Abstract
In recent years, there has been an increased recognition that vitamin D is not only a risk factor for poor musculoskeletal health but also a possible important contributor in the development of different autoimmune diseases. This has been linked to multiple immunosuppressive properties of vitamin D. Vitamin D has got pleiotropic effects and this reflects the wide spread presence of vitamin D receptors (VDRs) throughout the body. Currently, there is much ongoing research with regard to its emerging role in immunopathology. VDR has not only been found in tissues involved in calcium homeostasis but also in a variety of cell lines involved primarily in immune regulation, e.g., mononuclear cells, dendritic cells, antigen-presenting cells, and activated B lymphocytes and CD4+ T cells. There have been several reports linking the presence of low vitamin D to various autoimmune diseases. However, for autoimmune/inflammatory disease outcomes, firm conclusions regarding cause and effect cannot be based on epidemiological association studies, and prospective, long-term, well-designed studies, including large intervention trials, are needed across all life stages. In this paper, we will describe the evidence base for this potential role of vitamin D in different aspects of autoimmune diseases. Because of the enormous breadth of this emerging field, our aim in this review is not to provide an exhaustive list of all literature pertinent to vitamin D and immunopathology. Instead, we will frame the reasons and rationale behind the development of several immunoregulatory activities for 1,25(OH)2D3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Most of rheumatic diseases are multifactorial, and Vitamin D is now known to have a multitude of effects on immune systems. The role of vitamin D deficiency as a potential environmental agent in the development of different autoimmune diseases is exciting and is gaining more and more recognition. This could be linked to multiple immunosuppressive properties of vitamin D; as in animal models, vitamin D supplementation has been found to be of therapeutic benefit in autoimmune encephalomyelitis [1], collagen-induced arthritis [2], type 1 diabetes mellitus [3], inflammatory bowel disease [4], autoimmune thyroiditis [5], and systemic lupus erythematosus (SLE) [6]. 1,25(OH)2D3 is the biologically active metabolite, which requires the hydroxylation of 25(OH)D3 by 25(OH)D3-1-α-hydroxylase occuring mainly in the proximal convoluted tubule cells of the kidney. Interestingly, there is widespread presence of extra-renal source of this enzyme (1α-hydroxylase), which is mainly regulated by immune signals rather than bone factors [7], and 1,25(OH)2D then acts in a paracrine manner on immune cells. This supports the recognition of 1,25 (OH)D as an important modulator of immune function. Vitamin D mediates its effect through binding to vitamin D receptor (VDR) and activation of VDR-responsive genes. There is widespread presence of VDR in the body, and the VDR gene polymorphism was found to associate with autoimmune diseases [8].
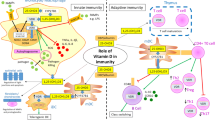
The immunomodulatory effects of 1,25(OH)2D3 are mediated mainly through its action on antigen presenting cells, especially on dendritic cells, where these effects are most potent and well described [9, 10]. This subsequently leads to its inhibited differentiation from monocytes or murine bone marrow-derived precursors and maturation and increased apoptosis with resultant inhibition of dendritic cell-dependent T-cell activation [11, 12]. Moreover, 1,25(OH)2D3 suppresses autoimmunity by inhibiting pro-inflammatory Th17 cells, and inducing the T regulatory cells (Tregs) and natural killer T cells [13]. With sufficient vitamin D stores, the active form of vitamin D hormone (1,25-dihydroxy vitamin D3) regulates T helper cell (Th1) and dendritic cell function. The net result is a decrease in the Th1-driven autoimmune response through reduced production of IL-2 and interferon-γ. However, the production of the Th2-associated cytokine IL4 has been shown to be upregulated by 1,25(OH)2D3 treatment in vivo. Conversely, with the inadequate vitamin D levels, the immune system favors the development of self-reactive T cells and autoimmunity [7]. Moreover, 1,25(OH)D induces the apoptosis and suppresses the proliferation and differentiation of activated B-cells [14]. Vitamin D has also been shown to have potent anti-inflammatory properties through inhibition of various inflammatory cytokines such as TNF alpha, interleukins, and the activity of macrophages [15, 16]. In experimental models, vitamin D hormone has been shown to suppress the release of pro-inflammatory cytokine TNF-alpha, and upregulate the production of anti-inflammatory cytokine, interleukin 10 [17, 18]. Interestingly, a double-blind, randomized, placebo-controlled trial has confirmed these findings by using 2,000 IU of vitamin D supplementation for 9 months [19]. Moreover, two studies have shown a significant inverse acute phase response and vitamin D status among patients undergoing uncomplicated orthopedic surgeries [20, 21].
