Abstract
Defects in dystroglycan post-translational modification result in congenital muscular dystrophy with or without additional eye and brain involvement, are referred to as secondary dystroglycanopathies and have been associated with mutations in 11 different genes encoding glycosyltransferases or associated proteins. However, only one patient with a mutation in the dystroglycan encoding gene DAG1 itself has been described before. We here report a homozygous novel DAG1 missense mutation c.2006G>T predicted to result in the amino acid substitution p.Cys669Phe in the β-subunit of dystroglycan in two Libyan siblings. The affected girls presented with a severe muscle–eye–brain disease-like phenotype with distinct additional findings of macrocephaly and extended bilateral multicystic white matter disease, overlapping with the cerebral findings in patients with megalencephalic leucoencephalopathy with subcortical cysts. This novel clinical phenotype observed in our patients further expands the clinical spectrum of dystroglycanopathies and suggests a role of DAG1 not only for dystroglycanopathies but also for some forms of more extensive and multicystic leucodystrophy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dystroglycan plays an essential role as an anchor for diverse extracellular proteins (e.g. laminin) to the cytoskeleton. It is encoded by the DAG1 gene and post-translationally cleaved into a transmembrane β-subunit (β-dystroglycan, βDG) and a highly glycosylated extracellular α-subunit (α-dystroglycan, αDG) [1]. O-glycosylation of αDG with various oligosaccharides is crucial for its normal binding function to the extracellular matrix [2]. In congenital muscular dystrophies with defective O-glycosylation of α-dystroglycan (dystroglycanopathies), hypoglycosylation of αDG in muscle biopsy is detected with immunohistochemical methods. There is a broad clinical spectrum of dystroglycanopathies with the most severe conditions, muscle–eye–brain disease (MEB) and Walker–Warburg syndrome (WWS), constantly being associated with characteristic brain malformations and eye involvement [3, 4]. Recently, the genotype–phenotype correlations were refined as over the past decade a more overlapping clinical spectrum became evident [3]. To date, mutations in 11 known or putative glycosyltransferase genes or associated proteins have been identified (POMT1, POMT2, POMGnT1, Fukutin, FKRP, LARGE, ISPD, GDTC2, B3GNT1, B3GALNT2 and TMEM5) and currently explain approximately 50 % of patients with characteristic clinical findings [3, 5–11]. The associated clinical manifestations are referred to as secondary dystroglycanopathies [2]. Unexpectedly, to date, only one patient with a primary dystroglycanopathy associated with a homozygous missense mutation (c.575C>T, p.T192M) in the DAG1 gene itself has been reported by Hara et al. [12]. The 16-year-old female patient was diagnosed with a limb-girdle muscular dystrophy with mental retardation and without structural brain malformation. Further in vitro and in vivo studies support the pathogenicity of the mutation confirming neuromuscular disease with muscular dystrophy, defective glycosylation of αDG and a marked reduction in αDG's ability to bind extracellular matrix components [12].
Here, we describe a Libyan family with two affected siblings suffering from a dystroglycanopathy resembling a MEB-like condition with the exceptional additional finding of extended bilateral multicystic white matter disease. This novel clinical phenotype is associated with a novel homozygous missense mutation in the DAG1 gene.
Materials and methods
Clinical data
Clinical evaluation was performed at the age of 2 years 8 months and 3 years 7 months, respectively. It included neurological and ophthalmological examination, electroencephalogram and measurement of creatine kinase values in both siblings. Magnetic resonance imaging of the brain (1,5 T Siemens Avanto, 12 channel head coil), nerve conduction studies and muscle biopsy were performed in the younger girl.
Muscle histology and immunohistochemistry
A muscle biopsy from vastus lateralis muscle was obtained. Standard stainings for histological analysis were prepared. For immunochemistry, unfixed 8-μm frozen sections were incubated with monoclonal antibodies to merosin (Novocastra; clone Mer3/22B2; diluted 1:100) and α-dystroglycan (Novocastra; clone VIA4-1; 1:100) for 1 h at room temperature, followed by a biotinylated rabbit anti-mouse antibody (Dako; 1:400) for 1 h. The reaction product was visualized using diaminobenzidine (Fluka) and H2O2 (Roth). Dilutions and washings were done with phosphate buffered saline. Examinations of the sections were performed with a Zeiss Axioskop microscope.
