Abstract
Muscle–eye–brain disease (MEB, OMIM 253280) is an autosomal recessive disorder characterized by a distinct triad of congenital muscular dystrophy, structural eye abnormalities, and cobblestone lissencephaly. Clinically, MEB patients present with early onset muscular hypotonia, severely compromised motor development, and mental retardation. Magnetic resonance imaging reveals a lissencephaly type II with hypoplasia of the brainstem and cerebellum. MEB is associated with mutations in the gene for protein O-mannose beta-1,2-N-acetylglucosaminyltransferase (POMGnT1, OMIM 606822). In this paper, we report the clinical findings of nine MEB patients from eight families. Eight of the nine patients presented typical features of MEB. However, a broad phenotypic variability was observed, ranging from two patients with severe autistic features to another patient with an unusually mild phenotype, initially diagnosed as congenital muscular dystrophy. Furthermore, severe hydrocephalus was reported in two families during a previous pregnancy, emphasizing the phenotypic overlap with Walker–Warburg syndrome. In addition to three previously reported mutations, we identified six novel POMGnT1 mutations (one missense, five truncating) in the present patient cohort. Our data suggest mutational hotspots within the minimal catalytic domain at arginine residue 442 (exon 16) and in intron 17. It is interesting to note that all mutations analyzed so far result in a complete loss of enzyme activity. Therefore, we conclude that the type and position of the POMGnT1 mutations are not of predictive value for the clinical severity. This supports the notion that additional environmental and/or genetic factors may contribute to the observed broad spectrum of POMGnT1-associated phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Defects in O-glycosylation of alpha-dystroglycan have recently been associated with a spectrum of autosomal recessive congenital disorders affecting both muscle function and the development of the central nervous system (CNS). O-Glycosylation results in the attachment of the tetrasaccharide NeuAc2–3Gal–4GlcNAc–2Man to alpha-dystroglycan [1]. The protein O-mannose beta-1,2-N-acetylglucosaminyltransferase (POMGnT1) catalyzes the transfer of an N-acetylglucosamine (GlcNAc) residue to the protein-bound mannose, whereas the protein O-mannosyltransferases 1 and 2 (POMT1, POMT2) are required for the attachment of the tetrasaccharides to alpha-dystroglycan. Additional enzymes likely to be involved in further extension of the tetrasaccharide chain are not yet identified; however, Fukutin and Fukutin-related protein (FKRP) are considered as potential candidates [1].
POMGnT1 mutations (OMIM 606822) result in muscle–eye–brain disease (MEB, OMIM 253280) [2] and have also occasionally been observed in patients with the severe form of Fukuyama-type congenital muscular dystrophy (FCMD, OMIM 253800) [3] or a mild form of Walker–Warburg syndrome (WWS, OMIM 236670) [3]. In addition, POMT1 and POMT2 mutations (POMT1, OMIM 607423; POMT2, OMIM 607439) have been associated with WWS [4, 5], while mutations in the Fukutin gene (OMIM 607440) result in the classical FCMD phenotype [6] and have also been associated with WWS-like phenotypes [7].
MEB was initially described in Finnish patients with an estimated incidence of 1:50,000 [8]. Patients with MEB present with a distinct triad of congenital muscular dystrophy, ocular abnormalities, and cobblestone lissencephaly (lissencephaly type II). Congenital muscular dystrophy usually results in a generalized early onset hypotonia with elevated serum creatine kinase (CK) levels. Muscle biopsies reveal dystrophic changes with reduced or absent glycosylation of alpha-dystroglycan [9]. During the course of the disease, the initially hypotonic patients develop severe spasticity and contractures [10].
A wide spectrum of ophthalmologic abnormalities resulting in severe visual impairment has been observed and includes progressive myopia, retinal degeneration, congenital glaucoma, and cataracts. Giant visual evoked potentials have been described as an important diagnostic feature [11].
Magnetic resonance (MR) imaging reveals several characteristic features such as cobblestone lissencephaly (lissencephaly type II) and hydrocephalus associated with a flat and kinking brainstem, mild cerebellar hypoplasia, and cysts [12]. Typically, both psychomotor and mental development is severely compromised, and seizures are commonly observed [10].