Latitude-related prevalence of autoimmune diseases is one of the strong arguments in the favor of pathogenic role of vitamin D in different autoimmune conditions. Epidemiological studies widely support this hypothesis; however, interventional studies have provided mixed results, largely not that convincing. We, the authors, put forward a plausible explanation that vitamin D has a role in setting off the inflammatory and/or autoimmune cascade of events, followed by many autocrine and paracrine factors leading to a vicious circle of autoimmunity. At the stage when autoimmune disease is established, vitamin D replacement will not have much therapeutic benefit. Our hypothesis has been shared by a very recent study, which has attempted to examine the role of low vitamin D levels on autoantibody production. This study has revealed that ANA-positive healthy controls and patients with SLE are significantly more likely to be deficient in vitamin D than ANA-negative healthy controls; moreover, it was noted that SLE patients with vitamin D deficiency has high B-cell activity and serum IFNα activity [22].
A 2004 prospective cohort study, with an 11 years follow-up, has shown an association between greater intake of vitamin D and the lower risk of development of rheumatoid arthritis [23]. Another study has revealed a significant negative correlation of 25(OH)D values with RA clinical status (DAS28) in both North and South European RA patients, suggesting possible effects of vitamin D among other factors on disease activity [24]. A more recent study has shown an inverse relationship between 25(OH)D levels and the tender joint count, DAS28 score, and HAQ scores [25]. However, two previous studies have shown no association between 25(OH)D and the CRP level or the ESR [26, 27], but no measurement was made of the joint counts or HAQ scores. Among African Americans with a recent onset of RA, no association was observed between vitamin D levels and the pain, swollen joints or the disease activity scores — DAS28 [28]. A more recent study has observed a surprisingly high incidence of vitamin D deficiency in inflammatory joint disease patients in a sunny Mediterranean country; however, no correlation was found between vitamin D status and the disease activity indices [29].
Multiple studies have confirmed that significant majority of patients with lupus suffer from vitamin D insufficiency or deficiency, even after taking vitamin D supplementation [30, 31]. The data from a single centre registry of lupus patients in Hungary has revealed a significant association of reduced vitamin D levels with pericarditis, deep venous thrombosis, neuropsychiatric symptoms, severity of disease measured by SLEDAI, disease-specific antibodies (anti-double-stranded DNA antibodies and anti-smith antigen), complement levels, and serum immunoglobulin levels [32]. Two previous largest studies to date have also showed a significant correlation between higher disease activity and lower 25(OH)D [33, 34]. Apart from the known risk factors playing a key role in SLE (e.g., ethnicity and avoidance of sun), there are some other emerging risk factors, such as, medications. For example, corticosteroids accelerate the catabolism of 25(OH)D and 1,25(OH)2D [35]. Very low levels of vitamin D have also been reported in patients with systemic sclerosis, along with its significant association with inflammatory markers, disease duration, disease severity, and end organ involvement [36]. Similarly, low levels of vitamin D have been reported in patients with mixed connective tissue disease, undifferentiated connective tissue disease, Bechet’s disease and anti-phospholipid antibody syndrome [37–40]. Given a large variation on the methodological quality of studies and relatively small number of patients examined, let us look at the one recent meta-analysis and one systematic review to help us find patterns across those studies which have examined vitamin D in rheumatic diseases. VDR polymorphism in a meta-analysis has been linked with the susceptibility to RA in Europeans population, and SLE along with lupus nephritis in Asians [41]. A systematic review has examined the epidemiological evidence linking vitamin D to human autoimmune disease risk [42]. This paper acknowledges the potential role of vitamin D in autoimmune disease prevention; however, the paucity of prospective interventional evidence in humans was noted.
There is a clear theoretical basis for an immunomodulatory role of vitamin D, and there are breaths of epidemiological studies to support this. However, several confounding factors should be bore in mind; for example, obesity, smoking, alcohol consumption, low socioeconomic class, which are known independent risk factors for both rheumatoid arthritis disease activity and low vitamin D levels [43, 44]. Similarly, as low vitamin D levels have been shown to inversely associate with disease activity, the potential of reverse causality is another plausible explanation given the patients with active disease spend more time indoors and have low activity levels [45].