Linkage and sequencing analysis
Genetic analysis was performed with informed consent on genomic DNA extracted from blood leukocytes. Initially, linkage analysis was applied using closely linked microsatellite markers for known candidate loci (POMT1, POMT2, POMGnT1, FKTN, FKRP, LARGE and ISPD) and failed to identify any strong candidate loci.
For whole exome sequencing, 50 ng of isolated genomic DNA from the two affected sisters and the healthy mother was processed with the Nextera® Exome Enrichment Kit (Illumina, Inc., San Diego, CA, USA) according to the manufacturer's protocol. Library Quantification was carried out with the High Sensitivity DNA Kit on a Bioanalyzer (Agilent Technologies, Böblingen, Germany) and the Qubit™ dsDNA HS Assay Kit (Life Technologies, Darmstadt, Germany). The Library was sequenced as a 150-bp paired-end run on a MiSeq™ system with the MiSeq Reagent Kit v2 (Illumina, Inc., San Diego, CA, USA). Reads were aligned to the human reference genome (UCSC hg19, NCBI build 37.1) using the DNASTAR Lasergene® SeqMan Pro™ software, and variant detection was performed with DNASTAR ArrayStar® (DNASTAR Inc., Madison, WI, USA). Variants were selected in terms of unregistered variants (excluding registered SNPs), variants excluding synonymous changes and variants with an allele frequency of at least 90 % (assuming a homozygous mutation) and a minimum ten-fold coverage. Evaluation was focused on genomic variants homozygous in both affected daughters and heterozygous in the healthy mother, assuming autosomal recessive inheritance (Supplemental Figure 1).
Furthermore, the genetic data obtained by whole exome sequencing were in particular analyzed for sequence variations within additional genes known to be associated with congenital muscular dystrophies due to defective O-glycosylation (POMT1, POMT2, POMGnT1, FKTN, FKRP, LARGE, ISPD, GTDC2, B3GnT1, B3GALNT2 and TMEM5), similar dystroglycanopathy phenotypes (COL4A1, LAMA2, DPM2, DPM3 and SGK196) as well as cystic leucoencephalopathies (MLC1 and HEPACAM).
The identified DAG1 base substitution homozygous in both siblings and heterozygous in their mother was confirmed by direct partial sequencing of the coding region of amino acids p.622-p-820 of exon 2 (reference cDNA sequence NM004393.4) according to the manufacturer's recommendations using an ABI Prism Big-Dye Terminator Cycle Sequencing Kit version 3.1 and ABI 3100Dx XL Avant sequencer (Applied Biosystems, Foster City, CA, USA). Primer sequences are available on request.
Results
Clinical findings
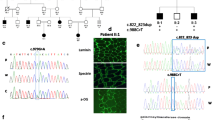
The two female siblings are the only children of Libyan, presumably non-consanguineous parents. Both pregnancies were uneventful resulting in normal vaginal deliveries at term. Antenatal ultrasound was not performed. The siblings had an identical course of disease with apparent onset of symptoms at the age of 4 months with developmental delay and general muscular hypotonia. Diagnostic work up at the age of 2 years 8 months and 3 years 7 months, respectively, showed severe mental retardation and muscular hypotonia without any ability to sit or walk (Table 1). While there was a lack of head control in the younger girl, the other sibling gained some limited ability to balance the head. They were both not able to speak. In the electroencephalography of the older girl, spike-waves discharges were detected, but none of the siblings needed antiepileptic therapy. Serum creatine kinase levels were approximately five-fold elevated (1,087 and 1,347 U/l). Ophthalmologic examinations revealed cataract, retinal dystrophy, severe myopia and buphthalmos, respectively. A nerve conduction study of the tibial nerve in the younger girl was normal.
Muscle histology and immunochemistry
Biopsy from vastus lateralis muscle showed muscle fibres of moderate size variability and some hypertrophic type 1 fibres (Fig. 1a). The diameter of the muscle fibres was 25 μm for type 1 fibres and 21 μm for type 2 fibres. Necrosis, phagocytosis or basophilia of myofibres were absent. There was no increase of internal myonuclei nor increase of endomysial connective tissue or inflammatory infiltrate. Immunohistochemical labelling for merosin appeared normal. However, no staining for αDG was detected (Fig. 1b), indicating either loss of αDG or deficient αDG glycosylation.
