Abstract
Rheumatoid arthritis (RA) is an autoimmune disease responsible for significant human morbidity in modern life. However, oxidative stress is one of the key markers for determining pathophysiology of patients with RA. The interaction between cellular immune system and body’s endogenous and/or exogenous antigens produce reactive oxygen species (ROS) and reactive nitrogen species (RNS) in autoimmune disease like RA. ROS and RNS include highly toxic superoxide (O2 −) and peroxynitrite (ONOO−) radicals, which activate the signaling cascades of inflammatory cells to synthesize pro-inflammatory cytokines and chemokines. Previous studies reported that Th1 cytokines could promote the development of autoimmune disorders like RA, whereas the Th2 cytokines may attenuate the same diseases. An increased awareness of the relationship between food and health led to a tremendous increase of antioxidant research in the last decade. Evaluation of the efficacy of dietary antioxidants is also becoming highly acceptable in RA research. A number of dietary phytomolecules are already established as having antioxidant activity in isolated synovial cellular infiltrate or peripheral blood neutrophils and lymphocytes. This review aims to highlight the oxidative stress in inflammatory cells of patients with RA and to summarize the clinical relevance of dietary antioxidants as a first step in assessing beneficial effect, safety and dose safety ratio in patients with RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease (CAID) which shows inflammatory responses through the involvement of inflammatory cells like monocytes, lymphocytes, neutrophils and macrophages (Kundu et al. 2011). Modern studies have enumerated a positive involvement of inflammatory mechanism in the body through synovial cellular infiltrate as well as peripheral blood inflammatory cells (Bala and Haldar 2013). Polymorphonuclear neutrophils and lymphocytes play a pivotal role in synovial inflammation and joint damage (Datta et al. 2014). Reactive oxygen species (ROS) and reactive nitrogen species (RNS) contribute directly to the destructive and proliferative synovitis in patients with RA (Kundu et al. 2011). As a result, the treatment of RA is becoming more advanced over the past decade with the development of new target on the inflammatory markers (Yamaoka 2016).
T- and B-lymphocyte cells are responsible for the body’s active soldiers in clinical RA, whereas most of the damages in this disease are due to the effector cells and their products including auto-antibodies, cytokines and other inflammatory mediators (Tineke et al. 2008, Mohammad and Abbas 2012). Th1 cytokines can promote the development of autoimmune disorders like RA, whereas the Th2 cytokines may attenuate the diseases by modifying the pathological targets (Giacomelli et al. 2016; Pandey et al. 2010). Oxidative stress refers to a situation where the production of oxidant exceeds the capacity to neutralize them and lead damage to cells membrane, lipids, nucleic acids and proteins (Bala et al. 2012b). The cytokines produced by Th1 cells directly or indirectly induce the oxidative stress in the cells of patient with RA; therefore; antioxidants play a vital role in reducing the oxidative stress. Thus, the antioxidant drug evaluation is becoming more acceptable in RA research now a day (Kundu et al. 2011; Datta et al. 2014; Bala et al. 2014).
Natural phytomolecules play a significant role in curing different diseases since ancient times (Abdullah et al. 2014). The World Health Organization (WHO) study reported that about 80% of world’s population relies on traditional medicine (WHO 2002). A number of phytomolecules found beneficial showing antioxidant activity in inflammatory cells of patients with RA (Kundu et al. 2011; Bala et al. 2014). The present review aims to assess the clinical etiology of RA and to highlight the oxidative stress in inflammatory cells of patients with RA. The review also summarizes the clinical relevance of dietary antioxidants as a first step in assessing beneficial effect, safety and dose safety ratio in patients with RA.
Etiology of RA
RA is one type of CAID that shows chronic responses, whereas inflammation is the body’s immediate immune response to eliminate toxic pathogens or other stimuli. Inflammation also restores the cells towards normal state or replace the damaged tissue (Kundu et al. 2011; Emmendoerffer et al. 2000). The cellular interaction with body’s endogenous and/or exogenous antigens generates reactive oxygen species (ROS) and reactive nitrogen species (RNS). Activation of cellular signaling cascade occurs in cellular inflammation via activation of NFκB for synthesizing proinflammatory cytokines and chemokines (Emmendoerffer et al. 2000; Ryan et al. 2004). In the innate immune system, mononuclear monocytes or macrophages play a significant role in eliminating the pathogen/antigen or in destroying cellular components through generation of ROS/RNS includes superoxide, nitric oxide, hydrogen peroxide, hydroxyl radical, peroxy nitrite and hydrochlorous acid (HOCl) (Ryan et al. 2004; Bala et al. 2014).
COX enzymes exist in two isoforms, one is COX-1 and another one is COX-2. COX-1 is constitutive enzyme while COX-2 is inducible enzyme and produced in high amount in activated inflammatory cells at the site of inflammation. Cyclooxygenase isoforms (Both COX-1 and COX-2) activated in all type of cellular stress and consider as the vital enzymes in inflammation. It is unregulated in the in inflammatory cells mainly macrophages, neutrophils and lymphocytes during inflammation (Bala et al. 2013; Sarkar et al. 2008). These enzymes are responsible for the formation of important inflammatory mediators called prostanoids (including prostaglandins, prostacyclin and thromboxane). COX enzymes convert arachidonic acid into prostaglandins, which generally shows vasodilatation in inflammation (Sarkar et al. 2008). The bioactive lipids, derived from arachidonic acid by COX-2, are potent inflammatory mediator for vasodilatation. PGE2, an oxygenated product of arachidonic acid, is dependent on ROS- and RNS-mediated activation of COX enzymes. PGE2 increases vascular permeability along with other vasoactive components such as histamine, bradykinin or nitric oxide thereby causing edema, pain and hyperalgesia at the local inflammatory sites during inflammation (Pandey et al. 2010). Therefore, a number of anti-inflammatory compounds show antioxidant property (Bala et al. 2013; Bala et al. 2014) and are consider as clinically effective in inflammation.
The role of inflammatory cytokines, such as tumor necrosis factor alpha (TNF- α), interleukin (IL)-1β and IL-6, in pathogenesis of arthritis is well documented. Increased production of TNF- α alters the disease etiology in RA. Levels of TNF-α, IL-1β and IL-6 are elevated in the synovium of patients with RA (Bala et al. 2013). The inflammatory cytokines produced through the activation of Nuclear Factor Kappa light chain enhancer of Activated B cells (NFκB). Interaction of activated T cells with synovial and peripheral blood monocytes/lymphocytes also triggers signal to produce cytokines in RA (Mirshafiey et al. 2008; Magari et al. 2004). The balance between Th1 and Th2 cytokines has significant interest in inflammation related to RA. The degree of polarization and heterogeneity of T-cell lymphocytes are important in the initiation and continuation of inflammation in RA via a direct relationship with NFκB (Magari et al. 2004) for inflammation.
