Abstract
The objective of this study is to investigate the current situation of hospital-acquired infection (HAI) in lupus patients from a southern Chinese population. A case-control study was performed. Data from Jan. 2007 to Jan. 2017 were collected. Each lupus patient with HAI was compared with two control individuals without infection selected from the same period of time. Three hundred and sixty episodes of HAI were analyzed. The average Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score at admission was 13.2 ± 7.2. The respiratory tract (58.8%) was most commonly involved, followed by the bloodstream (10.9%). Most episodes were bacteria-associated (50.0%), followed by viral infection (34.8%) and fungal infection (15.2%). Pathogenic bacteria were isolated in 87 episodes, among which 60 episodes were gram-negative bacteria (GNB)-related. Multidrug-resistant strains were detected in 46.4% of bacterial isolates. Fungi were isolated in 49 episodes. Candida albicans (46.9%) was the leading pathogen. Fifty-four episodes of virus infection were confirmed. In multivariate analysis, SLEDAI score (OR 1.1, 95% CI 1.1–1.2, P < 0.001), lupus nephritis (OR 3.7, 95% CI 2.7–5.1, P < 0.001), high dose of GC (OR 2.7, 95% CI 1.8–3.9, P < 0.001), and treatment with CYC (OR 2.9, 95% CI 2.1–4.0, P < 0.001) were risk factors for HAI. HAI in Chinese lupus patients had a unique epidemiology feature, which was characterized by common respiratory tract and bloodstream involvement and predominance of GNB with a high drug resistance rate. A variety of new pathogens including fungi and viruses emerged in the HAI patients. A history of nephritis or a higher SLEDAI score in SLE patients predicted HAI. Moreover, treatment with high dose of GC and CYC was also the main risk factor for HAI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease caused by immune system disorders. Infection is the most important cause of mortality in patients with SLE [1, 2]. A large retrospective study revealed that approximately 50% of lupus patients had experienced one serious infection, and 20% had two or more [3]. Our latest research also demonstrated that infection occurred in 34.6% of the lupus patients and about a quarter had at least two episodes of infection [4]. Study on lupus patients from the “Euro-Lupus Project” revealed that active SLE and infection (28.9%, each) appeared to be the first two causes of death within the initial 5 years of disease onset [1]. A study done recently in China, analyzing the cause of death in 3831 hospitalized patients with SLE from 1986 to 2012, demonstrated that infection (37.3%) was the biggest threat during the past 26 years [5].
According to the origin of pathogenic organisms, infection can be divided into two categories, namely community-acquired infection and hospital-acquired infection (HAI). Infection occurring in a hospital is usually more difficult to treat due to the complexity of environmental settings and pathogens. SLE per se entails several immunologic disorders, such as hyposplenism, hypocomplementemia, and altered innate and adaptive immune system, predisposing individuals to infection [6, 7]. Besides, medications used to treat active SLE, such as high dose of glucocorticoids (GC) and immunosuppressive drugs, have been shown to be independent risk factors for infection in hospitalized patients with SLE [3]. Therefore, HAI is a major complication in SLE. However, data about HAI in Chinese lupus patients were lacking. The objectives of this study were to determine the incidence, type, and outcomes of HAI in lupus patients; to discover the infective organisms and their antibiotic resistance pattern; and to identify risk factors for HAI.
Materials and methods
Patients and data collection
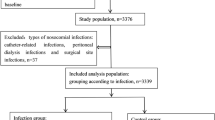
We performed a case-control study using medical records from the First Affiliated Hospital of Sun Yat-Sen University from Jan. 2007 to Jan. 2017. Each lupus patient with HAI was compared with two control individuals without infection selected from the same period of time. Diagnosis of SLE was made according to the revised American College of Rheumatology (ACR) criteria [8]. Disease activity at admission was retrospectively measured using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score according to the medical records [9]. The infective condition was systematically checked. Patients who were lost to follow-up or without complete records were excluded. Hypoalbuminemia was defined as level of serum albumin lower than 35 g/L. Leukopenia was defined as total white blood cell count less than 4000/mm3 on two or more occasions. In a retrospective chart review, we recorded leukopenia only if it was clinically attributed to active SLE but not to severe infection or adverse effect of medications. Anemia was defined as hemoglobin level lower than 110 g/L in female and 120 g/L in male. Thrombocytopenia was defined as platelet count less than 100,000/mm3, precluding other identifiable causes. Patients with hematological impairment due to disease activity or hypoalbuminemia due to immunosuppressant treatment were excluded. Usage of GC and immunosuppressants within 1 month prior to the occurrence of HAI was recorded. The dose of GC was converted using the following equation: 1 mg of prednisolone = 0.8 mg of methylprednisolone = 0.15 mg of dexamethasone.