Brain MR imaging
Cerebral magnetic resonance imaging showed severe abnormalities compatible with the clinical diagnosis of muscle–eye–brain disease. Infratentorial structural changes included flattening and kinking of the pons and brainstem, subcortical cysts in the cerebellar hemispheres and a hypoplastic vermis (Fig. 2a, c). The supratentorial alterations consisted of migration defects resulting in a thin cortical layer resembling diffuse polymicrogyria with frontal agyria, moderate ventricular dilatation and a thinning of the corpus callosum (Fig. 2b). Moreover, severe white matter abnormalities with a diffusely swollen and abnormal white matter of high T2 intensity and multiple cysts (Fig. 3a, b) were found and to our knowledge have previously not been described in MEB or other dystroglycanopathies before. In addition to multiple large bilateral cystic lesions of a somewhat radial orientation in the entire frontal to parietal subcortical white matter with irregular and confluent shape, there were striking bilateral subcortical cysts anterior-temporal with a diameter of approximately 2.5 cm (Fig. 3b, c).
T2-weighted MR images (slice thickness 2 mm) of a 2-year-8-month-old girl. Thin corpus callosum and hypoplasia of pons and cerebellar vermis (a). Neuronal migration disorder resembling polymicrogyria and frontal agyria, diffusely swollen white matter with abnormal high signal intensity, moderate dilatation of the ventricles (b). Cerebellar abnormalities with a hypoplastic vermis and multiple subcortical cysts in the cerebellar hemispheres (c)
Multicystic leucodystrophy with large and confluent cysts of variable signal intensity probably due to variable protein content [4-mm fluid attenuated inversion recovery (FLAIR) images, a]. The cysts are localized in the subcortical white matter, mainly in the frontoparietal region (b). Bilateral large subcortical cysts anteriorly in the temporal lobe (b, c; 2-mm T2-weighted images). Note the thin optic nerve with wide perioptic subarachnoid space (c)
Genetic findings
Whole exome sequencing identified four variants homozygous in both patients and heterozygous in the mother (Supplemental Figure 1 and Table 2). Three of these variants appeared to represent single nucleotide polymorphisms based on their frequency in control cohorts, their annotation in the NCBI dbSNP database as well as in silico analysis by both SIFT and Polyphen2. Both siblings are homozygous for a base substitution c.2006G>T in exon 2 of the DAG1 gene, predicted to result in the amino acid substitution p.Cys669Phe (Fig. 4). This base substitution could not be found in any of the tested 52 control subjects nor has it been reported so far as mutation or polymorphism in any of the publicly available databases including HGMD (http://www.hgmd.cf.ac.uk), Leiden Open Variation Database 2.0 (http://www.lovd.nl/2.0/), NCBI dbSNP (http://www.ncbi.nlm.nih.gov/snp/) and PubMed (http://www.ncbi.nlm.nih.gov/pubmed/). This cysteine at position 669 is localized within the extracellular portion (amino acids p.654-749) of the β-dystroglycan domain (p.654-895) and evolutionary highly conserved down to Caenorhabditis elegans. In the Torpedo paralog, this cysteine at p.669 has been postulated to form a covalent disulphide bound with p.Cys713 within ß-dystroglycan important for the tertiary structure of ß-dystroglycan and thus most likely also for the function of the α- and ß-dystroglycan complex [13]. Disruption of this disulphide bond is predicted to cause an altered secondary structure of βDG substituting a helix structure in the wild-type protein by a beta-strand structure [protein prediction program PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred)]. In silico analysis using different algorithms including Polyphen and SIFT predicts this missense mutation to be probably damaging (score 1.0) and deleterious (score 0.00), respectively.
No additional functionally relevant heterozygous or homozygous sequence variations were observed in any of the coding sequences of the particularly evaluated 18 genes, previously associated with congenital dystroglycanopathies or cystic leucoencephalopathies (269 targets; 95 % of all targets were covered at least 10×; 83 % at least 20×). Causal mutations in 17 of these 18 genes were further excluded by heterozygous sequence variants and/or discordant haplotypes between both affected siblings. No heterozygous variants were detected within DPM3 with a coverage of at least 10× over the entire coding region.