Role of NFκB in inflammation and Rheumatoid arthritis
NFκB is the protein complex present in all cell cytoplasm as a heterodimer made up with P50 and Rel A or P52 and Rel B protein (Prajapati et al. 2010). Inactivated NFkB located in the cytosol by binding with the inhibitory protein IkB (Prajapati et al. 2010; Laura et al. 2012). Due to intermediacy of integral membrane receptors, a variety of extracellular signals can activate the enzyme known as IkB kinase (IKK). IKK leads to phosphorylation of IkB protein, which in turn dissociates IkB from NFkB and eventually degrades IkB by the proteosomes (Laura et al. 2012). The activated NFκB translocated into the nucleus where it binds to specific sequence of DNA called response element (RE). The DNA/NFκB complexes then recruit other proteins such as coactivators and RNA polymerase and transcribe downstream DNA into mRNA. mRNA further translocated into proteins and result in a change of cell functions (Bastian and Johannes 2013; Laura et al. 2012). NFκB signal regulates the following biological functions during inflammatory response:
-
Upregulates the production of proinflammatory (TNF-α,IFN-γ, IL 1) and anti-inflammatory cytokines (IL4, IL10, IL13).
-
It regulates the synthesis of pro/anti apoptotic signaling proteins that control the natural apoptotic programmed cell death.
-
Upregulates the synthesis of different growth factors for repair and healing in inflammation.
-
Regulates the synthesis of proteolytic proteins that play a key role in rheumatoid arthritis.
-
Increased ROS and RNS production in cells and control oxidative stress during RA.
Role of prostaglandin in Inflammation and rheumatoid arthritis
Activation of COX enzymes occurs in inflammation and becomes extremely unregulated in the chondrocytes. Chondrocytes then accumulate in synovium in patients with RA (Bala et al. 2013; Sarkar et al. 2008). Arachidonic acid synthesized from membrane phospholipids through Phospholipase A2 (PL-A2) and PL-A2 converts to prostaglandins by the biological action of COX enzymes. Prostaglandins (PGs) show the following biological role in inflammation and RA (Mohammad and Abbas 2012; Emanuela and Garret 2011):
-
Elevate the movement of plasma and leucocytes in the affected site or synovial joints by virtue of vasodilatation capacity of prostaglandin mainly prostaglandin E2 (PGE2) (Emanuela and Garret 2011).
-
Inhibit the platelets aggregation that rise the facility to accumulate the leucocytes mainly neutrophils in affected site or synovial joints in inflammatory disease or RA (Bala et al. 2013). Platelets aggregation inhibits the facility of chondrocyte’s movement to synovium in patients with RA.
-
Prostaglandins directly activate the NADPH Oxidase enzymes, thus increasing ROS and RNS. ROS and RNS are the main key players in healing process in inflammation. It also plays a key role in cartilage damage in RA (Sarkar et al. 2008).
-
Elevated COX expression as well as increased PG formation activate the NFκB pathway and thus increase cytokines synthesis (Bastian and Johannes 2013; Laura et al. 2012).
Role of Bradykinin in Inflammation and rheumatoid arthritis
Bradykinin is a protein vasodilator peptide synthesized from high molecular weight kininogen with the help of kallikrein and Hageman factor. It is the most important component in kinin group (Zhanli et al. 2014). The role of bradykinin in inflammation and RA is summarized below (Kidd and Urban 2001).
-
Bradykinin activates the PL-A2 and increases PG synthesis. This action is mediated by G protein-coupled B2 receptor (Bradykinin 2 Receptor; GPCR) present in visceral smooth muscle, endothelium and sensory nerve through phospholipase C, IP3/DAG as secondary messengers (Zhanli et al. 2014).
-
B2 receptor causes visceral smooth muscle relaxation and vasodilatation and promote cell migration in inflammation and RA (Mohammad and Abbas 2012).
-
B2 receptor in sensory nerve causes pain perception in inflammation and RA (Kidd and Urban 2001; Zhanli et al. 2014).
-
Increased level of ROS/RNS/cytokines by B1 receptor (Bradykinin 1 Receptor; GPCR) activates the PG synthesis and responsible for tissue repairing in inflammation and tissue damage in RA (Emanuela and Garret 2011; Zhanli et al. 2014).
B-cell activation and autoantibodies
B lymphocytes activate through interaction with activated T cells and soluble inflammatory cytokines/ROS or RNS and enhance their proliferation and differentiation in RA (Bala et al. 2014; Kundu et al. 2011; Do¨rner and Burmester 2003). B cells correlate the link between lymphoid tissue and the autoimmune antibody in the sinovium (Do¨rner and Burmester 2003). The CD20 receptor expressed in differentiated B cells on their surfaces and lost upon in the terminal differentiation to antibody-forming plasma cells (Benito et al. 2009; Tineke et al. 2008). Autoimmune antibodies made by plasma cell represent the terminal stage of differentiation for B-lymphocytes.
Clinical RA characterized by the presence of auto immunoantibodies known as rheumatoid factors (RF). Auto immunoantibodies produced by B cells and/or anti-citrullinated peptide antibodies (Marta et al. 2012; Do¨rner and Burmester 2003). Modern hypothesis concludes that B-cell depletion in circulating peripheral blood has a regulatory role in controlling patients with RA. Different diverse immunoglobulin (Ig) molecules are generated by recombination of gene segment in B cells as it express Ig receptors and secrete the same Ig after activation in autoimmune disease. These immunoantibodies work as destructive molecule in RA and are responsible for the disease severity in patients with RA.
Therefore, T cells and B cells play an important role in pathogenesis in RA. The damage in RA is due to effector cells and it interacts to form inflammatory products including autoantibodies, cytokines and other mediators like ROS and RNS (Tineke et al. 2008; Mohammad and Abbas 2012). The concrete synovial lining in RA represented as an expansion of fibroblast like cells and macrophages (Mohammad and Abbas 2012; Kundu et al. 2011). Macrophages are producer of proinflammatory mediators like ROS, RNS and cytokines including TNF, IL-1, IL-6, IL-8 (Kobayashi et al. 2010). These cytokines further stimulate other monocytes/macrophage, as well as other inflammatory cells in the body’s microenvironment. However, cells like fibroblasts, osteoclasts, chondrocytes and hepatocytes are responsible for the generation of C-reactive protein. The synovial fibroblast also secretes cytokines including IL-6, IL-8 and GM-CSF, and other mediators including destructive proteases and collagenases (Lally et al. 2005; Mohammad and Abbas 2012).