Definition of infection
HAI was defined as infection acquired in a hospital or infection which originates in a hospital but displays symptoms after discharge. Accordingly, infection happening 48 h after admission was considered HAI in our study. Bacterial infection was diagnosed on the basis of clinical manifestations, radiographic imaging, laboratory test results such as white blood cell count, and treatment response to antibiotic therapy. Body fluid samples from suspected sites of infection such as sputum, blood, or urine were collected for pathogen detection. Bacterial infection was confirmed if a pathogen was identified by microscopy or culture; otherwise, it was diagnosed according to clinical findings. Drug sensitivity analysis was performed using the BD Phoenix Automated Microbiology System (BD Diagnostic Systems).
Invasive fungal disease (IFD) was diagnosed according to the 2008 European Organisation for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group Consensus Group criteria [10]. Diagnosis of oral candidiasis was made according to the presence of classic pseudomembranous lesions characterized by creamy white, curd-like patches on the tongue or oral mucosa [11].
Acute viral infection was diagnosed on the basis of (i) clinical features (e.g., prolonged fever, pharyngitis, arthralgia, cutaneous rash, acute hepatitis, or gastroenteritis); (ii) specific radiographic findings (e.g., interstitial pneumonia) highly suggesting acute viral infection; (iii) elevated titers of virus-specific immunoglobulin (Ig) M and subsequent IgG, or enhancing replication of virus nucleic acid confirmed by polymerase chain reaction (PCR) assay; and (iv) improvement after anti-virus therapy. In particular, since symptoms of Herpes zoster infection are relatively specific, diagnosis of Herpes zoster infection was clinically established if the patient presents painful skin rashes with blisters in a localized area. Virology test for Herpes zoster infection is not necessarily required. Mumps is diagnosed once a patient displays a swelling parotid in the absence of other viral infections, suppurative infection, tumors, salivary stones, Mikulicz’s disease, or secondary Sjögren’s syndrome, with serum test for mumps IgM positive within 5 days after disease onset.
Statistical analysis
Statistical analyses were performed using the SPSS 16.0 package. Quantitative variables were described as mean ± standard deviation and analyzed by t test. Categorical variables were described as frequency and percentage. Two-by-two tables were analyzed by the chi-square or Fisher exact test, as appropriate. Odds ratios (OR) and corresponding 95% confidence intervals (CI) of the variables with a P < 0.1 in univariate analysis were adjusted by multivariate logistic regression analysis to identify the risk factors associated with HAI. A P < 0.05 was considered to be statistically significant.
Results
Demographic data
Records for 3956 lupus patients were reviewed. Three hundred and thirty-four patients with HAI were identified, 54 male and 280 female included. The mean age was 34.6 ± 15.3 years. The incidence rate of HAI was 9.1%. Patient characteristics are shown in Table 1. Considering some patients were admitted to the hospital more than once, a total of 360 episodes of infection were analyzed. The average duration of SLE was 36.4 ± 56.8 months. The average SLEDAI score at admission was 13.2 ± 7.2, indicating high disease activity. Leukopenia was present in 17.5% of the cases. GC and cyclophosphamide (CYC) were the main medications, which were prescribed to 99.2 and 41.7% of the patients, respectively. The average dose of prednisone within 1 month prior to HAI was 0.8 mg/kg/day. Fifteen out of 360 patients (4.2%) received more than one immunosuppressive drugs. Twenty patients (5.6%) were sent to the intensive care unit (ICU) because of severe infection.