Discussion
Current spectrum of dystroglycanopathies
Congenital muscular dystrophies with defective O-glycosylation of α-dystroglycan (dystroglycanopathies) are a heterogeneous group of autosomal recessive diseases with a broad clinical spectrum [3]. The clinical phenotype is characterized by a combination of muscular disease with functional and/or structural brain and eye involvement of variable degree. The phenotypic spectrum ranges from the most severe conditions, muscle–eye–brain disease (MEB) and Walker–Warburg syndrome (WWS), to mild cases of limb girdle muscular dystrophy (LGMD) without mental retardation. The clinical diagnosis of a dystroglycanopathy is confirmed by immunohistochemical detection of hypoglycosylated αDG in muscle biopsy using specific antibodies against glycolysated α-dystroglycan. While initially the clinically well-defined entities of WWS and MEB were considered to be associated with mutations in specific genes, recently a broader and overlapping genotype–phenotype correlation became widely accepted [3, 14]. In 2007, Godfrey et al. defined a clinical classification of dystroglycanopathies consisting of seven phenotypic categories dependent on onset and severity of muscle disease and further subcategorized by the degree of functional and structural brain involvement. The MEB/FCMD (Fukuyama congenital muscular dystrophy) subtype is characterized by brain abnormalities including pachygyria, polymicrogyria, cerebellar hypo- and dysplasia and frequent flattening of the pons and brainstem, eye involvement consisting of congenital glaucoma, myopia, retinal atrophy and/or juvenile cataracts as well as severe motor and cognitive impairments and can also be assigned to the siblings presented in this report [3, 14].
Homozygous mutations in genes of glycosyltransferases involved in post-translational O-glycosylation of αDG like POMT1, POMT2, POMGnT1 and LARGE or in genes of associated proteins of unknown function (Fukutin and FKRP) are well known causes of different types of dystroglycanopathies [5]. Since 2012, another five genes of involved putative enzymes were identified: homozygous mutations in ISPD, GDTC2, B3GNT1, B3GALNT2 and TMEM5 [6–11] were reported to interfere with the αDG glycosylation process leading to a WWS-phenotype in humans. Mutations in these 11 genes are referred to as secondary dystroglycanopathies [2, 12]. They are estimated to be responsible for approximately 50 % of diseases while the genetic aetiology of the other half of affected patients remains unsolved.
DAG1 mutations in human disorders
In 2011, Hara et al. reported the first patient and to date only patient with a homozygous mutation in the dystroglycan-encoding gene DAG1 itself (primary dystroglycanopathy) [12]. They found a homozygous missense mutation (c.C575T) leading to the amino acid change methionine to threonine at amino acid residue 192 (p.T192M) that affected a highly conserved residue in the N-terminal of the α-subunit of dystroglycan. The mutation could not be found in any of the tested 100 control subjects. Furthermore, in vitro and in vivo studies in a knock-in mouse model showed histological hallmarks of muscular dystrophy and hypoglycosylation of αDG leading to a marked reduction in αDG's ability to bind extracellular matrix proteins in the mutant mice. This female patient was diagnosed at the age of 16 years with a relatively mild LGMD with severe mental retardation and normal brain imaging (see Table 1). More recently, this sequence variation has been reported in the dbSNP database under the reference number rs193922955 so far without any information on population frequencies.
In a 16-year-old female with cognitive impairment, facial hypotonia, raised serum creatine kinase and subcortical white matter changes, a contiguous gene syndrome was confirmed by array-CGH resulting from a heterozygous de novo deletion of 1.9 Mb in chromosomal region 3p21.31 including the entire DAG1 gene [15]. However, in the absence of a second identified DAG1 mutation on the other allele, the immunohistochemical staining of αDG was normal, and the functional relevance of the observed mildly to moderately reduced dystroglycan transcripts (60 % of control, assessed by quantitative RT-PCR on extracted RNA from the patients muscle biopsy material) remains unclear.
The patients we report here are the offspring of unaffected, presumably non-consanguineous Libyan parents and have a considerably more severe clinical course with onset of symptoms at 4 months of age, compatible with the “MEB/FCMD-like” phenotype according to Godfrey [3]. At the age of 2 years 8 months and 3 years 7 months, respectively, they presented with severe mental and motor retardation and were unable to sit or walk. There was involvement of the eyes with complex malformations and signs of muscular dystrophy with significantly reduced immunohistochemical labelling for α-dystroglycan in muscle biopsy. Brain MRI showed severe malformations compatible with MEB. In addition, there were multiple cysts in the subcortical white matter possibly representing a novel cerebral phenotype of dystroglycanopathy.
