in CS (p = 0.025); similarly, significant differences were identified for IL-6 cytokine between both study groups (SSc median 20.39 pg/mL vs. CS median 9.33 pg/mL, p < 0.01).
Additionally, the correlation of the Th1/Th2 and Th17 cytokines profiles with MIF serum levels in SSc patients and CS was analyzed (Table 4). In the SSc group, we found positive correlations of MIF with the IFN-γ (r = 0.63; p = 0.04) and TNF-α (r = 0.69; p < 0.01) cytokines of Th1 profile, but in the CS, this correlation was not observed. Regarding the Th2 profile (IL-4 and IL-10), we did not observe any correlations with MIF in both study groups. Meanwhile in the Th17 profile, a positive correlation of MIF with IL-17A (r = 0.59; p = 0.02), IL17F (r = 0.66; p < 0.01), IL-1β (r = 0.68; p < 0.01), and IL-21 (r = 0.51; p = 0.01) was found only in the patient’s group.
Discussion
SSc is an autoimmune, inflammatory, chronic, and multifactorial disease of unknown etiology [1], but it can be influenced by environmental and genetic factors [6]. Within the genetic factors, several genetic polymorphisms involved in SSc susceptibility and severity have been described in the HLA-DR locus. Other associated genes comprise IRF5 (rs607218), STAT4 (rs600558), IL12RB2 (rs601642), PXK (rs611450), and MIF (rs755622) [17, 18, 30].
Two independent studies observed that the MIF − 173G<C polymorphism was strongly associated with dcSSc [17, 18], and this polymorphism has been also associated with a high production of MIF in other autoimmune diseases such as RA [22] and SLE [31]. In our study, we identified an association between MIF genetic variants (− 794 CATT7 and − 173*C) and its haplotype C7 with SSc, so we suggest that these polymorphisms may confer susceptibility to develop of SSc. However, this result should be interpreted with caution due to the moderate (58–70%) statistical power observed [32]; this does not reduce the reliability of the association obtained, but it is known that the effect size can be overestimated when the power is moderate or low [33].
These results are according to those reported in the North American and Caucasian (eight European populations) populations, where they have confirmed not only an association between the − 173*C MIF allele with SSc risk, but they have been observed a greater frequency of C allele in the dcSSc subgroup of patients [17, 18]. One of the limitations of our study for this analysis was that we did not stratify the SSc into localized and diffuse types since the latter had a very low frequency (14%).
Regarding SSc, the MIF − 794 CATT5–8 polymorphism has been evaluated only by Wu et al., in the North American population (The United States of America and Canada), but unlike our population, they did not observe an association between the polymorphism and SSc [17]. This discrepancy could be explained by the different genetic structure that exists between both populations, as the population evaluated by Wu et al. was white population from The United States of America and Canada, which ancestry could be mainly European [34, 35], while the ancestry of our study population is predominantly Amerindian (48%), followed by Europe (38%), Asian (10%), and African (4%) [36]. Therefore, there is a need to further investigate the impact of genetic variations at the MIF gene in additional populations, with greater sample sizes, and with a better definition of clinical phenotypes.
In RA, it has been observed that carriers of the − 173*C and − 794 CATT7MIF alleles have a high activity of the disease classified by a DAS28 score in comparison with those patients carrying another allele [10, 14, 22]. Our research group have found several genetic associations of these MIF polymorphisms with susceptibility to RA [10], SLE [12], PsA [11], and MS [13] in population from western Mexico, which provides evidence that MIF gene could be a susceptibility biomarker of autoimmunity in the Mexican population. In the present study, the association of MIF polymorphisms with clinical evaluation indices, clinical manifestations, and autoantibodies in SSc patients was investigated, but we did not observe significant differences. In this regard, the previous similar studies only have investigated the association of MIF polymorphisms with anti-Scl70 and CENP-B autoantibodies without significant differences observed [17, 18]; for this reason, we cannot rule out such associations.