Neutrophil and lymphocyte served as the important predictive markers for sustained remission in RA (Chandrashekara et al. 2015). Neutrophils accumulated to the rheumatoid synovial cavity and aspirated mostly in the synovial fluid in large in number (Kundu et al. 2011). Neutrophils involvement in the joint synovium is purely driven by IL-8, leukotriene B4, and possibly localized plasma complement activation through C5a (Marta et al. 2012; Lally et al. 2005). Activated neutrophils in the synovial fluid also release oxygen-derived free radicals like ROS and RNS. Chondrocytes like synovial fibroblasts and monocytes are activated by IL1 and TNF-α to secrete proteolytic enzymes in RA. Thus, chondrocytes contribute to destruction of cartilage matrix and lead a progressive narrowing of joint spaces as observed radiographically in-patient with RA (Lally et al. 2005).
Increased amount of ROS/RNS is present in the synovial joint infiltrates as well as peripheral blood inflammatory cells of the patients with RA (Datta et al. 2014; Kundu et al. 2011). Peripheral blood primarily comprises of neutrophils, monocytes and lymphocytes. Therefore, previous studies mentioned the use of phytomolecules to suppress inflammation in peripheral blood mononuclear cells (PBMC) and polymorph nuclear (PMNC) cells. Studies also mentioned the direct involvement of ROS and RNS in RA as evaluated by using modern techniques like Flow Cytometry, Western Blot ELISA etc., (Bala et al. 2014; Datta et al. 2014; Kundu et al. 2011).
Inflammatory mediators in RA
As discussed above, the direct involvement of inflammatory mediators in clinical RA is well established. The most important inflammatory mediators in RA are ROS, RNS cytokines whereas the most active of these are O2 −, ONOO−,TNF-α, IL-1, and IL-6 (Martin et al. 2014, Mohammad and Abbas 2012). Cytokines, released in the synovial joint in RA, activate the T cells as well as other nearby cells including macrophages and neutrophils. The cytokines TNF-α, IL-1, and IL-6 upregulate the expression of the other mediators as their shared function (Mohammad and Abbas 2012).
The important effects of cytokines are:
-
Activate lymphocytes for cytokine synthesis.
-
Upregulate the adhesion molecule synthesis.
-
Activate the osteoclasts in joints.
-
Induce other inflammatory mediators including prostaglandins and nitric oxide.
-
Increased the matrix metalloproteinase synthesis in endothelial cell lining.
-
Induce the acute phase of response through C-reactive protein synthesis.
-
Regulate the systemic features like fatigue, fever, cachexia and pain.
-
Activate B cells (IL-6) to synthesize autoantibody as RF.
-
Upregulate the production of toxic peroxynitrite and hypochlorous acid in cells.
In addition to this, some of the important soluble mediators of inflammation including prostaglandins, leukotrienes, matrix metalloproteinase may diffuse to blood and settle in joint cavity (Raimund et al. 2007; Martin et al. 2014). Prostaglandins regulate vasodilatation; pain sensitization in localized inflammation, whereas leukotrienes play vital roles in vascular permeability and chemotaxis in RA. The proteolytic Matrix metalloproteinase (MMPs) enzymes degrade the collagen matrix of cartilage in joints (Burrage et al. 2006). Bradykinin causes release of prostaglandins from synovial fibroblasts, and potent algesic agent. Bradykinin acts on G-protein-coupled receptors (GPCR) i.e., bradykinin 2 receptors (B2) presents in visceral smooth muscle, endothelium and sensory nerve and are responsible for pain perception in RA. In addition, plasma complementary proteins are also available for interaction with immune complexes to generate additional chemo tactic stimuli during cell–cell interaction. The neuropeptide substance P is a potent vasoactive and proinflammatory peptide implicated in RA (Mohammad and Abbas 2012).
Involvement of ROS and RNS in Rheumatoid Arthritis
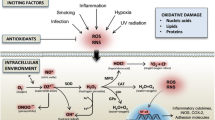
Free radical biology plays an important role in the pathophysiology of rheumatoid arthritis (Kundu et al. 2011). Free radicals are defined as any chemical species that bear unpaired electron(s) in its outer orbit. Free radicals are highly reactive due to the presence of unpaired electrons. They readily take part in chemical chain reactions with virtually all cell components (lipids, proteins, complex carbohydrates and nucleic acids) in the body. These reactions occur through a chain of oxidative reaction to cause tissue injury (Bala et al. 2013). In most biological systems, the O2 or N2 derived free radicals often referred to as reactive oxygen species (ROS) and reactive nitrogen species (RNS), respectively. The most significant biologically active free radicals derived from oxygen. ROS produced in cells include superoxide anion (O2 −), hydroxyl radical (·OH) and hydrogen peroxide (H2O2). Regulatory role of free radical biology starts with the superoxide anion radical (O2 −) formation and its degradation by superoxide dismutase (SOD) enzyme as describing in Fig. 1.
Generation of free radicals in inflammatory cells: the biological oxygen (O2) utilized by the mitochondria for the electron transport chain (ETC) system to generate ATP in cells. However, the membrane bound NADPH Oxidase enzyme activated during stress condition and utilized the free O2 to generate toxic O2 − radicals (Fig. 1) (Bala et al. 2013). The body’s defensive enzyme superoxide dismutase (SOD) immediately converts O2 − to less toxic hydrogen peroxide (H2O2) to prove its antioxidant activity. In addition, toxic hypochlorite (HOCl) generated from H2O2 and Cl− by a heme enzyme myeloperoxidase (MPO) particularly in immunologically activated macrophages cells. H2O2 produces the toxic hydroxyl radical via a Fe++-mediated reaction, which is known as Fenton reaction (Kavian et al. 2012; Bala et al. 2013). H2O2 immediately neutralized by catalase enzyme in the cells. Free radicals can also be formed in cell by some of the non-enzymatic reactions of oxygen with organic compounds as well as those initiated by ionizing reactions (Lobo et al. 2010). Nitric oxide formed from L-arginine through different isoform of NOS (as described in section “Involvement of ROS and RNS in Rheumatoid Arthritis”). Freely available NO can react with O2 − or H2O2 to form high molecular weight peroxynitrite (ONOO−), whose oxidant potential is higher than that of O2 − or H2O2 alone probably due to inability to escape from the cells (Bala et al. 2013; Bala et al. 2012a)
Some internally generated sources of free radicals are
-
Mitochondria: mitochondrial electron transport chain generates superoxide anion (O2 −) as a lead ROS and circulated in the body. Oxidative damage to cellular macromolecules or micromolecules is mainly caused by O2 − and leads to the dysfunction of normal cellular activity (Jeffrey et al. 2014).
-
Xanthine oxidase: mitochondrial O2 − production does not significantly increased in many diseases. However, xanthine oxidase activity to generate O2 − is higher in liver and plasma in different chronic diseases (Maria et al. 2016). The increase of plasma xanthine oxidase activity explained by the increase in the hepatic release of this enzyme, which is not due to nonspecific membrane damage but due to increase of cellular oxidative stress (Loba et al. 2010).