Sites of infection
The respiratory tract was the most affected site. Upper respiratory tract infection (147 episodes) and pneumonia (120 episodes) accounted for 58.8% of the infection episodes. Bloodstream infection (51 episodes) was in the third place, accounting for 11.2%. Other affected sites included the skin/soft tissue (47 episodes), genitourinary tract (46 episodes), oral cavity (21 episodes), and gastrointestinal tract (17 episodes). Infection involving the central nervous system (3 episodes) was relatively rare. In particular, 76 episodes had concurrent infection in two sites, and 9 episodes in three sites.
Pathogen distributions (bacterial infections and fungal infections)
Four hundred and twenty samples, including throat swab, sputum, blood, urine, stool, secretion, cerebrospinal fluid, and soft tissue, were sent for pathogenic organism culture. Pathogen distributions are shown in Table 2. Bacterial infection was confirmed in 87 episodes based on positive culture and classified as probable in 140 episodes based on clinical judgment. Among the isolated bacteria, 27 episodes were gram-positive and 60 episodes were gram-negative. Escherichia coli were the most common gram-negative bacteria (GNB) (33.3%), followed by Klebsiella pneumoniae (18.3%), and Acinetobacter baumannii (15%). As for gram-positive bacteria (GPB), Staphylococcus aureus (33.3%) and coagulase-negative staphylococci (CNS) (25.9%) were the main pathogens. Besides, 49 episodes were fungus-related. About 89.8% of fungal infections was caused by Candida spp. (44 episodes), followed by Aspergillus (3 episodes) and Penicillium marneffei (2 episodes). Sites of Candida spp. infection included the oral cavity (21 episodes), esophagus (15 episodes), and urinary tract (8 episodes). Compared with bacterial infection, fungal infection tended to develop at the early stage of SLE. The SLEDAI score and incidence rate of neuropsychiatric lupus were higher in lupus patients with fungal infection. Moreover, intravenous (IV) pulse methylprednisolone was more frequently used in lupus patients who developed fungal infection. No difference was found in age, other organ involvement, or other medications (Table 3).
Viral infection and associated factors
Nonspecific viral infection was diagnosed in 104 episodes. Fifty-four episodes of virus infection were confirmed by either elevated titers of virus-specific IgM and subsequent IgG, or high levels of virus nucleic acid replication accompanied with clinical findings. Herpes zoster infection was diagnosed in 24 episodes, cytomegalovirus (CMV) infection in 12 episodes, influenza infection in 10 episodes, and herpes simplex infection in six episodes. Two patients had Mumps virus infection. Within 1 month prior to viral infection, 23 patients (95.8%) were on GC treatment with an average dose of 0.8 mg/kg/day. IV pulse CYC, mycophenolate mofetil, methotrexate, and azathioprine were administered to ten (41.7%), six (25.0%), two (8.3%), and one (4.2%) patients, respectively. IV CYC was more frequently used (41.7 vs 20.1%, P < 0.001), and the average dose of GC was also higher (0.8 vs 0.3 mg/kg/day, P < 0.001) in lupus patients with viral infection compared with the control group. No significant difference was found in other treatment, including mycophenolate mofetil, methotrexate, and azathioprine.
Factors associated with HAI in patients with SLE
A comparison of characteristics between patients with and without HAI is shown in Table 1. The SLEDAI score at admission in patients with HAI was higher than that in patients without infection. With regard to clinical features, the incidence rate of thrombocytopenia, hypoproteinemia, and renal involvement was higher in patients with HAI. Moreover, patients with HAI received higher dose of CYC and GC. In multivariate analysis, the SLEDAI score (OR 1.1, 95% CI 1.1–1.2, P < 0.001), lupus nephritis (OR 3.7, 95% CI 2.7–5.1, P < 0.001), high dose of GC (OR 2.7, 95% CI 1.8–3.9, P < 0.001), and treatment with CYC (OR 2.9, 95% CI 2.1–4.0, P < 0.001) were risk factors for HAI (Table 4).