Conclusion
Immunoregulatory effects of vitamin D are well-described on cellular and cytokine levels, and there is mounting evidence that low vitamin D levels contribute to mortality and morbidity in different autoimmune diseases. Exactly, how low vitamin D levels exerts its immunomodulatory effects is not clear. For autoimmune/inflammatory disease outcomes, firm conclusions regarding cause and effect cannot be based on epidemiological association studies, and prospective, long-term, well-designed studies, including large intervention trials, are needed across all life stages.
References
Van Etten E, Branisteanu DD, Overbergh L, Bouillon R, Verstuyf A, Mathieu C (2003) Combination of a 1,25-dihydroxyvitamin D3 analog and a bisphosphonates prevents experimental autoimmune encephalomyelitis and preserves bone. Bone 32:397–404
Cantorna MT, Hayes CE, DeLuca HF (1998) 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr 128:68–72
Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R (1994) Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia 37:552–558
Cantorna MT, Munsick C, Bemiss C, Mahon BD (2000) 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 130:2648–2652
Fournier C, Gepner P, Sadouk M, Charreire J (1990) In vivo beneficial effects of cyclosporin A and 1,25-dihydroxyvitamin D3 on the induction of experimental autoimmune thyroiditis. Clin Immunol Immunopathol 54:53–63
Lemire JM, Ince A, Takashima M (1992) 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity 12:143–148
Arnson Y, Amital H, Shoenfeld Y (2007) Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis 66(9):1137–1142
Garcia-Lozano JR, Gonzalez-Escribano MF, Valenzuela A, Garcia A, Nunez-Roldan A (2001) Association of vitamin D receptor genotypes with early onset rheumatoid arthritis. Eur J Immunogenet 28:89–93
Penna G, Adorini L (2000) 1_,25-Dihydroxyvitamin D-3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164:2405–2411
van Halteren AGS, van Etten E, de Jong EC, Bouillon R, Roep BO, Mathieu C (2002) Redirection of human autoreactive T-cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D-3. Diabetes 51:2119–2125
Van Etten E, Mathieu C (2005) Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol 97:93–101
Cutolo M (2009) Vitamin D and autoimmune rheumatic diseases. Rheumatology (Oxford) 48(3):210–212
Yu S, Cantorna MT (2008) The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci USA 105:5207–5212
Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE (2007) Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179:1634–1647
Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S (2007) The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J Steroid Biochem Mol Biol 103(3–5):558–562
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281
Zhu Y, Mahon BD, Froicu M, Cantorna MT (2005) Calcium and 1alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol 35:217–224
Canning MO, Grotenhuis K, de Wit H, Ruwhof C, Drexhage HA (2001) 1-alpha,25-Dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. Eur J Endocrinol 145:351–357
Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R (2006) Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 83(4):754–759
Reid D, Knox S, Talwar D, O’Reilly DJ, Blackwell S, Kinsella J et al (2010) Acute changes in the systemic inflammatory response is associated with transient decreases in circulating 25-hydroxyvitamin D concentrations following elective knee arthoplasty. Ann Clin Biochem 47(Suppl 1):95–96
Louw JA, Werbeck A, Louw ME, Kotze TJ, Cooper R, Labadarios D (1992) Blood vitamin concentrations during the acute-phase response. Crit Care Med 20:934–941
Ritterhouse LL, Crowe SR, Niewold TB, Kamen DL, Macwana SR, Roberts VC, Dedeke AB, Harley JB, Scofield RH, Guthridge JM, James JA (2011) Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann Rheum Dis 70(9):1569–1574, Epub 2011 May 17
Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG (2004) Iowa women’s health study. Arthritis Rheum 50:72–77
Cutolo M, Otsa K, Laas K et al (2006) Circannual vitamin D serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin Exp Rheumatol 24:702–704
Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D (2007) Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum 56:2143–2149
Kroger H, Penttila IM, Alhava EM (1993) Low serum vitamin D metabolites in women with rheumatoid arthritis. Scand J Rheumatol 22:172–177
Oelzner P, Miller A, Deschner F, Huller M, Abendroth K, Hein G et al (1998) Relationship between disease activity and serum levels of vitamin D metabolites and PTH in rheumatoid arthritis. Calcif Tissue Int 62:193–198
Craig SM, Yu F, Curtis JR et al (2010) Vitamin D status and its associations with disease activity and severity in African Americans with recent-onset rheumatoid arthritis. J Rheumatol 37:275–281, Epub 2009 Dec 23
Braun-Moscovici Y, Toledano K, Markovits D, Rozin A, Nahir AM, Balbir-Gurman A (2009) Vitamin D level: is it related to disease activity in inflammatory joint disease? Rheumatol Int 31:493–499, Epub 2009 Dec 23
Kamen DL, Cooper GS, Bouali H et al (2006) Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev 5(2):114–117
Toloza SM, Cole DE, Gladman DD et al (2010) Vitamin D insufficiency in a large female SLE cohort. Lupus 19(1):13–19
Szodoray P, Tarr T, Bazso A, Poor G, Szegedi G, Kiss E (2010) The immunopathological role of vitamin D in patients with SLE: data from a single centre registry in Hungary. Scand J Rheumatol 40(2):122–126, Epub 2010 Oct 26
Ben-Zvi I, Aranow C, Mackay M et al (2010) The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS One 5(2):e9193
Amital H, Szekanecz Z, Szucs G et al (2010) Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis 69(6):1155–1157
Kamen DL (2010) Vitamin D in lupus — new kid on the block? Bull NYU Hosp Jt Dis 68(3):218–222
Caramaschi P, Dalla Gassa A, Ruzzenente O, Volpe A, Ravagnani V, Tinazzi I, Barausse G, Bambara LM, Biasi D (2010) Very low levels of vitamin D in systemic sclerosis patients. Clin Rheumatol 29(12):1419–1425
Hajas A, Sandor J, Csathy L, Csipo I, Barath S, Paragh G, Seres I, Szegedi G, Shoenfeld Y, Bodolay E (2010) Vitamin D insufficiency in a large MCTD population. Autoimmun Rev 10(6):317–324, Epub 2010 Dec 13
Zold E, Szodoray P, Gaal J, Kappelmayer J, Csathy L, Gyimesi E, Zeher M, Szegedi G, Bodolay E (2008) Vitamin D deficiency in undifferentiated connective tissue disease. Arthritis Res Ther 10(5):R123
Hamzaoui K, Ben Dhifallah I, Karray E, Sassi FH, Hamzaoui A (2010) Vitamin D modulates peripheral immunity in patients with Behçet’s disease. Clin Exp Rheumatol 28(4 Suppl 60):S50–S57
Agmon-Levin N, Blank M, Zandman-Goddard G, Orbach H, Meroni PL, Tincani A, Doria A, Cervera R, Miesbach W, Stojanovich L, Barak V, Porat-Katz BS, Amital H, Shoenfeld Y (2011) Vitamin D: an instrumental factor in the anti-phospholipid syndrome by inhibition of tissue factor expression. Ann Rheum Dis 70(1):145–150
Lee YH, Bae SC, Choi SJ, Ji JD, Song GG (2010) Associations between vitamin D receptor polymorphisms and susceptibility to rheumatoid arthritis and systemic lupus erythematosus: a meta-analysis. Mol Biol Rep 38(6):3643–3651, Epub 2010 Nov 26
Kriegel MA, Manson JE, Costenbader KH (2010) Does vitamin D affect risk of developing autoimmune disease? A systematic review. Semin Arthritis Rheum 40(6):512–531, Epub 2010 Nov 2
Hypponen E, Berry D, Cortina-Borja M, Power C (2010) 25-Hydroxyvitamin D and pre-clinical alterations in inflammatory and hemostatic markers: a cross sectional analysis in the 1958 British Birth Cohort. PLoS One 5:e10801
McEntegart A, Morrison E, Capell HA, Duncan MR, Porter D, Madhok R et al (1997) Effect of social deprivation on disease severity and outcome in patients with rheumatoid arthritis. Ann Rheum Dis 56:410–413
Welsh P, Peters MJ, Sattar N (2011) Is vitamin D in rheumatoid arthritis a magic bullet or a mirage? The need to improve the evidence base prior to calls for supplementation. Arthritis Rheum 63(7):1763–1769
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haroon, M., FitzGerald, O. Vitamin D and its emerging role in immunopathology. Clin Rheumatol 31, 199–202 (2012). https://doi.org/10.1007/s10067-011-1880-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-011-1880-5