Thus far, 28 different POMGnT1 mutations have been identified (see Table 1). In Finnish patients, a common founder mutation in intron 17 (c.1539+1G>A) was found in 37 of 38 analyzed alleles from 14 families [8]. The clinical spectrum varies widely among MEB patients with identified POMGnT1 mutations [3]. The latter study proposed a genotype–phenotype correlation with more severe cerebral malformations being associated with POMGnT1 mutations near the 5′ terminus. In contrast, no obvious correlation was observed in regard to the severity of the muscular dystrophy.
In this paper, we present the clinical, neuroradiological, and molecular genetic findings in nine MEB patients from eight independent families of various ethnic origins (German, Turkish, and English). Six novel POMGnT1 mutations are reported (Tables 1 and 2). The goals of the present study were (1) to further characterize the spectrum of identified human POMGnT1 mutations and (2) to analyze our patient cohort for a genotype–phenotype correlation.
Materials and methods
Clinical evaluation included neurological and ophthalmologic examination, electroencephalogram, repeated measurement of CK values, and assessment of the psychomotor development. For three patients, a muscle biopsy and immunohistochemical evaluation has been performed. T1- and T2-weighted cerebral MR images were provided from different institutions with variable sequence protocols and evaluated by a neuroradiologist blinded to the genetic data. Evaluation included the following structures (criteria): cortex (thickness, pachygyria, agyria, polymicrogyria, interhemispheric fusion), white matter (gray–white boundary, extent of myelinization), ventricular system, corpus callosum (hypoplasia, dysgenesis), brainstem (hypoplasia, kinking), and cerebellum (hypoplasia, cystic formation). Peripheral blood samples were obtained after informed consent from the parents. Deoxyribonucleic acid was extracted from ethylenediamine tetraacetic acid blood using standard protocols. The 21 coding exons (exons 2 to 22) and the intron–exon boundaries of the POMGnT1 gene were amplified by polymerase chain reaction (PCR). PCR products were cycle sequenced using the ABI Prism BigDye Terminator Cycle Sequencing Kit (version 1.1) and analyzed on an ABI 3100 Avant sequencer (Applied Biosystems, Foster City, CA). Mutations were confirmed by restriction enzyme digestion when possible or by sequencing of a second independent PCR product. PCR products of closely linked polymorphic markers D1S2797, D1S451, and D1S3175 were amplified for linkage analysis using FAM-labeled primers and separated on an ABI 3100 Avant sequencer (Applied Biosystems).
Results
Clinical findings
Eight of the nine analyzed patients were referred for molecular testing with clinical signs of MEB. In the present study, the age at the time of clinical examination varied between 3 months and 16 years (Table 2). For seven of these eight MEB patients, clinical data are available on psychomotor and mental development (age range = 10 months to 16 years), uniformly indicating moderate to severe global retardation. For example, patient 6 had not even obtained head control up to the age of 6 years, while patients 4, 5, and 7 were reported to sit with support between 1 and 2 years. Patient 5 walked a few steps with assistance at the age of 15 years and spoke some words. Seizures were reported in seven patients. The onset of seizures was observed in five of these seven cases within the first year, while no seizures were present in patients 4 and 6 up to the age of 4 or 6 years, respectively (Table 2). Patients 3 and 5 showed severe autistic behavioral changes in the second decade of life [13]. All patients presented a severe muscular hypotonia at birth. CK values were markedly elevated in eight patients (range 430–2,700 U/l), while patient 6 at the age of 4 months showed CK values within normal range despite characteristic clinical features of MEB. The clinical symptoms of four of these MEB patients (patients 2a and b, 5, 7) have previously been described [13, 14].
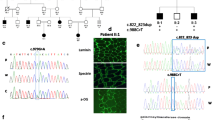
A congenital muscular dystrophy was diagnosed in patient 8 associated with mild mental retardation and psychomotor developmental delay. Muscle biopsy of the tibialis anterior muscle revealed degenerative myopathy. Immunohistochemistry showed reduced staining for merosin and alpha-dystroglycan (Fig. 1a–c), while staining for dystrophin, adhalin, dysferlin, caveolin, and collagen-6 were found within normal range. Immunoblotting for merosin and alpha-dystroglycan showed reduced bands with normal molecular weight (data not shown). White matter changes were initially described as symmetric leukodystrophy with normal myelinization matching the age of the patient, without affecting the corpus callosum or basal ganglia (Fig. 1d, e). Furthermore, discrete polymicrogyria of the frontal cortex, enlarged ventricles, and multiple cerebellar cysts were detected (Fig. 1d, e).