A striking observation in our study was a higher MIF mRNA expression in CS than in SSc patients. It is interesting to note that this is the first study reporting MIF mRNA expression in peripheral leukocytes from SSc patients. However, Corallo et al. showed that MIF mRNA expression is higher in cultures of fibroblasts from SSc patients than those from CS [37]. A possible explanation for this finding might be that in SSc patients, the production of MIF mRNA can be higher in skin cells than in leukocytes. It is possible, therefore, that MIF may have an important role in the localized inflammatory process (the skin) in SSc patients but not at the systemic level. Also, this finding could be also explained by the treatment of the SSc patients, since it has been reported that some anti-rheumatic drugs, such as chloroquine, can negatively regulate the mRNA expression of proinflammatory cytokines (TNF-α and IL-1β) that correlate positively with the expression of MIF [38]. Thus, it is possible that this kind of drugs downregulated the MIF expression; however, this hypothesis should be tested in a larger cohort.
Another important finding was that patients with the susceptibility MIF haplotype (7C) had higher expression of MIF mRNA in comparison with carriers of other haplotypes. These changes in expression could be due to differences in the transcription factor interactions with the two polymorphisms of the MIF promoter that were studied. In the case of STR − 794 CATT5–8, the transcription factor ICBP90 binds to these repetitions, regulating positively the expression of this gene [15].
We did not find significant differences between MIF serum levels in both study groups; unlike other studies, it has been observed increased MIF serum levels in patients with SSc [5, 9, 17, 19]. We suggest that this discrepancy may be due to various factors, such as the significant differences observed in BMI (body mass index) between both groups (p = 0.02, data not shown). Although our CS does not attend to obesity, the average of their BMI places them in overweight (28 kg/m2, data not shown), while the SSc patients were placed in normal weight (24 kg/m2). Excess weight and obesity are characterized by a state of chronic low-level systemic inflammation [39, 40], which is caused by the expansion of adipose tissue. It is accompanied by a progressive infiltration of leukocytes in the adipose tissue (AT), which can be attributed to hypersecretion by the AT of proinflammatory cytokines, such as MIF, IL-6, TNF-β, IL-1β, and MCP-1 [41, 42]. In addition, there are more factors that can modulate MIF such as levels of antidiabetic drugs [43], glucocorticoids [44], or even drastic weight loss [45]. On the other hand, the Toll-like receptor 4 (TLR4), induces a release of MIF through the signal transduction of the LPS receptor complex; however, a recent study has shown that the long-term use of chloroquine decreased both the expression of mRNA and TLR-4 proteins in monocytes [38, 46,47,48]. In this way, it could indirectly affect the MIF levels.
Regarding the cytokines involved in the pathogenesis of SSc, the participation of cytokines from the Th2 and Th17 profiles has been observed, among which TGFβ, IL-6, IL-1β, IL-21, IL-10, IL-13, among others are the most associated [49,50,51,52]. It has been reported higher levels of IL-10, TNFα, and Th17 cytokine profile in serum and exhaled breath condensate (EBC) from patients with dcSSc and lcSSc than in controls. Furthermore, the cytokines are higher in dcSSc than in lcSSc [53]. In accordance with those results, we observed high levels of Th17 cytokine profile members (IL-1β and IL-6) in the SSc group, but we did not find differences between serum levels of all cytokines evaluated according to the type of SSc.
The increased levels of the Th17 cytokine members may explain the relatively good correlation between Th17/Th1 cells and some adhesion molecules such as L-selectin and ICAM, which are overexpressed in SSc, and both regulate the accumulation of Th2 and Th17 cells in the skin and lung, which leads to the development of fibrosis. On the other hand, other adhesion molecules such as P-selectin, E-selectin, and PSGL-1 regulate the infiltration of Th1 cells, which results in the inhibition of fibrosis [50].
Controversially, in our study, we did not find significant differences between SSc patients and controls in the Th2 cytokine profile, where IL-10 and IL-4 were measured. However, several studies have consistently described the involvement of Th2 cells in the pathogenesis of SSc and elevated levels of their cytokines such as IL-4 and IL-13. It is known that IL-4 is critical for the polarization of Th2 cells; however, IL-13 is also necessary to mount an appropriate Th2 response in SSc [52]. Therefore, we suggest that it would be important to measure other cytokines of the Th2 profile, such as IL-13 in the future, to corroborate our results. In addition, we investigated whether there were differences between the patients with and without treatment, and curiously, we observed that the levels of IL-4 are diminished in the group of patients with treatment (p = 0.02; data not shown). Due to the above, we suggest that the treatment could be affecting the levels of IL-4, based on a recent study that states that drugs such as glucocorticoids (dexamethasone) synergy with biological drugs are directed to IL-4 (F8-IL4) in the treatment of chronic inflammatory conditions [54].