Drug resistance patterns of the main isolated bacteria
All the pathogenic bacteria isolated were analyzed for drug sensitivity in vitro. Results of the first three types of the gram-negative bacteria (GNB) and first two types of GPB are shown in Table 5. Multidrug-resistant (MDR) strains were detected in 46.4% (26/56) of bacterial isolates. Extended-spectrum β-lactamase (ESBL) was produced mainly in E. coli (9/20, 45.0%) and K. pneumoniae (3/11, 27.3%). What was so astounding was that Enterobacter cloacae, which was resistant to carbapenem, accounted for 5.0% (1/20) of the cases. The proportion of extensively drug-resistant Acinetobacter baumannii (XDRAB) was 77.8% (7/9). Two strains of methicillin-resistant S. aureus (MRSA) (2/9, 22.2%) and five strains of methicillin-resistant CNS (MRCNS) (5/7, 71.4%) were found.
Disease mortality related to HAI
Patients who died of severe infection were analyzed. Infection sites and pathogens are shown in Table 6. Twelve deaths were estimated, among which seven patients had infection caused by mixed pathogens, and five patients had disseminated infection involving more than one organ. Severe pneumonia and sepsis were two main causes of mortality. In the control group, eight patients died during the study period, among which three died of renal involvement, two of interstitial lung disease, one of lupus encephalopathy, one of cardiovascular disease, and one of multiple-organ failure. Mortality was higher in HAI patients than those without any infection (3.3 vs 1.1%, P = 0.004).
Discussion
Infection has emerged as the leading cause of mortality in SLE. Lupus patients, who are hospitalized because of high disease activity and need intensive immunosuppressive therapy, are predisposed to HAI. However, there is limited knowledge on the HAI profile in such population. Our study showed that the incidence rate of HAI was as high as 9.1%, consistent with another report (11.7%) [12]. We further analyzed the infection patterns. The respiratory tract was frequently involved, followed by the circulatory system. Bacteria, GNB in particular, were predominant. MDR strains, XDR strains, and even CRE strains were detected. Severe infection caused by mixed pathogens or involving multiple sites was usually fatal. It seems clear that HAI increased the mortality of hospitalized patients with SLE. The analysis of HAI in lupus patients is of great significance and may help to guide clinical treatment in order to prolong long-term survival and improve life quality.
In our research, the respiratory tract, including the upper respiratory tract and lungs, was the most commonly affected site. The incidence rate of respiratory infection (58.8%) was higher than that reported in another study (27%) [13]. Difference in disease activity and treatment regimen could explain this discrepancy. The incidence rate of bloodstream infections ranked third on the list, which is much higher than that of community-acquired infection [4, 13]. Immunity dysregulation, greater vascular permeability, and frequent invasive intervention (e.g., intravenous injection) may all predispose patients to bloodstream infection. Therefore, it is not surprising that pneumonia and sepsis are the leading causes for which lupus patients are admitted to the ICU [14]. Some are even fatal. In our research, 9/12 deaths were attributed to severe pneumonia, sepsis, or co-infection. About 23.6% of the episodes had concurrent infection involving two organs or more, and 4/12 deaths were attributed to multiple-site infection. The incidence rate of HAI with multiple-organ involvement is even higher in another study [13], suggesting that HAI in lupus patients is usually more severe and difficult to treat.
Most HAI were caused by GNB, which are also the predominant pathogens that cause infection in lupus patients in other Asian countries [15, 16]. In our research, the common pathogens such as E. coli, K. pneumoniae, and Acinetobacter baumannii were resistant to ceftriaxone, levofloxacin, ciprofloxacin, and ampicillin. According to antibiotic resistance reports, these antibiotics may not be the first choice to treat HAI in lupus patients in the southern region. Antimicrobial regimen should be reassessed on the basis of bacterial culture results. Due to widespread use, or abuse in some circumstances, of antibiotics, the carbapenem resistance rate in GNB seems to keep increasing [17, 18]. Acinetobacter baumannii was the main strain which developed carbapenem resistance. Besides, Acinetobacter baumannii isolated in two cases were resistant to tigecycline. Considering the drug resistance rate of GNB is still increasing, the analysis of infection patterns is necessary to guide antibiotic preference. Different from another report [12], Salmonella spp. were seldom found. It could be due to the difference in sanitary conditions and dietary habits. As for GPB, S. aureus and CNS, as two leading pathogens, were all sensitive to vancomycin, linezolid, and tigecycline. Therefore, vancomycin or linezolid is still the first choice to treat GPB-associated HAI in lupus patients. In order to reduce the drug resistance rate, tigecycline, which is also toxic to GNB, is not suggested as the first-line treatment.
