Abstract
Rheumatoid arthritis (RA) is the most common form of inflammatory arthritis, a systemic autoimmune disease characterized by chronic inflammation of the synovial joints, ultimately leading to joint destruction and permanent disability, affecting 1% of the world population. Oxidative stress in rheumatoid inflammation, due to the fact that antioxidant systems are impaired in RA and caused by fee radicals, might have an essential role in etiology of RA. This review includes the interrelation of antioxidants against free radicals in RA patients. There is much evidence that antioxidant team that covers glutathione reductase, catalase, glutathione peroxidase, superoxide dismutase, and glucose-6-phopshate destroy reactive oxygen species and other free radicals through enzymatic as well as nonenzymatic means. The change in relative levels of antioxidants vis-à-vis free radical formation and level could be used as indicators for effective and earlier diagnosis of RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic inflammation of the synovial joints, ultimately leading to joint destruction and permanent disability [1]. It is the most common form of inflammatory arthritis and has a substantial social effect in terms of cost, disability, and lost productivity. The course of RA varies ranging from a mild to an aggressive form. The long-term outcome of this disease is characterized by significant morbidity, loss of functional capacity, and increased mortality. It is one of the most prevalent autoimmune diseases, affecting about 1% of the adult population in Western countries [2]. Early diagnosis and treatment reduce joint destruction, preserve function, and improve survival [3].
RA is a debilitating autoimmune disease where diagnosis is based on clinical and radiological features and the presence of rheumatoid factor in the serum. Although the pathogenesis of RA remains incompletely understood, much insight into the cellular and molecular mechanisms involved has been gained in the past decade. Its precise etiology remains to be elucidated, although both environmental and genetic components are believed to influence the development of disease. Of the former, cigarette smoking appears to be of importance in both disease susceptibility and severity [1].
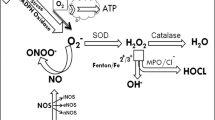
Some investigators have focused on oxidative stress in rheumatoid inflammation. Free radicals might have an essential role when possible alterations in the matrix, and the enzymes that degrade oxygen consumption are taken into consideration [4]. A free radical is any atom, molecule, or ion that contains one or more unpaired electrons that might be formed during a number of physiologic or pathologic reactions. Since pairing of electrons in orbitals depict a lower energy state than the same electrons unpaired, free radicals are usually more reactive than their parent species [4]. These unmapped electrons can initiate destructive reactions against proteins or lipid and nucleic acids in the body, reactivating the free radical molecules [5, 6], which increases the essence of these radicals in physiopathology of many diseases including RA [7, 8]. In healthy cells and tissues, any free radicals generated are likely to encounter and react with nonradicals since most cellular constituents are nonradicals, and the chances of most radicals meeting are low. Nevertheless, chemical reactivity of radicals varies enormously. Free radicals and byproducts of free radicals are among essential mediators of inflammation-related diseases [9]. The effects of free radicals on RA have been evaluated since 1970s [8, 10, 11].
Free radicals from oxygen metabolism destroy antioxidant systems [12]. Researchers such as Heliövaara et al. [13] have suggested that enzymatic and/or nonenzymatic antioxidant systems are impaired in RA. RA patients are therefore exposed to oxidant stress [12]. Consequently, a number of different activities of antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase, and glutathione reductase (GR) have been reported to be effective in treating RA [14–16]. Some other researchers found that RA patients were more inclined to lipid peroxidation because of the reduced antioxidant defense system [17, 18].
The synovial fluids taken from patients with RA were found to be exposed to high levels of antioxidant damages [19, 20]. According to Babior [21], reactive oxidants are essential tools for the pathogenesis of RA. Through evolution, mammalian cells have developed antioxidant defense systems for survival in an aerobic environment [22]. Cells carry varying antioxidant systems, including low-molecular-weight antioxidant molecules such as glutathione [23] and other antioxidant enzymes to protect themselves against free radicals, most frequently observed of which are defined in Table 1. SOD, which is an essential zinc and copper-containing metalloenzyme, is a primary defense element against oxygen-derived free radicals, which catalyzes the dismutation of the superoxide anion (\({\text{O}}\prime ^{{\text{ - }}}_{{\text{2}}} \)) into hydrogen peroxide (H2O2). H2O2 can be transformed to H2O and O2 through catalase [24]. In many studies, the level of SOD has shown varying concentration levels. While researchers such as Kalavacherla et al. [25] and Bae et al. [26] found lower levels of SOD, Olivieri et al. [27] found unchanged SOD and thiobarbituric acid levels.