Whole exome sequencing identified a homozygous missense mutation p.Cys669Phe in the extracellular portion of the β-subunit of dystroglycan affecting a highly conserved cysteine residue that is predicted to form a covalent intra-chain disulphide bond [13]. To our knowledge, it is the first reported β-dystroglycan mutation associated with a human phenotype.
DAG1 mutations in animal models
In 1997, the importance of dystroglycan in early embryonic development was demonstrated in dystroglycan null mice. The homozygous DAG-null mutation resulted in early embryonic lethality; the nulls exhibited severe abnormalities in early basement membrane formation [16]. Furthermore, selective deletion of CNS dystroglycan in mice produced cerebral cortex malformations resembling cobblestone lissencephaly typically found in dystroglycanopathies like MEB, FCMD and WWS [17]. However, deletion of only the C-terminal located cytoplasmatic domain of the β-subunit of dystroglycan did not interfere with normal cerebral cortex organization [18]. Therefore, the function of the dystroglycan complex during neuronal migration was postulated to critically depend on extracellular dystroglycan interactions. Cysteine at position p.669 is located within the N-terminal extracellular ßDG region, which together with the C-terminus of αDG has been recognized as the interphase between the two DG subunits [19]. Furthermore, the missense mutation p.Cys669Phe, reported in this paper, removes a cysteine residue postulated to form a critical covalent intra-chain disulphide bond within this extracellular portion of ßDG [13]. Disruption of this disulphide bond at position p.669 is predicted to causes a substantial change in the secondary structure of βDG which we propose to result in a critically disturbed interaction between βDG and αDG and a defectively glycosylated dystroglycan protein.
Knock-in mice bearing the only DAG1 mutation p.T192M described so far in a patient with mild manifestation of a LGMD before showed the hallmarks of muscular dystrophy in skeletal muscle while no structural brain malformation, but features of functional CNS impairment were evident [12].
Likewise, in zebrafish, the homozygous DAG1 missense mutation c.T1700A (p.V567D) was shown to result in muscle defects and abnormal eye and brain development reminiscent of human WWS, FCMD and MEB [20]. The mutation was considered to interfere with the interaction of the dystroglycan α- and β-subunit; in the mutant embryos, there was no detectable expression of αDG or βDG.
Selective disruption of αDG and βDG expression in Schwann cells has been show in mice to result in an age-dependent reduction of nerve conduction velocity [21]. Interestingly, in our patient, a normal nerve conduction velocity of the tibial nerve was found at the age of 2 years 8 months. This discrepancy might be explained by the young age at examination of our patient as conduction abnormalities in the mouse model worsened with age, and by the fact that the tibial nerve for unknown reasons was less severely affected in those mice than other nerves.
Neuroradiological brain involvement in dystroglycanopathies
Structural and functional involvement of the brain is a common feature in patients with dystroglycanopathies. Supratentorial as well as infratentorial structures can be affected, and the severe brain malformation can in fact be the clinical finding guiding the early diagnosis of a dystroglycanopathy, e.g. as prenatal sonographic diagnosis of Walker–Warburg syndrome. In a brain magnetic resonance imaging study of patients with dystroglycanopathy resulting from mutations in POMT1, POMT2, POMGnT1, Fukutin or LARGE, 15 of 27 had cortical abnormalities like polymicrogyria, pachygyria or cobblestone lissencephaly; 17 of 27 ventricular dilatation; 16 of 27 abnormalities of the pons and/or brainstem; and 17 of 27 cerebellar involvement with cysts, dysplasia or hypoplasia, respectively [22]. White matter changes were found in the MRI of 20 of 27 (74 %) patients consisting of diffuse or regionally (especially periventricular) pronounced abnormally high T2 signal intensity or reduced white matter volume in two cases. However, neither by Clement et al. [22] nor in any other article so far, supratentorial cysts in the white matter were described. In the cerebral MRI of our patient, there are marked large bilateral multicystic lesions in the subcortical white matter that are particularly pronounced in the frontal to parietal regions. In addition, subcortical cysts in the anterior temporal region are present. Appearance of the white matter lesions is different from white matter injuries in premature infants with cerebral palsy but shares some characteristic MRI features with megalencephalic leucoencephalopathy with subcortical cysts (MLC) related to MLC1 mutations [23]. Similar MRI findings in our patient are the diffusely abnormal and swollen white matter, the anterior temporal subcortical cysts and the clinical finding of macrocephaly. However, the clinical and brain phenotype is more severe in our siblings who in addition present with multiple large bilateral cysts throughout the entire frontal to parietal subcortical white matter. These lesions appear to have a radial orientation possibly corresponding to the radial neuronal migration during cortex formation. Furthermore, it is noteworthy that MEB-like cortical abnormalities, infratentorial CNS malformations, eye malformations and confirmed muscular dystrophy as found in our patient are not described as part of the MLC phenotypic spectrum.