Muscle biopsy of the tibialis anterior muscle revealed a degenerative myopathy with variation of muscle fiber size and increased endomysial connective tissue (a, H&E 1:100). Immunohistochemistry showed mildly reduced staining for merosin (b) and markedly reduced staining for alpha-dystroglycan (c). T1-weighted MR images revealed a bilateral, frontal, and occipital pronounced polymicrogyria with a central leukodystrophy (d) with multiple cerebellar cysts in the T2-weighted MR images (e). Mutation analysis confirmed this patient to be compound heterozygous for the truncating mutation R580X in exon 20 (f) and the missense mutation R605H in exon 21 (g; wild type in upper lane).
Non-CNS associated features included an anorectal atresia in patient 2a and gallbladder hydrops in patient 2b, neither of which have been associated previously with MEB. However, congenital absence of the gallbladder has been noted in one patient with WWS [15].
Ophthalmologic findings
A wide variety of ocular abnormalities were reported in all nine patients resulting in severe visual impairment. The most frequent finding was a severe myopia (n = 6) during early development. Congenital structural eye abnormalities included: hypoplasia/dysplasia or atrophy of the optic nerve (n = 5), macula (n = 2), papilla (n = 4) or chorioidea (n = 1), cataract (n = 3), coloboma (n = 2), and microphthalmus (n = 1).
Structural cerebral findings (ultrasound, MR imaging)
Prenatal ultrasound data are available for five cases revealing a hydrocephalus in four of them (patients 2a, 3, 4, and 6) as well as in a previous pregnancy of families 3 and 4. In patient 1, repeated sonographic examinations were reported without any structural abnormalities.
Cerebral MR images were available for seven cases. Neuroradiological evaluation revealed as the most prominent and consistent feature in all patients marked alterations of the white matter as well as a hypoplasia of the brainstem with a characteristic flattening and kinking of the pons (Fig. 2b,d). In addition, all patients had a migration defect of variable extent with predominant pachygyria of the frontoparietal cortex and occipital polymicrogyria (Figs. 1d, 2a, c, and 3c). All patients had hydrocephalic changes of varying degree (Figs. 1d, 2a, c, and 3c), partially because of stenosis of the aqueduct (patients 2a and 2b in [14]). Furthermore, a thinning of the corpus callosum was a common feature in this patient cohort (Figs. 2b and 3d). A Dandy–Walker malformation was observed in three cases. Cerebellar cysts were detected in three cases only (patients 3, 5, and 8; Fig. 1e). Two of our index patients (2a and 2b) presented with a complex brain malformation including absence of the septum pellucidum and small chiasma, consistent with the diagnosis of septo-optic dysplasia. In addition, the hippocampus and hypophysis appeared to be malformed in both cases (patients 2a and 2b in [14]).
T1-weighted MR images of patient 1 (heterozygous POMGnT1 mutations c.593delG in exon 7 and c.1539+1G>A in intron 17) shows moderate hydrocephalus with frontal pachygyria and occipital polymicrogyria with partially confluent white matter lesions (a) as well as moderate hypoplasia of the pons with a kinking of the brainstem and the cerebellum (b). T2-weighted MR images of patient 3 with shunt implantation (homozygous POMGnT1 missense mutation R442C in exon 16) depict also moderate frontal pachygyria, occipital polymicrogyria (c), and moderate pontine hypoplasia. Note severe thickening of the diploe in particular frontal and occipital (d)
Genetic findings
We identified three previously reported and six novel POMGnT1 mutations (Tables 1 and 2). Three patients of German descent (patients 2a, 2b, and 3) were found to be homozygous for the previously reported POMGnT1 missense mutation Arg442Cys (ESM Fig. 1a and b). Both families are living in the same German midwest area. To assess the possibility of a founder mutation, we analyzed intragenic single-nucleotide polymorphisms and three closely linked polymorphic markers. All three patients are homozygous for ten intragenic sequence variants commonly observed as a conserved haplotype in our patient population and for a D1S3175 allele of identical size (approximately 0.67 Mb distal to POMGnT1). These data may be compatible with a common founder allele in this German region. However, both families are discordant and/or heterozygous in regard to the allele size of the neighboring markers D1S451 and D1S2797, approximately 0.93 Mb distal or 0.27 Mb proximal to POMGnT1, respectively. In addition, homozygosity for a novel arginine to histidine substitution at the same codon 442 (Arg442His) was observed in a Turkish patient of consanguineous parents (patient 7; ESM Fig. 1a and c).