In our research, 25.8% of the HAI were fungus-related. Consistent with another report, the majority were caused by either Candida spp. or Aspergillus [19]. As a source of opportunistic infections, Candida albicans is most commonly seen in our research. Infection with Candida albicans may occur in the oral cavity, gastrointestinal tract, or genitourinary tract. Fungal pneumonia is usually caused by invasive fungi including Aspergillus (three episodes). In spite of the relatively low incidence rate, fungal infection could be life-threatening. It was responsible for 62.5% (10/16) of deaths according to previous reports [5]. Mortality of IFD is also high (53%) [20]. Therefore, fungal infection should be suspected when lupus patients with pulmonary infiltrates show no improvement after antibiotic therapy.
Virus infection is not rare in lupus patients with HAI. Herpes zoster is the most common, followed by Cytomegalovirus (CMV). Compared with other autoimmune diseases, the incidence rates of both Herpes zoster infection and CMV infection are highest in lupus patients [21, 22]. In particular, rapidly progressing CMV infection can be fatal. In our previous research, 5/12 (41.7%) patients died of CMV-associated interstitial pneumonia [22]. Patients with CMV infection usually display typical symptoms, including persistent fever, dyspnea, or diarrhea. However, it could be asymptomatic in some immunocompromised hosts. Since viruses such as Herpes zoster or CMV could be in their latency and are reactivated when a patient is in an immunosuppressive state, vaccines to protect immunocompromised individuals from Herpes zoster infection can be considered. Unfortunately, no conclusions have been made on whether preemptive anti-CMV therapy should be prescribed in patients who are serologically positive but asymptomatic. In our research, patients were treated with anti-virus medications once CMV infection was highly suspected (i.e., symptoms + typical imaging + no improvement to antibiotics). Further research is needed to evaluate the efficacy of prophylactic anti-CMV therapy in hospitalized lupus patients.
Of note, fungal infection occurs at the early stage of SLE. Patients with fungal infection commonly have high disease activity and nervous system involvement and receive IV pulse methylprednisolone therapy. Similar results have been shown in other studies [20, 23]. Moreover, we found that patients with viral infection received higher doses of CYC and GC. In the present research, consistent with previous studies, major organ involvement, such as renal involvement, and high disease activity defined by the SLEDAI score are predisposing factors for HAI. We also found a significant association between the onset of HAI and treatment strategy. This finding was comparable with other reports [24]. Our study highlights the importance of disease control to prevent HAI. Intensive therapy, including IV CYC and a high dose of GC, could increase the risk of HAI. Our study suggests that alternative therapy should be considered when disease achieves remission. Treatment strategy to control disease activity and reduce the risk of HAI at the same time remains a challenge.
HAI is common in lupus patients and remains one of the primary causes of mortality. There are some notable features of HAI in Chinese lupus patients, including a high incidence rate of respiratory tract and bloodstream involvement and predominance of GNB with a high drug resistance rate. A variety of new pathogens including fungi and viruses emerged in the HAI patients. Hospitalized lupus patients, who have major organ involvement or high disease activity, or receive high doses of GC and IV CYC, are prone to the development of HAI. To our knowledge, this could be the first report to investigate the current situation of HAI in lupus patients from a southern Chinese population. Empirical antibiotic therapy should be guided by local bacteriological surveillance reports and drug sensitivity analysis.