Furthermore, the two genes MLC1 and HEPACAM so far associated with MLC in humans were covered with our whole exome sequencing approach and for both siblings did not reveal any sequence alterations, thus suggesting an additional and independent MLC phenotype. A possible functional link between white matter pathology in our patients and MLC might be provided by co-localization and interference of the MLC1 gene product with proteins of the dystrophin–glycoprotein complex (DGC) in astrocytes of human brain tissue [24]. Additionally, the importance of dystroglycan expression in the radially oriented glia for brain development has been shown before in mice and might in fact explain the seemingly radial orientation of the cerebral cysts in the sisters of this report [18].
Interestingly, there are marked similarities of the MRI white matter changes observed in our patients with the findings described in congenital muscular dystrophy (CMD) with merosin deficiency (CMD1A). CMD1A results from mutations in the LAMA2 gene encoding laminin-α2, a chain of merosin [25]. CMD1A patients present with a muscle dystrophy phenotype with elevated creatine kinase, dystrophic appearance in muscle biopsy with partial or complete laminin-α2 deficiency in immunohistochemistry as well as bilateral increase in T2 signal intensity in the white matter of the cerebral hemispheres [26, 27]. Few patients were reported with associated cortical malformation, especially occipital pachygyria and agyria [27]; in those, the presence of these cortical malformations correlated with cognitive impairment and seizures. On a molecular basis, the high affinity binding of glycosylated αDG to extracellular laminin is well defined [1], and disruption of this interaction is considered to play a crucial role in the pathogenesis of dystroglycanopathies [5].
In summary, we present here for the first time a more complex MEB-like dystroglycanopathy with extended bilateral multicystic leucodystrophy associated with a novel homozygous missense mutation in the β-subunit of the DAG1 gene. Our findings expand the genotypic and phenotypic spectrum of dystroglycanopathies in humans and support the central role of dystroglycan itself in their pathogenesis. The unexpectedly low number of patients with causal DAG1 mutations reported so far might point to a more severe and perhaps different phenotypic spectrum of other DAG1 mutations with more severe functional consequences.
References
Barresi R, Campbell KP (2006) Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci 119(Pt 2):199–207
Muntoni F, Torelli S, Wells DJ, Brown SC (2011) Muscular dystrophies due to glycosylation defects: diagnosis and therapeutic strategies. Curr Opin Neurol 24(5):437–42
Godfrey C, Clement E, Mein R, Brockington M, Smith J, Talim B, Straub V, Robb S, Quinlivan R, Feng L, Jimenez-Mallebrera C, Mercuri E, Manzur AY, Kinali M, Torelli S, Brown SC, Sewry CA, Bushby K, Topaloglu H, North K, Abbs S, Muntoni F (2007) Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain 130(Pt 10):2725–35
Cormand B, Pihko H, Bayés M, Valanne L, Santavuori P, Talim B, Gershoni-Baruch R, Ahmad A, van Bokhoven H, Brunner HG, Voit T, Topaloglu H, Dobyns WB, Lehesjoki AE (2001) Clinical and genetic distinction between Walker-Warburg syndrome and muscle-eye-brain disease. Neurology 56(8):1059–69
Wells L (2013) The o-mannosylation pathway: glycosyltransferases and proteins implicated in congenital muscular dystrophy. J Biol Chem 288(10):6930–5