According to Jira et al. [28], there is a possibility that the lowered levels of SOD activity may be due to the inhibition of the enzyme by hydrogen peroxide, which might be an indicator of high degree of superoxide anion production resulting in increased hydrogen peroxide liberation through dismutation reaction. Rheumatoid patients undergoing therapy with nonsteroidal anti-inflammatory drug (NSAID) are usually good examples of significant correlation between SOD activity and intracellular lysate thiol (LSH) concentrations [29, 30]. However, the correlation vanishes when sulphasalazine is added, which is an indicator of interference with free radical defense mechanisms, similar to the actions of sodium aurothiomalate and auranofin [30]. Similarly, Nivsarkar [31] found that in RA patients, SOD activity was lower than controls, but levels of circulating SOD were up after NSAID therapy. Rister and Bauermeister [32] found that SOD content in polymorphonuclear leukocytes of children with RA was diminished compared to healthy controls. On the other hand, Ozkan et al. [33], Gambhir et al. [18], or Scudder et al. [34] were not able to observe a significant change in SOD levels.
Along with the SOD activity, Rasool and Varalakshmi [35] found that the activity of GSH-Px is decreased in arthritic animals that could be a consequence of enormous production of free radicals. Glutathione is essential in the stability of lysosomal and other cell membranes. Moreover, tripeptide is thought to protect cell membranes against damage from superoxides and free radicals [36]. The tissue damage resulting from the inflammatory effects of RA is partly due to lipid peroxidation and prostaglandin synthesis. Thus the protective effect of glutathione may be due to the inhibition of these reactions as suggested by Munthe et al. [37]. According to this study, the depressed level of erythrocyte glutathione observed in patients with active RA goes up in good responders to d-penicillamine, while no similar increases are observed in nonresponders to the drug unless supplemented by l-cysteine. The concentration of glutathione is regulated through glutathione synthetase, GSH-Px, and GR [36].
GSH-Px is localized in the cytoplasm and mitochondria of cells. It catalyzes the degradation of various peroxides by oxidizing glutathione with the formation of its conjugates [38]. Munthe et al. [37] found depressed values of erythrocyte GSH-Px activity during active phases of RA, similar to the findings by Tarp [39]. Mèzes et al. [40] observed an upwards movement in GSH-Px activity in (female) rheumatoid patients, while male patients were neutral. Abella et al. [41] did not observe any differences in GSH-Px activity in patients with RA, in contradiction to Vanella et al. [42] who found that both erythrocyte GSH-Px and GR levels were reduced in the observed patients.
Vijayalakshmi et al. [43] found supporting evidences of low GSH-Px activity in liver, kidney, and heart in arthritis, the findings of which were supported by Shah and Vohora [16]. Braven et al. [36] found that the level of erythrocyte GSH-Px activity in patients with RA was high comparatively. This view was opposed to by Tarp [44] for Braven et al. [36] might not have taken into account the potential differences in selenium status between RA patients and controls as also noted by Vijayalakshmi [43]. On the other hand, Cimen et al. [45] found that GSH-Px might not be playing an essential role in rheumatic events. The affinity of GSH-Px for H2O2 is stronger than the affinity of catalase, which makes GSH-Px more efficient at low levels of H2O2 concentrations.
Catalase is another effective enzyme that functions together with SOD and GSH-Px through enzymic antioxidant defense system and catalyses the decomposition of hydroperoxide to water and oxygen to protect cells against O2 toxicity and lipid peroxidation [46].
Reactive oxygen species (ROS) play a role in the pathogenesis of a number of inflammatory diseases such as RA. Endogenous antioxidant enzymes such as catalase protect cells against damage caused by ROS. Catalase plays an essential role in the reduction of lipid peroxides and hydrogen peroxides in a reaction involving H2O2 as an absolute substrate. SOD, catalase, and GSH-Px are thought to be the fundamental antioxidant enzymes, for they are closely related to the direct elimination of ROS [16]. Despite many reports that do not find catalase activity in serum of patients with RA, Kerimova et al. [47] reported decreased erythrocyte catalase activity in RA patients. These findings are also in line with Taysi et al. [24]. Nevertheless, Gambhir et al. [18] and Cimen et al. [45] found unchanged catalase activity in erythrocytes of RA patients.