-
Peroxisomes: the presence of a novel group of enzyme known as peroxisome involved in the metabolism of oxygen free radicals. In addition to hydrogen peroxide, peroxisomes also generate O2 − radicals and nitric oxide. O2 − and NO are the cellular messengers with a variety of physiological roles in intra- and inter-cellular communication (Corpas et al. 2001).
-
Inflammation: inflammation is the body’s way of initiating healing response. However, unregulated inflammation can result in excessive free radicals activity and cause tissue destruction. Immune signaling/inflammation is one of the primary mechanisms for initiating the inflammatory pathway. The notable immune-signaling cytokines are generated/activated by free radicals (Woo et al. 2000).
-
Phagocytosis: phagocytes mediate innate immunological response by releasing products to damage invading microorganisms (Loba et al. 2010).Oxidative stress is the main factor responsible for key changes leading to the repairing and degeneration of cells involved in phagocytosis of macromolecules (Olchawa et al. 2016).
Some externally generated sources of free radicals are (Loba et al. 2010)
-
Cigarette smoking.
-
Environmental/chemical pollutants.
-
Radiation contact to cells.
-
Stress.
RNS refer to nitrogen-centered radicals and these include nitric acid (NO), peroxynitrite (ONOO−) and nitrogen dioxide radical (·NO2). Nitric oxide (NO) acts as one of the lead RNS, that play significant beneficial role in human cells/systems (Seaman et al. 2016). Biologically active NO is formed from the amino acid l-arginine by one of the three available isoforms of Nitric Oxide synthase (NOS) (nNOS, iNOS, and eNOS). nNOS is originally identified as constitutive in neuronal tissue. It is also known as NOS-I or NOS-1. iNOS is originally identified as being inducible by cytokines in activated macrophages and liver. It is also known as NOS-II or NOS-2. eNOS is originally identified as constitutive in vascular endothelial cells. It is also known as NOS-III or NOS-3 (Seaman et al. 2016; Bala et al. 2013). NOS requires oxygen, tetrahydrobiopterin, NADPH, calmodulin, flavin adenine dinucleotide (oxidised; FAD), flavin mononucleotide (FMN) and heme for catalytic reaction and activation, whereas Ca2++ is essential for nNOS and eNOS activity (Mirshafiey et al. 2008). RNS and ROS are always reactive as they carry a free electron in outer orbital. Freely available NO can react with O2 − or H2O2 to form peroxynitrite (ONOO−). The oxidant potential of high molecular weight ONOO− is greater than that of O2 − or H2O2 alone probably due to inability to escape from the cells (Bala et al. 2013; Bala et al. 2012a).
Antioxidant enzymes work as the protective cellular defense against oxidative damage in inflammation. Catalase (CAT), GSH (Reduced Glutathione) peroxidases (GP), superoxide dismutase (SOD) are the most important antioxidant defense enzymes in cell. Reduced glutathione (GSH) is the most important non-enzymatic antioxidant defense in inflammatory cells (Halliwell 1992; Bala et al. 2013; Bala et al. 2012a). The tripeptide glutathione in its reduced form (GSH; c-l-glutamyl-l-cysteinyl-glycine) acts as antioxidant by virtue of its ability to donate H+. GSH is considered as the principal cellular antioxidant thereof in cells (Bala et al. 2012b).
Cells make glutathione in two adenosine triphosphate-dependent steps (Couto et al. 2013):
-
Gamma-glutamyl-cysteine is synthesized from l-glutamate and cysteine via the enzyme gamma-glutamylcysteine synthetase (glutamate cysteine ligase, GCL). The reaction is considered as the rate-limiting step in glutathione synthesis.
-
Glycine added to the C-terminal of gamma-glutamylcysteine via the enzyme glutathione synthetase.
Glutathione is the thiol-containing low molecular weight molecule, synthesized from glutamate, cysteine and glycine. N-acetyl cysteine is a stable, effective precursor of cysteine for intracellular GSH synthesis (Bala et al. 2012a). As the major component of the cellular antioxidant system, GSH (reduced) reacts with all ROS and RNS and stabilizes them by donating H+ and itself converted into GSSG (Oxidized Glutathione). GSSG again get back to GSH by the action of glutathione reductase (GR) with the coenzyme NADH (Bala et al. 2014). Several studies mentioned the direct reduction of GSH level during inflammatory diseases like RA.
Accumulation of inflammatory cells in synovial joints and increased oxidative damage observed clinically in patients with RA. Protein carbonylation, lipid peroxidation as detected by spectrophotometer and S-nitrosothiols detected by flow cytometry show evidence to oxidative damage in RA (Datta et al. 2014). Neutrophils constituted the major cellular component of the synovium of patients with RA. The levels of ROS and RNA also correlated strongly with protein carbonylation and lipid peroxidation (Kundu et al. 2011). Scientific studies on phytomolecules found effective as inhibiting different O2 and N2 radicals (Bala et al. 2014; Kundu et al. 2011). Therefore, the role of ROS and RNS in the pathophysiology of RA is documented in modern advanced research.
Sources and classification of natural dietary antioxidants according to their antioxidant mechanism
Plants are rich source of naturally occurring antioxidants like phenols, phenolic acid, flavonoids and their derivatives. Fruits and vegetables contain abundant source of these types of compounds as antioxidant (Carocho et al. 2013; Liu 2013). Several studies have reported the antioxidant potential of a wide variety of dietary green vegetables. Vegetable provides rich source of antioxidants, specially anthocyanins, flavonones, flavonols and vitamin C (Liu 2013). Fruits, especially berries, cherries and citrus fruits contain high levels of polyphenolic compound and the polyphenol content in berries varies from 30 to 2000 mg/100 g, which include anthocyanins, proanthocyanidins and flavonols (Atreyi and Uma 2016; Suhaj 2006). Spices and herbs like garlic, ginger, curcumin also have antioxidant potential (Moure et al. 2001; Suhaj 2006). A comprehensive study on the role of phenolic compounds in the oxidative process of fruits reveals the effects of processing and storage on its antioxidative capacity (Moure et al. 2001). Wines contain a variety of polyphenolic compounds, the most abundant being anthocyanins whose antioxidant activity was also reported in whiskeys (Suhaj 2006).
Antioxidants comprise a broad class of compounds that counteract free radicals, inhibit oxidative chain reactions and can react with damaged DNA. Their chemical structures and particular mechanism of antioxidants activity differ widely (Kushi et al. 2012). The growing interest in the role of antioxidants in health care and disease is well documented. Antioxidants work in multiple pathways—act as radical scavenger, hydrogen donor, electron donor, peroxide decomposer, singlet oxygen quencher, enzyme inhibitor, synergist, and as metal chelating agents. Both enzymatic and no enzymatic antioxidants exist in the intracellular and extracellular environment to detoxify ROS & RNS (Bala et al. 2013; Moure et al. 2001).