References
Cervera R, Abarca-Costalago M, Abramovicz D, Allegri F, Annunziata P, Aydintug AO, Bacarelli MR, Bellisai F, Bernardino I, Biernat-Kaluza E, Blockmans D, Boki K, Bracci L, Campanella V, Camps MT, Carcassi C, Cattaneo R, Cauli A, Cervera R, Chwalinska-Sadowska H, Contu L, Cosyns JP, Danieli MG, DCruz D, Depresseux G, Direskeneli H, Domènech I, Espinosa G, Fernández-Nebro A, Ferrara GB, Font J, Frutos MA, Galeazzi M, Garcìa-Carrasco M, García Iglesias MF, García-Tobaruela A, George J, Gil A, González-Santos P, Grana M, Gül A, Haga HJ, de Haro-Liger M, Houssiau F, Hughes GR, Ingelmo M, Jedryka-Góral A, Khamashta MA, Lavilla P, Levi Y, López-Dulpa M, López-Soto A, Maldykowa H, Marcolongo R, Mathieu A, Morozzi G, Nicolopoulou N, Papasteriades C, Passiu G, Perelló I, Petera P, Petrovic R, Piette JC, Pintado V, de Pita O, Popovic R, Pucci G, Puddu P, de Ramón E, Ramos-Casals M, Rodríguez-Andreu J, Ruiz-Irastorza G, Sanchez-Lora J, Sanna G, Scorza R, Sebastiani GD, Sherer Y, Shoenfeld Y, Simpatico A, Sinico RA, Smolen J, Tincani A, Tokgöz G, Urbano-Márquez A, Vasconcelos C, Vázquez JJ, Veronesi J, Vianna J, Vivancos J, European Working Party on Systemic Lupus Erythematosus (2006) Systemic lupus erythematosus in Europe at the change of the millennium: lessons from the “Euro-Lupus Project”. Autoimmun Rev 5(3):180–186. https://doi.org/10.1016/j.autrev.2005.06.004
Jakes RW, Bae SC, Louthrenoo W, Mok CC, Navarra SV, Kwon N (2012) Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res (Hoboken) 64(2):159–168. https://doi.org/10.1002/acr.20683
Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM, Kim SC, Costenbader KH (2015) Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol 67(6):1577–1585. https://doi.org/10.1002/art.39070
Chen D, Xie J, Chen H, Yang Y, Zhan Z, Liang L, Yang X (2016) Infection in southern Chinese patients with systemic lupus erythematosus: spectrum, drug resistance, outcomes, and risk factors. J Rheumatol 43(9):1650–1656. https://doi.org/10.3899/jrheum.151523
Fei Y, Shi X, Gan F, Li X, Zhang W, Li M, Hou Y, Zhang X, Zhao Y, Zeng X, Zhang F (2014) Death causes and pathogens analysis of systemic lupus erythematosus during the past 26 years. Clin Rheumatol 33(1):57–63. https://doi.org/10.1007/s10067-013-2383-3
Doaty S, Agrawal H, Bauer E, Furst DE (2016) Infection and lupus: which causes which? Curr Rheumatol Rep 18(3):13. https://doi.org/10.1007/s11926-016-0561-4
Danza A, Ruiz-Irastorza G (2013) Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus 22(12):1286–1294. https://doi.org/10.1177/0961203313493032
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40(9):1725. https://doi.org/10.1002/art.1780400928
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35(6):630–640. https://doi.org/10.1002/art.1780350606
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46(12):1813–1821. https://doi.org/10.1086/588660
Fangtham M, Magder LS, Petri MA (2014) Oral candidiasis in systemic lupus erythematosus. Lupus 23(7):684–690. https://doi.org/10.1177/0961203314525247
Chen MJ, Tseng HM, Huang YL, Hsu WN, Yeh KW, Wu TL, See LC, Huang JL (2008) Long-term outcome and short-term survival of patients with systemic lupus erythematosus after bacteraemia episodes: 6-yr follow-up. Rheumatology (Oxford) 47(9):1352–1357. https://doi.org/10.1093/rheumatology/ken196