Buysse K, Riemersma M, Powell G, van Reeuwijk J, Chitayat D, Roscioli T, Kamsteeg EJ, van den Elzen C, van Beusekom E, Blaser S, Babul-Hirji R, Halliday W, Wright GJ, Stemple DL, Lin YY, Lefeber DJ, van Bokhoven H (2013) Missense mutations in β-1,3-N-acetylglucosaminyltransferase 1 (B3GNT1) cause Walker-Warburg syndrome. Hum Mol Genet 22(9):1746–54
Roscioli T, Kamsteeg EJ, Buysse K, Maystadt I, van Reeuwijk J, van den Elzen C, van Beusekom E, Riemersma M, Pfundt R, Vissers LE, Schraders M, Altunoglu U, Buckley MF, Brunner HG, Grisart B, Zhou H, Veltman JA, Gilissen C, Mancini GM, Delrée P, Willemsen MA, Ramadža DP, Chitayat D, Bennett C, Sheridan E, Peeters EA, Tan-Sindhunata GM, de Die-Smulders CE, Devriendt K, Kayserili H, El-Hashash OA, Stemple DL, Lefeber DJ, Lin YY, van Bokhoven H (2012) Mutations in ISPD cause Walker-Warburg syndrome and defective glycosylation of α-dystroglycan. Nat Genet 44(5):581–5
Willer T, Lee H, Lommel M, Yoshida-Moriguchi T, de Bernabe DB, Venzke D, Cirak S, Schachter H, Vajsar J, Voit T, Muntoni F, Loder AS, Dobyns WB, Winder TL, Strahl S, Mathews KD, Nelson SF, Moore SA, Campbell KP (2012) ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker-Warburg syndrome. Nat Genet 44(5):575–80
Manzini MC, Tambunan DE, Hill RS, Yu TW, Maynard TM, Heinzen EL, Shianna KV, Stevens CR, Partlow JN, Barry BJ, Rodriguez J, Gupta VA, Al-Qudah AK, Eyaid WM, Friedman JM, Salih MA, Clark R, Moroni I, Mora M, Beggs AH, Gabriel SB, Walsh CA (2012) Exome sequencing and functional validation in zebrafish identify GTDC2 mutations as a cause of Walker-Warburg syndrome. Am J Hum Genet 91(3):541–7
Stevens E, Carss KJ, Cirak S, Foley AR, Torelli S, Willer T, Tambunan DE, Yau S, Brodd L, Sewry CA, Feng L, Haliloglu G, Orhan D, Dobyns WB, Enns GM, Manning M, Krause A, Salih MA, Walsh CA, Hurles M, Campbell KP, Manzini MC; UK10K Consortium, Stemple D, Lin YY, Muntoni F (2013) Mutations in B3GALNT2 cause congenital muscular dystrophy and hypoglycosylation of α-dystroglycan. Am J Hum Genet 92(3):354–65
Jae LT, Raaben M, Riemersma M, van Beusekom E, Blomen VA, Velds A, Kerkhoven RM, Carette JE, Topaloglu H, Meinecke P, Wessels MW, Lefeber DJ, Whelan SP, van Bokhoven H, Brummelkamp TR (2013) Deciphering the glycosylome of dystroglycanopathies using haploid screens for lassa virus entry. Science 340(6131):479–83
Hara Y, Balci-Hayta B, Yoshida-Moriguchi T, Kanagawa M, Beltrán-Valero de Bernabé D, Gündeşli H, Willer T, Satz JS, Crawford RW, Burden SJ, Kunz S, Oldstone MB, Accardi A, Talim B, Muntoni F, Topaloğlu H, Dinçer P, Campbell KP (2011) A dystroglycan mutation associated with limb-girdle muscular dystrophy. N Engl J Med 364(10):939–46
Deyst KA, Bowe MA, Leszyk JD, Fallon JR (1995) The alpha-dystroglycan-beta-dystroglycan complex. Membrane organization and relationship to an agrin receptor. J Biol Chem 270(43):25956–9
Hehr U, Uyanik G, Gross C, Walter MC, Bohring A, Cohen M, Oehl-Jaschkowitz B, Bird LM, Shamdeen GM, Bogdahn U, Schuierer G, Topaloglu H, Aigner L, Lochmüller H, Winkler J (2007) Novel POMGnT1 mutations define broader phenotypic spectrum of muscle-eye-brain disease. Neurogenetics 8(4):279–88
Frost AR, Böhm SV, Sewduth RN, Josifova D, Ogilvie CM, Izatt L, Roberts RG (2010) Heterozygous deletion of a 2-Mb region including the dystroglycan gene in a patient with mild myopathy, facial hypotonia, oral-motor dyspraxia and white matter abnormalities. Eur J Hum Genet 18(7):852–5
Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP (1997) Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum Mol Genet 6(6):831–41
Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP (2002) Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418(6896):422–5
Satz JS, Ostendorf AP, Hou S, Turner A, Kusano H, Lee JC, Turk R, Nguyen H, Ross-Barta SE, Westra S, Hoshi T, Moore SA, Campbell KP (2010) Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J Neurosci 30(43):14560–72
Sciandra F, Bozzi M, Morlacchi S, Galtieri A, Giardina B, Brancaccio A (2009) Mutagenesis at the alpha-beta interface impairs the cleavage of the dystroglycan precursor. FEBS J 276(17):4933–45
Gupta V, Kawahara G, Gundry SR, Chen AT, Lencer WI, Zhou Y, Zon LI, Kunkel LM, Beggs AH (2011) The zebrafish dag1 mutant: a novel genetic model for dystroglycanopathies. Hum Mol Genet 20(9):1712–25
Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, Brophy PJ, Schmelzer JD, Low PA, Wrabetz L, Feltri ML, Campbell KP (2003) Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron 38(5):747–58
Clement E, Mercuri E, Godfrey C, Smith J, Robb S, Kinali M, Straub V, Bushby K, Manzur A, Talim B, Cowan F, Quinlivan R, Klein A, Longman C, McWilliam R, Topaloglu H, Mein R, Abbs S, North K, Barkovich AJ, Rutherford M, Muntoni F (2008) Brain involvement in muscular dystrophies with defective dystroglycan glycosylation. Ann Neurol 64(5):573–82
van der Knaap MS, Boor I, Estévez R (2012) Megalencephalic leukoencephalopathy with subcortical cysts: chronic white matter oedema due to a defect in brain ion and water homoeostasis. Lancet Neurol 11(11):973–85
Boor I, Nagtegaal M, Kamphorst W, van der Valk P, Pronk JC, van Horssen J, Dinopoulos A, Bove KE, Pascual-Castroviejo I, Muntoni F, Estévez R, Scheper GC, van der Knaap MS (2007) MLC1 is associated with the dystrophin-glycoprotein complex at astrocytic endfeet. Acta Neuropathol 114(4):403–10
Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tomé FM, Schwartz K, Fardeau M, Tryggvason K et al (1995) Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet 11(2):216–8
Gavassini BF, Carboni N, Nielsen JE, Danielsen ER, Thomsen C, Svenstrup K, Bello L, Maioli MA, Marrosu G, Ticca AF, Mura M, Marrosu MG, Soraru G, Angelini C, Vissing J, Pegoraro E (2011) Clinical and molecular characterization of limb-girdle muscular dystrophy due to LAMA2 mutations. Muscle Nerve 44(5):703–9
Geranmayeh F, Clement E, Feng LH, Sewry C, Pagan J, Mein R, Abbs S, Brueton L, Childs AM, Jungbluth H, De Goede CG, Lynch B, Lin JP, Chow G, Sousa CD, O'Mahony O, Majumdar A, Straub V, Bushby K, Muntoni F (2010) Genotype-phenotype correlation in a large population of muscular dystrophy patients with LAMA2 mutations. Neuromuscul Disord 20(4):241–50
Acknowledgements
The authors wish to thank the family for their participation in this study and M. Becher and F. Müller for excellent technical assistance. The authors declare that the study complies with the laws of the country in which it was performed.
Conflict of interest
The authors declare that they do not have a conflict of interest.
Electronic supplementary material
Author information
Authors and Affiliations
Corresponding author
Additional information
Tobias Geis and Klaus Marquard contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPT 100 kb)
Rights and permissions
About this article
Cite this article
Geis, T., Marquard, K., Rödl, T. et al. Homozygous dystroglycan mutation associated with a novel muscle–eye–brain disease-like phenotype with multicystic leucodystrophy. Neurogenetics 14, 205–213 (2013). https://doi.org/10.1007/s10048-013-0374-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-013-0374-9