Classification of natural antioxidants according to their antioxidant mechanism
The mechanism of action of antioxidants differs widely. Many of them act as radical scavenger others as prooxidant enzyme inhibitors (CYP2E1 or NADPH Oxidase). Some of them inactivate of the NF-κB pathway to inhibit cytokine synthesis. However, antioxidant activity does not necessarily imply in a single mechanism in biological system always (Bala et al. 2012b). The mechanism of action of antioxidants is described below and summarized in Table 1.
-
Antioxidants scavenge/directly inhibit the active free radicals or toxic non-radicals to suppress chain initiation and/or break the chain propagation reaction. Some antioxidants are hydrophilic and some others are lipophilic. Vitamin C, uric acid, bilirubin, albumin, and thiols are hydrophilic. However, vitamin E and ubiquinol are lipophilic radical scavenging antioxidants. Whereas vitamin E is known as the most active lipophilic antioxidant (Kushi et al. 2012; Bala et al. 2013; Lobo et al. 2010).
-
The preventive antioxidant suppresses the formation of free radicals in the cell (Lobo et al. 2010). These are the second line of defense in body. Although, the precise mechanism and site of in vivo radical formation are not clearly well documented. The metal-induced decomposition of hydro peroxides and hydrogen peroxide must be one of the important sources (Bala et al. 2013). Glutathione peroxidase, glutathione-s-transferase, phospholipid hydro peroxide glutathione peroxidase are known to decompose lipid hydro peroxides to corresponding alcohols by antioxidant. Glutathione peroxidase and catalase reduce hydrogen peroxide to water (Christine and Joseph 2010).
-
DNA repairs system plays an important role as defensive against oxidative damage. Various kinds of enzymes such as glycosylases and nucleases, which repair the damaged DNA, are also known as antioxidant (Rock et al. 2012; Diplock 1995; Lobo et al. 2010).
-
The proteolytic enzymes like proteinases, proteases, and peptidases, present in the cytosol and in the mitochondria of mammalian cells, recognize, degrade, and remove damaged/modified proteins to prevent the accumulation of oxidized proteins (Bala et al. 2013; Mai et al. 1990; Lobo et al. 2010).
Generally, biological antioxidants act as free radical scavengers and are, thus, found to play a significant protective role during oxidative stress in a variety of diseases such as liver cirrhosis, inflammation, atherosclerosis, diabetes, cancer, neurodegenerative diseases and also in RA (Hitchon and El-Gabalawy 2004). Many important molecules including proteins, lipids and nucleic acid chains are very susceptible to oxidizing reaction by free radicals (Khansari et al. 2009). It is not expected to see many deteriorating events following oxidative stress leading to decreased longevity of the cells as well as destroying the normal physiological function of cells (Kundu et al. 2011). Pro-inflammatory cytokines like TNF-α, IFN-γ, interleukin (IL)-1β and IL-6 are key elements in abnormal transformation of the cells and autoimmune activities leading to RA. Therefore, chronic inflammation should be considered as a high risk factor for RA. The antioxidant system within the body can easily try to handle free radicals; however, dietary antioxidant supplements are considered as acquired defense in body (Bonnefont 2002).
Effectiveness of phytomolecules in Rheumatoid Arthritis
As discussed, RA is responsible for significant human morbidity in modern life. Oxidative stress is considered as a potential biomarker for detecting disease pathophysiology in patients with RA (Kundu et al. 2011; Datta et al. 2014). Th1 and Th2 cytokines balance attract the great interest as described earlier. The degree of polarization and heterogeneity of mononuclear T lymphocytes or polymorphonuclear neutrophils may be important in the initiation and perpetuation of inflammation in RA (Magari et al. 2004). Therefore, the studies on non-toxic natural antioxidants are of great interest now a days. Some of the important clinically effective natural antioxidants are mentioned below and summarized in Table 2.
-
5-Hydroxy-3,4′,7-trimethoxyflavone: it is isolated from Cleome gynandra L. (Capparidaceae). It is equally effective to decrease oxidative damage by inhibiting superoxide radical, hydroxyl radical and NO in the isolated peripheral blood mononuclear lymphocytes of patients with RA and human erythrocytes (Bala et al. 2014; Bala et al. 2012a).
-
Allylpyrocatechol (APC; 3-prop-2-enylbenzene-1,2-diol): it is isolated from Piper betle (Family-Piperaceae). The effect of APC on nitric oxide measured in macrophages using 4,5-diaminofluorescein diacetate by flow cytometry whereas, it decreased production of nitric oxide in synovial infiltrated cells. The effect also correlated with its in vitro nitric oxide scavenging activity (Kundu et al. 2011).
-
Curcumin: curcumin is yellow hydrophobic polyphenol compound derived from the herb turmeric. It is effective through somatostatin generation via cAMP/PKA and Ca2+/CaMKII signaling pathways. However, “thetracurmin” which is a highly bioavailable form of curcumin, is found effective in patients with osteoarthritis to suppress pain. (Yasuaki et al. 2014; Qingshan et al. 2013).
-
Curcumin analogues Diarylpentanoid (BDMC33) [2,6-bis(2,5-dimethoxybenzylidene) cyclohexanone]: it significantly inhibits the pro-gelatinase B (pro-MMP-9) and collagenase activities via suppression of MMP-1 in activated SF. In addition, BDMC33 strongly suppressed MMP-3 gene expression as well as inhibited COX-2 and IL-6 gene expression. BDMC33 abolished the p65 NF-κB nuclear translocation and NF-κB DNA binding activity in PMA-stimulated SF (Lee et al., 2015).
-
Epigallocatechin-3-gallate: green tea polyphenol Epigallocatechin-3-gallate regulates inducible nitric oxide synthase. It also inhibits IL-1β- induced phosphorylation and proteasomal degradation of IκBα to suppress NF-κB nuclear translocation (Singh et al. 2002; Ahmed et al. 2002).
-
Genistein: isoflavones such as genistein and daidzein are found in a number of plants including lupin, fava beans, soybeans, kudzu, and psoralea. It is also found as the primary food source in the medicinal plants like Flemingia vestita, F. macrophylla, and coffee. Genistein inhibits cytokines or growth factor-induced proliferation and transformation phenotype in fibroblast-like synoviocytes in RA (Lui et al. 2015).
-
Icaritin: Epimedii herba is one of the most widely studied herbs for treatment of bone metabolic and related diseases in China. Epimedium flavonoid is composed of seven flavonoid compounds and its main active compound extracted as Icaritin. Icaritin enhances bone-healing (Yao et al. 2012). Icaritin possessed the potential for enhancing bone formation via its osteopromotive but not an osteoinductive mechanism.