Recently, it has been suggested that MIF is a cytokine with profibrotic functions, as an inhibitor of the apoptosis of the fibroblasts and playing an important role in the stimulation of fibroblasts for the production of collagen and extracellular matrix in SSc [9, 20]. In the same way, MIF is associated with immunomodulation of Th1, Th2, and Th17 cytokine profile in inflammatory diseases. A positive correlation of MIF with cytokine TGF-β has also been observed in SSc [9]. In our study, we observed a positive correlation between the MIF serum levels and cytokines of the Th1 (IFN-γ and TNF-α) and Th17 (IL-17A, IL-17F, IL-1β, and IL-21) profile in SSc patients but not in control subjects. Although to date there is only a report of the correlation between MIF and TGF-β in SSc [9], our research group has evaluated this correlation in other autoimmune diseases, such as PsA [55] and SLE [56]. In PsA, the correlation between MIF and TNF-α expression and the Th1, Th2, and Th17 cytokine profile was evaluated; we observed that high expression of TNF-α mRNA increases the profiles of Th1 cytokines (IFN-γ and TNF-α) and Th17 (IL-17 e IL-22), but this correlation was not observed with the MIF expression [55]. In PBMCs of SLE and CS, it was observed that MIF induces the inflammatory response in both physiological (CS) and pathological (SLE) conditions with a predominance of the Th17 profile in CS and an increase in TNF-α and IL-6 in active SLE. This suggests that MIF is an immunomodulatory cytokine that might not have predominance towards a specific Th profile [56].
In conclusion, this study provides evidence that the individual polymorphisms and functional haplotype in MIF are associated with susceptibility to SSc and high MIF mRNA expression in a Mexican-Mestizo population from southern Mexico. In the same way, the results obtained suggest that MIF is associated with a proinflammatory response in SSc, as it correlates positively with the Th1 (IFN-γ and TNF-α) and Th17 (IL-17A, IL-17F, IL-1β, and IL-21) cytokine profile. Further research should be undertaken to evaluate these cytokine profiles in cultures of PBMCs or skin cells to have more forceful results.
References
Barsotti S, Stagnaro C, d'Ascanio A et al (2016) One year in review 2016: systemic sclerosis. Clin Exp Rheumatol 34 Suppl 100(5):3–13
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA Jr, Carreira PE, Riemekasten G, Clements PJ, Denton CP, Distler O, Allanore Y, Furst DE, Gabrielli A, Mayes MD, van Laar JM, Seibold JR, Czirjak L, Steen VD, Inanc M, Kowal-Bielecka O, Müller-Ladner U, Valentini G, Veale DJ, Vonk MC, Walker UA, Chung L, Collier DH, Csuka ME, Fessler BJ, Guiducci S, Herrick A, Hsu VM, Jimenez S, Kahaleh B, Merkel PA, Sierakowski S, Silver RM, Simms RW, Varga J, Pope JE (2013) 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 65(11):2737–2747
Katsumoto TR, Whitfield ML, Connolly MK (2011) The pathogenesis of systemic sclerosis. Annu Rev Pathol 6:509–537
Stern EP, Denton CP (2015) The pathogenesis of systemic sclerosis. Rheum Dis Clin N Am 41(3):367–382
Selvi E, Tripodi SA, Catenaccio M, Lorenzini S, Chindamo D, Manganelli S, Romagnoli R, Ietta F, Paulesu L, Miracco C, Cintorino M, Marcolongo R (2003) Expression of macrophage migration inhibitory factor in diffuse systemic sclerosis. Ann Rheum Dis 62(5):460–464
Ramos PS, Silver RM, Feghali-Bostwick CA (2015) Genetics of systemic sclerosis: recent advances. Curr Opin Rheumatol 27(6):521–529
Calandra T, Roger T (2003) Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 3(10):791–800
Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R (2002) Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci U S A 99(1):345–350
Kim JY, Kwok SK, Hur KH, Kim HJ, Kim NS, Yoo SA, Kim WU, Cho CS (2008) Up-regulated macrophage migration inhibitory factor protects apoptosis of dermal fibroblasts in patients with systemic sclerosis. Clin Exp Immunol 152(2):328–335