One patient from Southern California (English and German ancestry) was found to be heterozygous for the Finnish founder mutation c.1539+1G>A (patient 1). Furthermore, a novel splice site mutation affecting the splice acceptor site of intron 17 (c.1540−2A>G) was observed in the homozygous state in a Turkish patient from a consanguineous marriage (patient 5; ESM Fig. 1d). Because adenine is 100% conserved at this position of mammalian acceptor splice sites, altered splicing of intron 17 and/or the neighboring exons can be expected.
For the first time, we report a heterozygous truncating mutation in exon 2 (c.25_26insC; Fig. 3a) together with a novel heterozygous truncating mutation in exon 7 (c.593delG; Fig. 3b) in a 2-year-old German boy (patient 4) with spastic paraparesis, moderate hydrocephalus, and polymicrogyria (Fig. 3c). In this family, a previous pregnancy was terminated at 22 weeks postconception because of a severe fetal hydrocephalus. The c.593delG mutation was also observed in the heterozygous state in patient 1 from Southern California (Fig. 2a, b). Congenital hydrocephalus was also noted in two pregnancies of family 3 (patient 3 and fetus; Fig. 2c), homozygous for the previously reported POMGnT1 mutation Arg442Cys (exon 16; ESM Fig. 1a and b). The neuropathological autopsy of this fetus revealed agyria, massively enlarged ventricles, and agenesis of the corpus callosum. At the same position, a homozygous POMGnT1 missense mutation Arg442His in patient 7 was also associated with severe hydrocephalus [13] (ESM Fig. 1a and c). The 8-year-old Turkish patient of consanguineous parents (patient 6) showed classical MEB associated with a homozygous 5-bp deletion c.1350_1354delCTGGG within exon 16 (ESM Fig. 1e). Despite the location of the mutation in the 3′ half of the gene, he also presented with marked hydrocephalus.
a Patient 4, heterozygous for two frameshift mutations, c.25_26insC in exon 2 and b c.593delG in exon 7 (wild type sequence in upper lane), presented with moderate hydrocephalus, moderate frontoparietal pachygyria and occipital polymicrogyria, and discrete periventricular white matter lesions (c; T1-weighted MR images) and severe hypoplasia (flattening and kinking) of the brainstem with a Dandy–Walker malformation (d; T2-weighted MR images).
Patient 8 with congenital muscular dystrophy was found to be compound heterozygous for the nonsense mutation Arg580X in exon 20 (Fig. 1f) and the missense mutation Arg605His (Fig. 1g) in exon 21, both mutations being located at the 3′ end of the POMGnT1 gene.
Discussion
Current status of POMGnT1 mutations
Including this report, a total of 34 different mutations distributed across the entire POMGnT1 gene have now been reported in MEB patients (Table 1). Twenty-three are predicted to result in a premature protein truncation, the remaining 11 being missense mutations located at highly conserved amino acid positions and/or within the putative catalytic domain.
The base substitution c.1539 + 1G>A at the splice donor site in intron 17 was first identified as a Finnish founder mutation and should affect the minimal catalytic domain in the C-terminal half of the protein [18]. It has also been observed in patients from Turkey, Belgium, and in North America [2, 3, 8]. The functional relevance of this recurrent intron 17 splice site mutation had been analyzed in human skeletal muscle [2]. Reverse transcriptase PCR revealed the generation of two different messenger ribonucleic acids (mRNAs), one read-through of the sequences of intron 17 with introduction of a premature termination codon at position 484. The second transcript lacked the upstream exon 17, resulting in an in-frame deletion of 42 amino acids. Both mRNAs were demonstrated to result in POMGnT1 mutants with a residual enzyme activity below 1% when compared to the wild-type protein [2]. In our cohort, this mutation was also observed once (patient 1). We now report a novel base substitution at the acceptor site of intron 17 (c.1540−2A>G) in the homozygous state in a Turkish patient (patient 5; ESM Fig. 1d) with an MEB phenotype associated with severe autistic features [13].