Catalase is expressed predominantly within peroxisomes, where it dismutates the ROS and H2O2 formed as a result of oxidation of long-chain fatty acids [48]. Expression of catalase in vitro and in vivo affects the expression of genes influencing inflammation [49]. Therefore, increased catalase activity can be protective against RA through limiting the production of ROS or through regulation of the expression of genes involved in inflammation [50]. El Sohemy et al. [50] also found that differences in catalase activity did not play a significant role in the onset of RA.
Similar to the enzymes such as GSH-Px and SOD, RA is associated also with low antioxidant status related to low activities of GR [51] and linked also to decreased levels of reduced glutathione that is an intracellular antioxidant in the synovial fluid T cells of patients with RA [52].
Mulherin et al. [51] reported that higher basal and stimulated GR might be expected in patients with RA in response to chronic oxidative stress due to synovial inflammation. The association of riboflavin deficiency with increased disease activity suggests that impaired GR activity could facilitate continuing inflammation in these patients. Another study reported impaired GR activity in synovial fluid in RA [53].
The glucose-6-phosphate (G6P) provides the reducing equivalents required for GR conversion of glutathione sulfide back to serum reduced glutathione levels [54]. Hassan et al. [54] also suggest that a state of oxidative stress exists in RA as well as the depletion of serum reduced glutathione levels and that the decrease of detoxifying enzymes such as GR and GSH-Px are thought to be contributing factors. Hassan et al. [54] found similar results in which the RA patients they observed had significantly lower levels of GR, similar to the propositions by Chow and Tappel [55], who thought that G6P dehydrogenase, GR, and GSH-Px work in harmony against oxidative cellular damage. Herve et al. [56] found in a specific study on G6P isomerase (GPI) that GPI was not a specific auto-antigen in RA.
Conclusions
Although there remains much to be gone through regarding the etiology of RA, there is much evidence that antioxidant team that covers GR, CAT, GSH-Px, SOD, and GPI destroy ROS and other free radicals through enzymatic as well as nonenzymatic means. The change in relative levels of antioxidants vis-à-vis free radical formation and level could be used as indicators for effective and earlier diagnosis of RA.
References
Glossop JR, Dawes PT, Mattey DL (2006) Association between cigarette smoking and release of tumour necrosis factor and its soluble receptors by peripheral blood mononuclear cells in patients with rheumatoid arthritis. Rheumatology 45:1223–1229
Martinez A, Valdivia A, Pascual-Salcedo D et al (2005) PADI4 polymorphisms are not associated with rheumatoid arthritis in the Spanish population. Rheumatology 44:1263–1266
American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines (2002) Guidelines for the management of rheumatoid arthritis. Arthritis Rheuma 46:328–346
Evans P, Halliwell B (1999) Free radicals and hearing: cause, consequence, and criterie. Ann NY Acad Sci 884:19–40
Freeman BA, Crapo JD (1982) Free radicals and tissue injury. Lab Invest 47:412–426
Lunec J, Blake D (1990) Oxygen free radicals: their relevance to disease processes. In: Cohen D, Lewis B, Albert KGMM (eds) The metabolic and moleculer basis of acquired disease. Balliere Tindall, London, pp 189–212
Barbor DA, Harris SR (1994) Oxygen free radicals and antioxidants: a review. Am Pharm 34:26–35
Greenwald RA (1991) Oxygen radicals, inflammation, and arthritis: pathophysiological considerations and implications for treatment. Semin Arthritis Rheum 20:219–240
Jasin HE (1997) Mechanism of tissue damage in rheumatoid arthritis. In: Koopman WJ (ed) Arthritis and allied conditions: textbook of rheumatology. Williams Wilkies, Baltimore, pp 1017–1036
Imadaya A, Terasawa K, Tosa H (1988) Erythrocyte antioxydant enzymes are reduced in patients with rheumatoid arthritis. J Rheumatol 15:1628–1631