Llamas-Covarrubias MA, Valle Y, Bucala R, Navarro-Hernández RE, Palafox-Sánchez CA, Padilla-Gutiérrez JR, Parra-Rojas I, Bernard-Medina AG, Reyes-Castillo Z, Muñoz-Valle JF (2013) Macrophage migration inhibitory factor (MIF): genetic evidence for participation in early onset and early stage rheumatoid arthritis. Cytokine 61(3):759–765
Morales-Zambrano R, Bautista-Herrera LA, De la Cruz-Mosso U et al (2014) Macrophage migration inhibitory factor (MIF) promoter polymorphisms (−794 CATT5-8 and −173 G>C): association with MIF and TNFalpha in psoriatic arthritis. Int J Clin Exp Med 7(9):2605–2614
De la Cruz-Mosso U, Bucala R, Palafox-Sanchez CA et al (2014) Macrophage migration inhibitory factor: association of −794 CATT5-8 and −173 G>C polymorphisms with TNF-alpha in systemic lupus erythematosus. Hum Immunol 75(5):433–439
Castaneda-Moreno VA, De la Cruz-Mosso U, Torres-Carrillo N et al (2018) MIF functional polymorphisms (−794 CATT5-8 and −173 G>C) are associated with MIF serum levels, severity and progression in male multiple sclerosis from western Mexican population. J Neuroimmunol 320:117–124
Baugh JA, Chitnis S, Donnelly SC, Monteiro J, Lin X, Plant BJ, Wolfe F, Gregersen PK, Bucala R (2002) A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun 3(3):170–176
Yao J, Leng L, Sauler M, Fu W, Zheng J, Zhang Y, du X, Yu X, Lee P, Bucala R (2016) Transcription factor ICBP90 regulates the MIF promoter and immune susceptibility locus. J Clin Invest 126(2):732–744
Gregersen PK, Bucala R (2003) Macrophage migration inhibitory factor, MIF alleles, and the genetics of inflammatory disorders: incorporating disease outcome into the definition of phenotype. Arthritis Rheum 48(5):1171–1176
Wu SP, Leng L, Feng Z, Liu N, Zhao H, McDonald C, Lee A, Arnett FC, Gregersen PK, Mayes MD, Bucala R (2006) Macrophage migration inhibitory factor promoter polymorphisms and the clinical expression of scleroderma. Arthritis Rheum 54(11):3661–3669
Bossini-Castillo L, Simeon CP, Beretta L, Vonk MC, Callejas-Rubio JL, Espinosa G, Carreira P, Camps MT, Rodríguez-Rodríguez L, Rodríguez-Carballeira M, García-Hernández FJ, López-Longo FJ, Hernández-Hernández V, Sáez-Comet L, Egurbide MV, Hesselstrand R, Nordin A, Hoffmann-Vold AM, Vanthuyne M, Smith V, de Langhe E, Kreuter A, Riemekasten G, Witte T, Hunzelmann N, Voskuyl AE, Schuerwegh AJ, Lunardi C, Airó P, Scorza R, Shiels P, van Laar JM, Fonseca C, Denton C, Herrick A, Worthington J, Koeleman BP, Rueda B, Radstake TRDJ, Martin J (2011) Confirmation of association of the macrophage migration inhibitory factor gene with systemic sclerosis in a large European population. Rheumatology 50(11):1976–1981
Becker H, Willeke P, Schotte H, Domschke W, Gaubitz M (2008) Macrophage migration inhibitory factor may contribute to vasculopathy in systemic sclerosis. Clin Rheumatol 27(10):1307–1311
Ningyan G, Xu Y, Hongfei S, Jingjing C, Min C (2015) The role of macrophage migration inhibitory factor in mast cell-stimulated fibroblast proliferation and collagen production. PLoS One 10(3):e0122482
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16(3):1215
Radstake TR, Sweep FC, Welsing P et al (2005) Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum 52(10):3020–3029
Makhija R, Kingsnorth A, Demaine A (2007) Gene polymorphisms of the macrophage migration inhibitory factor and acute pancreatitis. JOP 8(3):289–295
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408
Shi YY, He L (2005) SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 15(2):97–98
Hong EP, Park JW (2012) Sample size and statistical power calculation in genetic association studies. Genomics Inform 10(2):117–122
Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses using G*power 3.1: tests for correlation and regression analyses. Behav Res Methods 41(4):1149–1160