Wide genetic and phenotypic variability of disorders associated with defects in O-glycosylation of alpha-dystroglycan
MEB, WWS, and FCMD have been linked to defects in a family of genes, known to be involved in the O-glycosylation of alpha-dystroglycan. Mutations in all six genes known thus far (POMT1, POMT2, POMGnT1, Fukutin, FKRP, and LARGE) may result in the entire spectrum of these autosomal recessive disorders [19]. Furthermore, MEB shares clinical features with FCMD as well as with WWS. Patients with WWS present more severe cerebral malformations and die usually within the first years of life. In contrast to POMGnT1, recent data suggest a clear genotype–phenotype correlation for patients with POMT1 mutations: The severe WWS phenotype is caused by loss of function mutations, while the presence of one or two mutations conferring some residual POMT1 activity results in a spectrum of milder phenotypes including congenital muscular dystrophy with mental retardation (LGMD2K) without any obvious structural CNS defects [20, 21].
In addition, a wide overall clinical variability has been observed in 18 patients homozygous for the Finnish POMGnT1 founder mutation, although no particular reference was made to the cerebral malformations [8]. Likewise, two Finnish siblings with identical POMGnT1 genotype presented with similar clinical findings but strikingly different neurodevelopmental impairment [16]. Similarly, we also observed a broad variability within one family (family 4). Patient 4, heterozygous for novel POMGnT1 frameshift mutations in exons 2 and 7 (Fig. 3a, b), showed a moderate hydrocephalus (Fig. 3c). At the age of 4 years and 6 months, he is able to walk up to 300 m with aid, to eat and drink independently, is very social, cheerful, and can vocalize basic needs. In contrast, a previous pregnancy of this family was terminated during the second trimester because of a severe hydrocephalus, resembling a WWS phenotype.
Neuroradiological characteristics of MEB
Two studies describe in detail the cerebral MR imaging findings of clinically diagnosed MEB patients from Finland, probably related to the Finnish founder mutation c.1539 + 1G>A [10]. Based on a cohort of 20 patients, the following common features were noted: cortical pachygyric dysplasia combined with defects of the septum pellucidum and corpus callosum, as well as hypoplasia of the pons with no significant alterations in the gray–white matter relations. The initial Finnish study, however, noted also white matter abnormalities in the periventricular and deep white matter [12]. The absence of the pontine bulge as well as cerebellar hypoplasia is a prominent feature in this Finnish MEB population without definitive genetic diagnosis. In our POMGnT1 mutation-confirmed patients, we observed the combination of brainstem kinking with cortical polymicrogyria as well as white matter lesions with hydrocephalus of varying degree as the most frequent and characteristic neuroradiological hallmarks. The presence of brainstem kinking with pontine hypoplasia together with two of the above-described neuroradiological criteria should prompt a focused neurogenetic diagnostic workup for POMGnT1 mutations and, if negative, genetic testing of additional genes involved in O-glycosylation of alpha-dystroglycan.
Muscle findings and protein function
POMGnT1 catalyzes the second glycosyl transfer step in the biosynthesis of mammalian O-mannosyl glycans [2]. O-mannosylation of alpha-dystroglycan is required for the binding between alpha-dystroglycan and laminin [22]. Immunoreactivity to the glycans of alpha-dystroglycan has been demonstrated to be undetectable in skeletal muscle from patients with MEB as well as FCMD [9, 23]. In patients with POMGnT1 mutations, the immunoreactivity for alpha-dystroglycan in skeletal muscle biopsies did not correlate with the location of the mutation [3]. Muscle biopsies of the typical MEB patients 5 and 7 revealed myopathic changes with a reduction in glycosylated epitopes for alpha-dystroglycan [13]. Our patient 8 also presents characteristic changes in the muscle biopsy (Fig. 1a–c) despite a strikingly mild cerebral phenotype resulting from compound heterozygosity for a novel nonsense mutation Arg580X in exon 20 and a novel missense mutation Arg605His in exon 21 (Fig. 1d–g).
Knockout mouse
In contrast to the embryonic lethal homozygous knockout (KO) mice for POMT1 or fukutin [24, 25], the KO mouse homozygous for a POMGnT1 null mutation is viable and presents a complex developmental disorder closely resembling MEB [26]. The cerebellum is smaller with histological evidence of a cerebellar migration defect. The cerebral cortex shows an abnormal distribution of neural cells with overmigration of cortical neurons into layer I and the leptomeninges resembling lissencephaly type II. Hydrocephalus was seen in four out of seven KO mice indicating that this is a characteristic but not obligate feature in the presence of a POMGnT1 homozygous null mutation. Considering the identical genetic background of transgenic KO mice, the development of hydrocephalus seems to be modified by additional environmental factors. It is interesting to note that fusion of both cerebral hemispheres was observed in these homozygous KO mice. Such interhemispheric fusion has also been described as a characteristic feature in patients with lissencephaly type II [27] but was not present in our MEB probands with confirmed POMGnT1 mutations.