-
Paeoniflorin: total glycosides of paeony (TGP) are obtained from Radix paeoniae, in which paeoniflorin is the major active component. TGP/paeoniflorin suppresses inflammatory process by reducing the production of prostaglandin E2, leukotriene B4, nitric oxide, reactive oxygen species, proinflammatory cytokines and chemokines. TGP/paeoniflorin also inhibits the proliferation of lymphocytes and fibroblast-like sinoviocytes. The inhibitory effect on formation of new blood vessels, and the production of matrix metalloproteinase is also reported (Zhang et al. 2008).
Plants provide a rich resource for natural drug research and development. In recent years, the use of traditional medicine information on plant research received considerable interest. Additionally, the consequent use of dietary antioxidants is continuously increasing worldwide (Bala et al. 2011). The previous studies focused on the scientific and clinical etiology of RA in correlation with oxidative stress and the effectiveness of the plant-derived active molecules on all type of inflammatory cells is established (Kundu et al. 2011; Bala et al. 2014).
Safety of natural antioxidants
Natural antioxidants are preferred by consumers worldwide and may gain easy consumer acceptance and easy scientific approval than synthetic additives. However, any natural compound commonly found in food has no guarantee that it is entirely non-toxic for human health (Carocho et al. 2013). Synthetic antioxidants are tested for carcinogenic or mutagenic effect, but many natural food compounds have not been tested yet (Atreyi and Uma 2016; Carocho et al. 2013). The comparative advantages and disadvantages of synthetic and natural antioxidants are summarized in Table 3.
The clinical safety and efficacy of natural dietary antioxidants are controversial worldwide (Bala et al. 2013). Number of researchers are concerned that high-dose antioxidant supplement could decrease the oxidative stress in all type of inflammatory diseases (Rock et al. 2012). Antioxidant supplementation could selectively protect healthy cells from such damage or may protect the damage cells as well (Kushi et al. 2012).
In contrary, the toxicological risk of food-based dietary compounds are enquired. However, there are no such reports on the harmful toxicological aspect of the flavonoids. The acute toxicity test is the most basic method of testing of toxicology safety assessment, and plays a key role in chemical toxicity assessment (Ke-Zheng et al. 2015).
In one of our studies during the cellular toxicity test of an isolated plant flavonoid (CWF; 5-Hydroxy-3,4′,7-trimethoxyflavone isolated from Cleome gynandra L), we found no such significant cellular toxicity in mouse macrophage cells at 3 h of incubation in Krebs Hensleit (KH) buffer (Fig. 2a, unpublished data). 1.449% erythrocytes only lysed at a concentration of 500 µg/ml of the CWF at 1 h of incubation in controlled condition (Fig. 2b, unpublished data). Both the graphs are prepared in Graph Pad Prism software version 4.03., software. Linear regression for figure B analyzed with effect of “force zero” and unknown value interpolated from standard curve. All experiments are done in triplicate and final graph is prepared as showing mean ± SEM.
Cellular toxicity study of CWF (a): CWF (5-Hydroxy-3,4′,7-trimethoxyflavone at different concentration) was incubated with mouse macrophages cells up to 3 h in Krebs Hensleit buffer (In BOD incubator), cell viability was confirmed by trypan blue exclusion methods. Haemolytic study of CWF (b): CWF (at different concentration) was incubated with RBC (1% washed erythrocyte suspension in PBS) at 37 °C for 1 h. Distilled water (DW) and PBS were taken as positive and negative controls, respectively. OD taken at 540 nm
Furthermore, to understand the antioxidant conundrum, it is useful to review the basics. Antioxidants block the negative effects of free radicals. Free radical damages DNA, proteins, and lipids; augment the aging process; and plays a role in weakening the immune system towards many diseases such as cancer, diabetes, and cardiovascular complication. Conversely, naturally produced free radicals (by-products of metabolism) are essential for health. They destroy unhealthy cells, bacteria, and viruses and detoxify harmful chemicals (Liu et al. 2011).
Conclusion
The use of specific dietary components changes from treating deficiencies to preventing diseases (Aalt and Guido 2002). Many chronic diseases are associated with oxidative stress and researchers are trying to establish the beneficial role of antioxidants clinically (Couto et al. 2013). Therefore, the use of antioxidant-rich food or antioxidant food supplements became immensely popular worldwide. The term “nutraceuticals” is introduced to indicate the use of purified or concentrated food components in a pharmaceutical formulation with a presumed health or increasing effect (Bala et al. 2013; Couto et al. 2013).
Modern therapeutic drugs have a number of adverse or toxic effects. Since the scientists are continuously trying to find new traditional knowledge-based alternative for the treatment of RA, traditional medicine as multi-component and multi-target approaches perfectly matches with the holistic concept of ancient systems biology. It is also applicable in the treatment of RA. Appropriate knowledge on side effect, biotransformation or bio kinetics of the nutraceuticals, therapeutic dose and molecular mechanism of action of natural antioxidants is highly recommended in modern RA research. However, modernization of traditional knowledge for subsequent careless application of natural antioxidant is highly demanded.
This review highlighted recent studies describing the relationships between rheumatoid arthritis and free radicals, and the potential biological mechanisms to explain these associations. Based on existing research evidence, patients with RA should therapeutically utilize antioxidant supplement, especially in controlled dose to retard side or adverse effect. However, very few antioxidant dietary supplements reported for their side or adverse effect. Results from many clinical studies conducted to date have shown benefits and in all cases mentioned significant effect on appropriate biological targets (Shown in Fig. 3). Although, it is not yet clear that in which specific phase of RA, antioxidant consumption may be beneficial. Therefore, it is advisable to apply the cautionary principle and to advise patient not to consume excess antioxidant dietary supplement without medical supervision, until further studies bring greater clarity
.