Purcell S, Cherny SS, Sham PC (2003) Genetic power calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 19(1):149–150
Mayes MD, Bossini-Castillo L, Gorlova O, Martin JE, Zhou X, Chen WV, Assassi S, Ying J, Tan FK, Arnett FC, Reveille JD, Guerra S, Teruel M, Carmona FD, Gregersen PK, Lee AT, López-Isac E, Ochoa E, Carreira P, Simeón CP, Castellví I, González-Gay MÁ, Zhernakova A, Padyukov L, Alarcón-Riquelme M, Wijmenga C, Brown M, Beretta L, Riemekasten G, Witte T, Hunzelmann N, Kreuter A, Distler JHW, Voskuyl AE, Schuerwegh AJ, Hesselstrand R, Nordin A, Airó P, Lunardi C, Shiels P, van Laar JM, Herrick A, Worthington J, Denton C, Wigley FM, Hummers LK, Varga J, Hinchcliff ME, Baron M, Hudson M, Pope JE, Furst DE, Khanna D, Phillips K, Schiopu E, Segal BM, Molitor JA, Silver RM, Steen VD, Simms RW, Lafyatis RA, Fessler BJ, Frech TM, AlKassab F, Docherty P, Kaminska E, Khalidi N, Jones HN, Markland J, Robinson D, Broen J, Radstake TRDJ, Fonseca C, Koeleman BP, Martin J, Ortego-Centeno N, Ríos R, Callejas JL, Navarrete N, García Portales R, Camps MT, Fernández-Nebro A, González-Escribano MF, Sánchez-Román J, García-Hernández FJ, Castillo MJ, Aguirre MÁ, Gómez-Gracia I, Fernández-Gutiérrez B, Rodríguez-Rodríguez L, Vicente E, Andreu JL, Fernández de Castro M, García de la Peña P, López-Longo FJ, Martínez L, Fonollosa V, Espinosa G, Tolosa C, Pros A, Rodríguez Carballeira M, Narváez FJ, Rubio Rivas M, Ortiz Santamaría V, Díaz B, Trapiella L, Freire MC, Sousa A, Egurbide MV, Fanlo Mateo P, Sáez-Comet L, Díaz F, Hernández V, Beltrán E, Román-Ivorra JA, Grau E, Alegre Sancho JJ, Blanco García FJ, Oreiro N, Fernández Sueiro L (2014) Immunochip analysis identifies multiple susceptibility loci for systemic sclerosis. Am J Hum Genet 94(1):47–61
Sreih A, Ezzeddine R, Leng L, LaChance A, Yu G, Mizue Y, Subrahmanyan L, Pons-Estel BA, Abelson AK, Gunnarsson I, Svenungsson E, Cavett J, Glenn S, Zhang L, Montgomery R, Perl A, Salmon J, Alarcón-Riquelme ME, Harley JB, Bucala R (2011) Dual effect of the macrophage migration inhibitory factor gene on the development and severity of human systemic lupus erythematosus. Arthritis Rheum 63(12):3942–3951
Norton BJ, Strube MJ (2001) Understanding statistical power. J Orthop Sports Phys Ther 31(6):307–315
Button KS, Ioannidis JP, Mokrysz C et al (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14(5):365–376
Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL (2015) The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet 96(1):37–53
Roy-Gagnon MH, Moreau C, Bherer C, St-Onge P, Sinnett D, Laprise C, Vézina H, Labuda D (2011) Genomic and genealogical investigation of the French Canadian founder population structure. Hum Genet 129(5):521–531
Martinez-Cortes G, Salazar-Flores J, Fernandez-Rodriguez LG et al (2012) Admixture and population structure in Mexican-mestizos based on paternal lineages. J Hum Genet 57(9):568–574
Corallo C, Paulesu L, Cutolo M, Ietta F, Carotenuto C, Mannelli C, Romagnoli R, Nuti R, Giordano N (2015) Serum levels, tissue expression and cellular secretion of macrophage migration inhibitory factor in limited and diffuse systemic sclerosis. Clin Exp Rheumatol 33(4 Suppl 91):S98–S105
Karres I, Kremer JP, Dietl I et al (1998) Chloroquine inhibits proinflammatory cytokine release into human whole blood. Am J Phys 274(4 Pt 2):R1058–R1064
Church TS, Willis MS, Priest EL, LaMonte MJ, Earnest CP, Wilkinson WJ, Wilson DA, Giroir BP (2005) Obesity, macrophage migration inhibitory factor, and weight loss. Int J Obes 29(6):675–681
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444(7121):860–867
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112(12):1821–1830
Dandona P, Aljada A, Ghanim H, Mohanty P, Tripathy C, Hofmeyer D, Chaudhuri A (2004) Increased plasma concentration of macrophage migration inhibitory factor (MIF) and MIF mRNA in mononuclear cells in the obese and the suppressive action of metformin. J Clin Endocrinol Metab 89(10):5043–5047
Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R (1995) MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377(6544):68–71
Kamchybekov U, Figulla HR, Gerdes N, Jung C (2012) Macrophage migration inhibitory factor is elevated in obese adolescents. Arch Physiol Biochem 118(4):204–209
Llamas-Covarrubias MA, Valle Y, Navarro-Hernandez RE et al (2012) Serum levels of macrophage migration inhibitory factor are associated with rheumatoid arthritis course. Rheumatol Int 32(8):2307–2311
Roger T, David J, Glauser MP, Calandra T (2001) MIF regulates innate immune responses through modulation of toll-like receptor 4. Nature 414(6866):920–924
Hong Z, Jiang Z, Liangxi W, Guofu D, Ping L, Yongling L, Wendong P, Minghai W (2004) Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int Immunopharmacol 4(2):223–234
Meloni F, Solari N, Cavagna L et al (2009) Frequency of Th1, Th2 and Th17 producing T lymphocytes in bronchoalveolar lavage of patients with systemic sclerosis. Clin Exp Rheumatol 27(5):765–772
Yoshizaki A, Yanaba K, Iwata Y, Komura K, Ogawa A, Akiyama Y, Muroi E, Hara T, Ogawa F, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, Tedder TF, Sato S (2010) Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. J Immunol 185(4):2502–2515
Rodriguez-Reyna TS, Furuzawa-Carballeda J, Cabiedes J et al (2012) Th17 peripheral cells are increased in diffuse cutaneous systemic sclerosis compared with limited illness: a cross-sectional study. Rheumatol Int 32(9):2653–2660
O'Reilly S, Hugle T, van Laar JM (2012) T cells in systemic sclerosis: a reappraisal. Rheumatology 51(9):1540–1549
Rolla G, Fusaro E, Nicola S, Bucca C, Peroni C, Parisi S, Cassinis MC, Ferraris A, Angelino F, Heffler E, Boita M, Brussino L (2016) Th-17 cytokines and interstitial lung involvement in systemic sclerosis. J Breath Res 10(4):046013
Schmid AS, Hemmerle T, Pretto F, Kipar A, Neri D (2018) Antibody-based targeted delivery of interleukin-4 synergizes with dexamethasone for the reduction of inflammation in arthritis. Rheumatology 57(4):748–755
Bautista-Herrera LA, De la Cruz-Mosso U, Morales-Zambrano R et al (2018) Expression of MIF and TNFA in psoriatic arthritis: relationship with Th1/Th2/Th17 cytokine profiles and clinical variables. Clin Exp Med 18(2):229–235
De la Cruz-Mosso U, Garcia-Iglesias T, Bucala R et al (2018) MIF promotes a differential Th1/Th2/Th17 inflammatory response in human primary cell cultures: predominance of Th17 cytokine profile in PBMC from healthy subjects and increase of IL-6 and TNF-alpha in PBMC from active SLE patients. Cell Immunol 324:42–49
Funding
The study was supported by funding from the National Council of Science and Technology (CONACYT) Grant A1-S-8774 (CONACYT Ciencia Básica-Universidad de Guadalajara) assigned to JFMV. Also, CJH-B was a Ph.D. fellow at CONACYT-Mexico (#301502).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed written consent was obtained from all subjects before enrollment to the study. The investigation was performed according to the ethical guidelines of the 2013 Declaration of Helsinki and was approved by the ethical, investigation, and biosecurity committee of the Hospital General de Chilpancingo “Dr. Raymundo Abarca Alarcón,” Chilpancingo de los Bravo, State of Guerrero, Mexico (CI/317/2016).
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baños-Hernández, C.J., Navarro-Zarza, J.E., Bucala, R. et al. Macrophage migration inhibitory factor polymorphisms are a potential susceptibility marker in systemic sclerosis from southern Mexican population: association with MIF mRNA expression and cytokine profile. Clin Rheumatol 38, 1643–1654 (2019). https://doi.org/10.1007/s10067-019-04459-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04459-8