POMGnT1 mutations lead to loss of function
Two assay systems have been established to determine residual POMGnT1 enzyme activity. In particular, functional analysis of mutant constructs in a cell-based assay system revealed complete loss of POMGnT1 enzyme activity for ten truncating mutations as well as three missense mutations [28] (Table 1). In addition, the reduction in POMGnT1 activity by about 93% was detected in frozen muscle biopsies of three MEB patients [17] (Table 1). Severely reduced POMGnT1 enzyme activity can also be assumed for the three novel frameshift POMGnT1 mutations (c.25_26insC, c.593delG, c.1350_1354delCTGGG) and the nonsense mutation Arg580X described in our patients 1, 4, 6, and 8.
A correlation between the location of the mutation and the severity of the cerebral malformation has been proposed. In a previous study, patients harboring mutations near the 5′ terminus showed more severe cerebral malformations, in particular hydrocephalus, when compared to patients with mutations near the 3′ terminus of the gene [3]. In the present study, only one patient followed this proposed association: patient 8, a compound heterozygote for mutations in exons 20 and 21, showed a mild cerebral malformation (Fig. 1d–g). However, in conjunction with the previous functional studies on enzyme activity, our data of the other MEB patients argue against this previously suggested model, as truncating and missense mutations throughout the entire POMGnT1 gene uniformly result in complete loss of POMGnT1 enzyme activity [28]. For instance, we demonstrate one patient with moderate hydrocephalus and two POMGnT1 mutations at the 5′ end (patient 4; Fig. 3). Furthermore, a moderate to severe hydrocephalus appears to be associated with homozygous missense mutations at codon 442 in exon 16 within the minimal catalytic domain (patients 2a, 2b, 3, 7) [16]. These data suggest an important role of this highly conserved arginine residue at position 442. In combination with the described clinical variability and POMGnT1 enzyme activity, we assert there is no genotype–phenotype correlation for POMGnT1-confirmed MEB patients.
References
Lehle L, Strahl S, Tanner W (2006) Protein glycosylation, conserved from yeast to man: a model organism helps elucidate congenital human diseases. Angew Chem Int Ed Engl 45:6802–6818
Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, Herrmann R, Straub V, Talim B, Voit T, Topaloglu H, Toda T, Endo T (2001) Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell 1:717–724
Taniguchi K, Kobayashi K, Saito K, Yamanouchi H, Ohnuma A, Hayashi YK, Manya H, Jin DK, Lee M, Parano E, Falsaperla R, Pavone P, Van Coster R, Talim B, Steinbrecher A, Straub V, Nishino I, Topaloglu H, Voit T, Endo T, Toda T (2003) Worldwide distribution and broader clinical spectrum of muscle–eye–brain disease. Hum Mol Genet 12:527–534
Beltran-Valero de Bernabe D, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, Kayserili H, Merlini L, Chitayat D, Dobyns WB, Cormand B, Lehesjoki AE, Cruces J, Voit T, Walsh CA, van Bokhoven H, Brunner HG (2002) Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet 71:1033–1043
van Reeuwijk J, Janssen M, van den Elzen C, Beltran-Valero de Bernabe D, Sabatelli P, Merlini L (2005) POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker Warburg syndrome. J Med Genet 42:907–912
Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, Segawa M, Yoshioka M, Saito K, Osawa M, Hamano K, Sakakihara Y, Nonaka I, Nakagome Y, Kanazawa I, Nakamura Y, Tokunaga K, Toda T (1998) An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 394:388–392