Abbreviations
- RA:
-
Rheumatoid arthritis
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- O2 − :
-
Superoxide
- ONOO− :
-
Peroxinitrite
- CAID:
-
Chronic autoimmune disease
- COX:
-
Cyclooxygenase
- NFκB:
-
Nuclear factor kappa light chain enhancer of activated B cells
- IKK:
-
IkB kinase
- PL-A2:
-
Phospholipase A2
- MMPs:
-
Matrix metalloproteinase(s)
- NOS:
-
Nitric oxide synthase
- GPCR:
-
G-protein couple receptor
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
References
Aalt B, Guido RMMH (2002) The toxicity of antioxidants and their metabolites. Environ Toxicol Pharmacol 11:251–258
Ahmed S, Rahman A, Hasnain A, Lalonde M, Goldberg VM, Haqqi TM (2002) Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med 33:1097–1105
Al-N Abdullah, Rownak J, Mohammed R (2014) Zingiber officinale: a potential plant against rheumatoid arthritis. Arthritis 159089:1–8
Atreyi S, Uma G (2016) Natural antioxidants–the key to safe and sustainable life. Int J Latest Trends Eng Technol 6(3):460–466
Bala A, Haldar PK (2013) Free radical biology in cellular inflammation related to rheumatoid arthritis. OA Arthritis01 1(2):15
Bala A, Haldar PK, Kar B, Naskar S, Saha P, KunduSen S, Gupta M, Mazumder UK (2011) Antioxidant activity of the fractions of Cleome gynandra promotes antitumor activity in Ehrlich Ascites Carcinoma. Asian J Chem 23:5055–5060
Bala A, Kar B, Karmakar I, Kumar RBS, Haldar PK (2012a) Antioxidant activity of Cat’s whiskers flavonoid on some reactive oxygen and nitrogen species generating inflammatory cells is mediated by scavenging of free radical. Chin J Nat Med 10(5):1–7
Bala A, Haldar PK, Kar B, Naskar S, Mazumder UK (2012b) Carbon tetrachloride: a hepatotoxin causes oxidative stress in murine peritoneal macrophage and peripheral blood lymphocyte cells. Immunopharmacol Immunotoxicol 34:135–142
Bala A, Chetia P, Dolai N, Khandelwal B, Haldar PK (2014) Cat’s whiskersflavonoid attenuated oxidative DNA damage in macrophages cells: its importance in patients with rheumatoid arthritis. Inflammopharmacology 22(1):55–61
Bastian H, Johannes AS (2013) The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 12:86
Benito MM, Garcia-Carmona Y, Balsa A, Carlos PDA, Tatiana CI, Emilio MM, María E, Miranda C (2009) A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+ CD25+ regulatory T cells and CD4+ CD25− responder T cells. J Immunol 183:8268
Bonnefont RD (2002) Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care 5:561–568
Burrage PS, Mix KS, Brinckerhoff CE (2006) Matrix metalloproteinases: role in arthritis. Front Biosci 1(11):529–543
Carocho M, Ferreira ICFR (2013) A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol 51:15–25
Chandrashekara S, Rajendran A, Jaganath AB, Krishnamurthy R (2015) Neutrophil-lymphocyte ratio, pain perception, and disease activity score may serve as important predictive markers for sustained remission in rheumatoid arthritis. Reumatismo 67(3):109–115
Christine JW, Joseph JC (2010) Measurement of superoxide dismutase, catalase, and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5(1):51–66
Corpas FJ, Barroso JB, Río LA (2001) Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci 6(4):145–150
Couto N, Malys N, Gaskell S, Barber J (2013) Partition and turnover of glutathione reductase from Saccharomyces cerevisiae: a proteomic approach. J Proteome Res 12(6):2885–2894
Datta S, Kundu S, Ghosh P, De S, Ghosh A, Chatterjee M (2014) Correlation of oxidant status with oxidative tissue damage in patients with rheumatoid arthritis. Clin Rheumatol 33(11):1557–1564
Diplock AT (1995) Safety of antioxidant vitamins and beta- carotene. Am J Clin Nutr 62(6):1510S–1516S
Do¨rner T, Burmester GR (2003) The role of B cells in rheumatoid arthritis: mechanisms and therapeutic targets. Curr Opin Rheumatol 15:246–252
Emanuela R, Garret AF (2011) Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol 31(5):986–1000
Emmendoerffer A, Hecht M, Boeker T, Mueller M, Heinrich U (2000) Role of inflammation in chemical-induced lung cancer. Toxicol Lett 112:185–191
Giacomelli R, Ruscitti P, Alvaro S, Ciccia F, Liakouli V, Di Benedetto P, Guggino G, Berardicurti O, Carubbi F, Triolo G, Cipriani P (2016) IL-1β at the crossroad between rheumatoid arthritis and type 2 diabetes: may we kill two birds with one stone? Expert Rev Clin Immunol 12(8):849–855
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neuro-chem 59(1609–23):6
Hitchon CA, El-Gabalawy HS (2004) Oxidation in rheumatoid arthritis. Arthritis Res Therp 6:265–278
Jeffrey AS, Maddalena LA, Merilovich M, Ellen LR (2014) A midlife crisis for the mitochondrial free radical theory of aging. Longev Healthspan 3(4):2046–2395
Kavian N, Marut W, Servettaz A, Nicco C, Chereau C, Lemarechal H, Borderie D, Dupin N, Weill B, Batteux F (2012) Reactive oxygen species-mediated killing of activated fibroblasts by arsenic trioxide ameliorates fibrosis in a murine model of systemic sclerosis. Arthritis Rheum 64:3430–3440
Ke-Zheng P, Xiudong Y, Hong-Li Z, Shu-Xia P (2015) Safety evaluation, in vitro and in vivo antioxidant activity of the flavonoid-rich extract from Maydis stigma. Molecules 20:22102–22112
Khansari N, Shakiba Y, Mahmoudi M (2009) Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov 3:73–80
Kidd BL, Urban LA (2001) Mechanisms of inflammatory pain. Br J Anaesth 87(1):3–11
Kobayashi T, Murasawa A, Komatsu Y (2010) Serum cytokine and periodontal profiles in relation to disease activity of rheumatoid arthritis in Japanese adults. J Periodontol 81:650–657
Kundu S, Bala A, Ghosh P, Mitra A, Sarkar A, Bauri AK, Ghosh A, Chattopadhyay S, Chatterjee M (2011) Attenuation of oxidative stress by Allylpyrocatechol in synovial cellular infi ltrate of patients with Rheumatoid Arthritis. Free Radical Res 45:518–526
Kushi LH, Doyle C, McCullough M (2012) American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 62:30–67
Lally F, Smith E, Filer A, Stone MA, Shaw JS, Nash GB, Buckley CD, Rainger GE (2005) A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid sinovium. Arthritis Rheum 52:3460
Laura T, Anil KT, Jason B, Marta M, Guido F (2012) The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends in Cell Biol 22(11):557–566
Lee KH, Abas F, Mohamed ANB, Shaari K, Lajis NH, Israf DA, Syahida A (2015) Chemopreventive effects of a curcumin-like diarylpentanoid [2,6-bis(2,5-dimethoxybenzylidene)cyclohexanone] in cellular targets of rheumatoid arthritis in vitro. Int J Rheum Dis 18(6):616–627