Navarro-Zarza JE, Alvarez-Hernández E, Casasola-Vargas JC, Estrada-Castro E, Burgos-Vargas R (2010) Prevalence of community-acquired and nosocomial infections in hospitalized patients with systemic lupus erythematosus. Lupus 19(1):43–48. https://doi.org/10.1177/0961203309345776
Lee J, Dhillon N, Pope J (2013) All-cause hospitalizations in systemic lupus erythematosus from a large Canadian referral centre. Rheumatology (Oxford) 52(5):905–909. https://doi.org/10.1093/rheumatology/kes391
Teh CL, Ling GR (2013) Causes and predictors of mortality in hospitalized lupus patient in Sarawak General Hospital, Malaysia. Lupus 22(1):106–111. https://doi.org/10.1177/0961203312465780
Al-Rayes H, Al-Swailem R, Arfin M, Sobki S, Rizvi S, Tariq M (2007) Systemic lupus erythematosus and infections: a retrospective study in Saudis. Lupus 16(9):755–763. https://doi.org/10.1177/0961203307079943
Wang L, Wang Y, Fan X, Tang W, Hu J (2015) Prevalence of resistant gram-negative bacilli in bloodstream infection in febrile neutropenia patients undergoing hematopoietic stem cell transplantation: a single center retrospective cohort study. Medicine (Baltimore) 94(45):e1931. https://doi.org/10.1097/MD.0000000000001931
Chen CY, Tsay W, Tang JL, Tien HF, Chen YC, Chang SC, Hsueh PR (2010) Epidemiology of bloodstream infections in patients with haematological malignancies with and without neutropenia. Epidemiol Infect 138(7):1044–1051. https://doi.org/10.1017/S0950268809991208
Silva MF, Ferriani MP, Terreri MT, Pereira RM, Magalhaes CS, Bonfa E, Campos LM, Okuda EM, Appenzeller S, Ferriani VP, Barbosa CM, Ramos VC, Lotufo S, Silva CA (2015) A multicenter study of invasive fungal infections in patients with childhood-onset systemic lupus erythematosus. J Rheumatol 42(12):2296–2303. https://doi.org/10.3899/jrheum.150142
Wang LR, Barber CE, Johnson AS, Barnabe C (2014) Invasive fungal disease in systemic lupus erythematosus: a systematic review of disease characteristics, risk factors, and prognosis. Semin Arthritis Rheum 44(3):325–330. https://doi.org/10.1016/j.semarthrit.2014.06.001
Yun H, Yang S, Chen L, Xie F, Winthrop K, Baddley JW, Saag KG, Singh J, Curtis JR (2016) Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol 68(9):2328–2337. https://doi.org/10.1002/art.39670
Takizawa Y, Inokuma S, Tanaka Y, Saito K, Atsumi T, Hirakata M, Kameda H, Hirohata S, Kondo H, Kumagai S, Tanaka Y (2008) Clinical characteristics of cytomegalovirus infection in rheumatic diseases: multicentre survey in a large patient population. Rheumatology (Oxford) 47(9):1373–1378. https://doi.org/10.1093/rheumatology/ken231
Chen GL, Chen Y, Zhu CQ, Yang CD, Ye S (2012) Invasive fungal infection in Chinese patients with systemic lupus erythematosus. Clin Rheumatol 31(7):1087–1091. https://doi.org/10.1007/s10067-012-1980-x
Dubula T, Mody GM (2015) Spectrum of infections and outcome among hospitalized South Africans with systemic lupus erythematosus. Clin Rheumatol 34(3):479–488. https://doi.org/10.1007/s10067-014-2847-0
Funding
This project was supported by grants from the Guangdong Technology Project (No. 2014A020221009, No. 2016A020215043) and grants from the National Natural Science Foundation of China (81603435, 81601403).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Zhan, Z., Lao, M., Su, F. et al. Hospital-acquired infection in patients with systemic lupus erythematosus: a case-control study in a southern Chinese population. Clin Rheumatol 37, 709–717 (2018). https://doi.org/10.1007/s10067-017-3919-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3919-8