Semble LE (1995) Rheumatoid arthritis. New approaches for its evaluation and management. Arch Phys Med Rehabil 76:190–200
Harris ED (1993) Etiology and pathogenesis of rheumatoid arthritis. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds) Textbook of rheumatology. WB Saunders, Philadelphia, pp 833–873
Heliövaara M, Knekt P, Aho K et al (1994) Serum antioxidant and risk of rheumatoid arthritis. Ann Rheum Dis 53:51–53
Blake DR, Hall ND, Treby DA et al (1981) Protection against superoxide and hydrogen peroxide in synovial fluid from rheumatoid patients. Clin Sci 61:483–486
Mazetti I, Grigolo B, Borzi RM et al (1996) Serum copper/zinc superoxide dismutase levels in patients with rheumatoid arthritis. Int J Clin Lab Res 26:245–249
Shah ZA, Vohora SB (2002) Antioxidant/restorative effects of calcined gold preparations used in indian systems of medicine against global and focal models of ischaemia. Pharm & Toxicol 90:254–259
Rowley D, Gutteridge MC, Blake D et al (1984) Lipid peroxidation in rheumatoid arthritis: thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci 66:691–695
Gambhir JK, Lali P, Jain AK (1997) Correlation between blood antioxidant levels and lipid peroxidation in rheumatoid arthritis. Clin Biochem 30:351–355
Miyata T, Ishiguro N, Yasuda Y et al (1998) Increased pentosidine, an advanced glycation end product, in plasma and synovial fluid from patients with rheumatoid arthritis and its relation with inflammatory markers. Biochem Biophys Res Commun 244:45–49
Chapman AP, Antoniw P, Spitali M et al (1999) Therapeutic antibody fragments with prolonged in vivo half-lives. Nat Biotechnol 17:780–783
Babior BM (2000) Phagocytes and oxidative stres. Am J Med 109:33–44
Karincaoglu Y, Batcioglu K, Erdem T et al (2005) The levels of plasma and salivary antioxidants in the patient with recurrent aphthous stomatitis. J Oral Pathol & Med 34:7–12
Gul M, Kutay FZ, Temocin S et al (2000) Cellular and clinical implications of glutathione. Indian J Exp Biol 38:625–634
Taysi S, Polat F, Gul M et al (2002) Lipid peroxidation, some extracellular antioxidants, and antioxidant enzymes in serum of patients with rheumatoid arthritis. Rheumatol Int 21:200–204
Kalavacherla US, Ishaq M, Rao URK et al (1994) Malondialdehyde as a sensitive marker of inflammation in patients with rheumatoid arthritis. J Assoc Phys India 42:775–776
Bae SC, Kim SJ, Sung MK (2003) Inadequate antioxidant nutrient intake and altered plasma antioxidant status of rheumatoid arthritis patients. J Am Coll Nutr 22:311–315
Olivieri O, Girelli D, Trevisan MT et al (1991) Red blood cell susceptibility to lipid peroxidation, membrane lipid composition and antioxidant enzymes in patient with rheumatoid arthritis. J Rheumatol 18:1263–1264
Jira W, Spiteller G, Richter A (1997) Increased levels of lipid oxidation products in low density lipoproteins of patients suffering from rheumatoid arthritis. Chem Phys Lipids 87:81–89
Pullar T, Zoma A, Capell HA et al (1987) Alteration of thiol and superoxide dismutase status in rheumatoid arthritis treated with sulphasalazine. Br J Rheumatol 26:202–206
Rae KJ, Mackay CN, McNeil CJ et al (1986) Early and late changes in sulphydryl group and copper protein concentrations and activities during drug treatment with aurothiomalate and auranofin. Ann Rheum Dis 45:839–846
Nivsarkar M (2000) Improvement in circulating superoxide dismutase levels: role of nonsteroidal anti-inflammatory drugs in rheumatoid arthritis. Biochem Biophys Res Commun 270:714–716
Rister M, Bauermeister K (1982) Superoxid-Dismutase und Superoxid-Radikal-Freisetzung bei juveniler rheumatoider Arthritis. Klin Wochenschr 60:561–565
Ozkan Y, Yardım-Akaydın S, Sepici A et al (2007) Oxidative status in rheumatoid arthritis. Clin Rheumatol 26:64–68
Scudder PR, Al-Timimi D, McMurray W et al (1978) Serum copper and related variables in rheumatoid arthritis. Ann Rheum Dis 37:67–70
Rasool M, Varalakshmi P (2007) Protective effect of Withania somnifera root powder in relation to lipid peroxidation, antioxidant status, glycoproteins and bone collagen on adjuvant-induced arthritis in rats. Fundam Clin Pharmacol 21:157–164