Liu HR (2013) Health-promoting components of fruits and vegetables in the diet. Adv Nutr 4(3):384S–392S
Liu J, Wang C, Wang Z, Zhang C, Lu S, Liu J (2011) The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem 126:261–269
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 4(8):118–126
Lü S, Wang Q, Li G, Sun S, Guo Y, Kuang H (2015) The treatment of rheumatoid arthritis using Chinese medicinal plants: from pharmacology to potential molecular mechanisms. J Ethnopharmacol 176:177–206
Magari K, Miyata S, Ohkubo Y, Mutoh S (2004) Inflammatory cytokine levels in paw tissues during development of rat collagen-induced arthritis: effect of FK506, an inhibitor of T cell activation. Inflammation Res 53:469–474
Mai J, Sørensen PS, Hansen JC (1990) High dose antioxidant supplementation to MS patients. Effects on glutathione peroxidase, clinical safety, and absorption of selenium. Biol Trace Elem Res 24(2):109–117
Maria GB, Letizia P, Massimo B, Andrea B (2016) Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxid Med Cell Longev 2016:3527579–3527587
Marta BM, García-Carmona Y, Alejandro B, Belen M, Bautista C, Arroyo-Villa I, Cobo-Iba T, María Gema BH, de Pérez AC, Sanchez-Mateos P, Martín-Mol E, Miranda-Caru ME (2012) IL-15 Expression on RA synovial fibroblasts promotes B cell survival. PLoS One 7(7):e40620. doi:10.1371/journal.pone.0040620
Martin A, Mikael B, Karen E, Robin C, Kalle S, Niels S, Pieter S, Ulrik GWM, Torp-Pedersen S, Else MB, Danneskiold-Samsøe B, Nina V, Lars K, Henning B (2014) Synovial explants inflammatory mediator production corresponds to rheumatoid arthritis imaging hallmarks: a cross-sectional study. Arthritis Res Ther 16:R107
Mirshafiey A, Mohsenzadegan M (2008) The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran J Allergy Asthma Immunol 7:195–202
Mohammad J, Abbas M (2012) Prostaglandins and rheumatoid arthritis. Arthritis 2012:239310–239317
Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, Núñez MJ, Parajó JC (2001) Natural antioxidants from residual sources. Food Chem 72(2):145–171
Olchawa MM, Pilat AK, Szewczyk GM, Sarna TJ (2016) Inhibition of phagocytic activity of ARPE-19 cells by free radical mediated oxidative stress. Free Radic Res 50(8):887–897
Pandey A, Bani S, Dutt B, Suri KA (2010) Modulation of Th1/Th2 cytokines and inflammatory mediators by hydroxychavicol in adjuvant induced arthritis tissues. Cytokine 49:114–121
Prajapati B, Singhal M, Ganesh Y, Sharma N, Gupta V (2010) Role of NFkB in various immunological and inflammatory disorders. Int J Toxicol Pharmacol Res 2(1):35–39
Qingshan Y, Shujin W, Xinzhan M, Wanchun W, Huiping T (2013) Inhibition effect of curcumin on TNF-a and MMP-13 expression induced by advanced glycation end products in chondrocytes. Pharmacology 91(1–2):77–85
Raimund WK, Bruno S, Gerd RB (2007) Cells of the sinovium in rheumatoid arthritis. Macrophages. Arthritis Res Ther 9(6):224
Rock CL, Doyle C, Demark-Wahnefried W (2012) Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 62:243–274
Ryan KA, Smith MFJ, Sanders MK, Ernst PB (2004) Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NFkappaB and interleukin-8 expression. Infect Immun 72:2123–2130
Sarkar D, Saha P, Gamre S, Bhattacharjee S, Hariharan C, Ganguly S (2008) Antiinflammatory effect of allylpyrocatechol in LPS-induced macrophages is mediated by suppression of iNOS and COX-2 via the NF-κB pathway. Inter Immunopharmacol 8:1264–1271
Seaman A, Darrah E, Infantino M, Meacci F, Manfredi M, Benucci M, Mahler M (2016) Anti-peptidyl-arginine deaminase 3 (PAD3) antibodies as a promising marker to measure joint damage in patients with rheumatoid arthritis. Autoimmun Rev 9972(16):30073–30078
Singh R, Ahmed S, Islam N, Goldberg VM, Haqqi TM (2002) Epigallocatechin-3- gallate inhibits interleukin-1β-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor κB activation by degradation of the inhibitor of nuclear factor κB. Arthritis Rheum 46:2079–2086
Suhaj M (2006) Spice antioxidants isolation and their antiradical activity: a review. J Food Compos Anal 19:531–537
Tineke C, Johanna K, Trieneke T, Tineke CPK, Bernard V, Rogier M, Thurlings JDC, Anca IC, Theo O, Cor LV, Yiping Z, Paul PT, Dominique B (2008) B lymphocyte autoimmunity in rheumatoid synovitis is independent of ectopic lymphoid neogenesis. J Immunol 181:785–794
WHO (2002) Traditional medicine strategy launched vol. 80 of 610. WHO News, Geneva
Woo CH, Eom YW, Yoo MH, You HJ, Han HJ, Song WK, Yoo YJ, Chun JS, Kim JH (2000) Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J Biol Chem 13 275(41):32357–32362
Yamaoka K (2016) Janus kinase inhibitors for rheumatoid arthritis. Curr Opin Chem Biol 16(32):29–33
Yao D, Xin-Hui X, Xin-Luan W, Chao W, Yuk-Wai L, Shi-Hui C, Duan-Qing P, Yi-Xiang W, Gang L, Ling Q (2012) Icaritin, an exogenous phytomolecule, enhances osteogenesis but not angiogenesis—an in vitro efficacy study. PLoS One 7(8):e41264
Yasuaki N, Shogo, Shigeru Y, Masayuki M, Eri T, Tadashi H, ChiekoT, Atsushi I, Jun N, and Takashi N (2014) Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci 19(6):933–939
Zhang LLW, Wei NP, Wang QT, Wang JY, Chen YC, Wu H, Hu XY (2008) Paeoniflorin suppresses inflammatory mediator production and regulates G protein-coupled signaling in fibroblast-like synoviocytes of collagen induced arthritic rats. Inflamm Res 57:388–395
Zhanli X, Jihong D, Aizhen Y, Yi W (2014) A role for bradykinin in the development of anti-collagen antibody-induced arthritis. Rheumatology 53:1301–1306
Acknowledgements
Authors are thankful to University Grant Commission (Govt. of India). Himalayan Pharmacy Institute and Sikkim Manipal Institute of Medical Sciences, Sikkim India are highly acknowledged. Dr. Asis Bala sincerely thanks to Dr. M.G. Matsabisa (Research Director-IKS) & acknowledges University of Free State, Bloemfontein, South Africa for Post-Doctoral Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bala, A., Mondal, C., Haldar, P.K. et al. Oxidative stress in inflammatory cells of patient with rheumatoid arthritis: clinical efficacy of dietary antioxidants. Inflammopharmacol 25, 595–607 (2017). https://doi.org/10.1007/s10787-017-0397-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0397-1