Braven J, Ansari N, Figgitt DP et al (1989) A comparison of glutathione reductase and glutathione peroxidase activities in patients with rheumatoid arthritis and healthy adults. Br J Rheumatol 8:212–215
Munthe E, Jellum E, Aaseth J et al (1986) Gold salts and selenium in the treatment of rheumatoid arthritis. In: Swaak AJG, Koster JF (eds) Free radicals in arthritis diseases. EURAGE, Rijswijk, The Netherlands, pp 121–128
Islamov BI, Balabanova RM, Funtikov VA (2002) Effect of bioresonance therapy on antioxidant system in lymphocytes in patients with rheumatoid arthritis. Bull Exp Biol Med 134:248–250
Tarp U (1986) Selenium and glutathione peroxidase in rheumatoid arthritis. In: Swaak AJG, Koster JF (eds) Free radicals in arthritis diseases. EURAGE, Rijswijk, The Netherlands, pp 69–73
Mèzes M, Par A, Bartosceiwiecz G et al (1987) Vitamin E content and lipid peroxidation of blood in some chronic inflammatory diseases. Acta Physiol Hung 69:133–138
Abella A, Clerc D, Chalas J et al (1987) Contration en superoxyde dismutase (cuivre et manganese) catalase et glutathion peroxydase dans les hematies, les plaquettes, et le plasma des sujets atteints de polyarthrite rhumatoide. Ann Biol Clin 45:152–155
Vanella A, Scalia R, Terminella C et al (1987) Antioxidant enzymatic systems in erythrocytes from patients with rheumatoid arthritis. Pharmacol Res 15:1181–1188
Vijayalakshmi T, Muthulakshmi V, Sachdanandam P (1997) Salubrious effect of Semecarpus anacardium against lipid peroxidative changes in adjuvant arthritis studied in rats. Mol Cell Biochem 175:65–69
Tarp U (1989) Selenium glutathione peroxidase in rheumatoid arthritis. Br J Rheumatol 29:158
Cimen MYB, Cimen OB, Kacmaz M et al (2000) Oxidant/antioxidant status of the erythrocytes from patients with rheumatoid arthritis. Clin Rheumatol 19:275–277
Ramprasad VR, Shanthi P, Sachdanandam P (2005) Evaluation of antioxidant effect of Semecarpus anacardium Linn.nut extract on the components of immune system in adjuvants arthritis. Vasc Pharmacol 42:179–186
Kerimova AA, Atalay M, Yusifov EY et al (2000) Antioxidant enzymes; possible mechanism of gold compound treatment in rheumatoid arthritis. Pathophysiology 7:209–213
Kunau WH, Dommes V, Schulz H (1995) Beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog Lipid Res 34:267–342
Benhamou PY, Moriscot C, Richard MJ et al (1998) Adenovirus-mediated catalase gene transcription reduces oxidant stress in human, porcine and rat pancreatic islets. Diabetologia 41:1093–1100
El-Sohemy A, Cornelis MC, Park YW et al (2006) Catalase and PPARc2 genotype and risk of rheumatoid arthritis in Koreans. Rheumatol Int 26:388–392
Mulherin D, Fitzgerald O, Bresnihan B (1996) Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum 39:115–124
Maurice M, Van der Voort E, Vilet A et al (1997) Chronic oxidative stress in rheumatoid arthritis: implication for T cell function. In: Montaguier L, Oliuier R, Pasquier C (eds) Oxidative stress in cancer, AIDS and neurodegenerative diseases. Marcel Dekker, New York, pp 537–546
Bazzichi L, Ciompi ML, Betti L et al (2002) Impaired glutathione reductase activity and level of collogenase and elastase in synovial fluid in rheumatoid arthritis. Clin Exp Rheumatol 20:761–766
Hassan MQ, Hadi RA, Al-Rawi ZS et al (2001) The glutathione defense system in the pathogenesis of rheumatoid arthritis. J Appl Toxicol 21:69–73
Chow CK, Tappel AL (1972) An enzymatic protective mechanism against lipid peroxidation damage to lung of ozone exposed rats. Lipids 7:518–524
Herve CA, Wait R, Venables PJ (2003) Glucose-6-phosphate isomerase is not a specific autoantigen in rheumatoid arthritis. Rheumatology 42:986–988
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalpakcioglu, B., Şenel, K. The interrelation of glutathione reductase, catalase, glutathione peroxidase, superoxide dismutase, and glucose-6-phosphate in the pathogenesis of rheumatoid arthritis. Clin Rheumatol 27, 141–145 (2008). https://doi.org/10.1007/s10067-007-0746-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-007-0746-3