Silan F, Yoshioka M, Kobayashi K, Simsek E, Tunc M, Alper M, Cam M, Guven A, Fukuda Y, Kinoshita M, Kocabay K, Toda T (2003) A New mutation of the fukutin gene in a non-Japanese patient. Ann Neurol 53:392–396
Diesen C, Saarinen A, Pihko H, Rosenlew C, Cormand B, Dobyns WB, Dieguez J, Valanne L, Joensuu T, Lehesjoki AE (2004) POMGnT1 mutation and phenotypic spectrum in muscle–eye–brain disease. J Med Genet 41:e115
Kano H, Kobayashi K, Herrmann R, Tachikawa M, Manya H, Nishino I, Nonaka I, Straub V, Talim B, Voit T, Topaloglu H, Endo T, Yoshikawa H, Toda T (2002) Deficiency of adystroglycan in muscle–eye–brain disease. Biochem Biophys Res Commun 291:1283–1286
Santavuori P, Valanne L, Autti T, Haltia M, Pihko H, Sainio K (1998) Muscle–eye–brain disease—clinical features, visual evoked potentials and brain imaging in 20 patients. Eur J Peadiatr Neurol 1:41–47
Pihko H, Lappi M, Raitta C, Sainio K, Valanne L, Somer H, Santavuori P (1995) Ocular findings in muscle–eye–brain (MEB) disease: a follow up study. Brain Dev 17:57–61
Valanne L, Pihko H, Katevuo K, Karttunen P, Somer H, Santavuori P (1994) MRI of the brain in muscle–eye–brain (MEB) disease. Neuroradiology 36:473–476
Haliloglu G, Gross C, Senbil N, Talim B, Hehr U, Uyanik G, Winkler J, Topaloglu H (2004) Clinical spectrum of muscle–eye–brain disease: from the typical presentation to severe autistic features. Acta Myol 23:137–139
Meyer S, Struffert T, Uyanik G, Oehl-Jaschkowitz B, Hehr U, Shamdeen MG (2005) Congenital muscular dystrophies: muscle–eye–brain disease. Klin Padiatr 217:68–69 (in German)
Kanoff RJ, Curless RG, Petito C, Falcone S, Siatkowski RM, Pegoraro E (2002) Walker–Warburg syndrome: neurologic features and muscle membrane structure. Pediatr Neurol 18:76–80
Vervoort VS, Holden KR, Ukadike KC, Collins JS, Saul RA, Srivastava AK (2004) POMGnT1 gene alterations in a family with neurological abnormalities. Ann Neurol 56:143–148
Zhang W, Vajsar J, Cao P, Breningstall G, Diesen C, Dobyns W, Herrmann R, Lehesjoki A-E, Steinbrecher A, Talim B, Toda T, Topaloglu H, Voit T, Schachter H (2003) Enzymatic diagnostic test for Muscle–Eye–Brain type congenital muscular dystrophy using commercially available reagents. Clin Biochem 36:339–344
Akasaka-Manya K, Manya H, Kobayashi K, Toda T, Endoa T (2004) Structure–function analysis of human protein O-linked mannose b1,2-N-acetylglucosaminyltransferase 1, POMGnT1. Biochem Biophys Res Commun 320:39–44
Godfrey C, Mein R, Brockington M, Elson E, Topaloglu H, Smith J, Escolar D, Bertini E, Merlini I, Mercuri E, Bushby K, Straub V, North K, Abbs S, Muntoni F (2006) Molecular genetic analysis of 6 glycosyltransferases in a large population of dystroglycanopathy patients significantly widens the spectrum of phenotypes resulting from POMT1, POMGnT1 and Fukutin mutations. Neuromuscul Disord 16:683
Balci B, Uyanik G, Dincer P, Gross C, Willer T, Talim B, Haliloglu G, Kale G, Hehr U, Winkler J, Topaloglu H (2005) An autosomal recessive limb girdle muscular dystrophy (LGMD2) with mild mental retardation is allelic to Walker-Warburg syndrome (WWS) caused by a mutation in the POMT1 gene. Neuromuscul Disord 15:271–275
van Reeuwijk J, Maugenre S, van den Elzen C, Verrips A, Bertini E, Muntoni F, Merlini L, Scheffer H, Brunner HG, Guicheney P, van Bokhoven H (2006) The expanding phenotype of POMT1 mutations: from Walker–Warburg syndrome to congenital muscular dystrophy, microcephaly, and mental retardation. Human Mutat 27:453–459
Chiba A, Matsumura K, Yamada H, Inazu T, Shimizu T, Kusunoki S, Kanazawa I, Kobata A, Endo T (1997) Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J Biol Chem 272:2156–2162
Hayashi YK, Ogawa M, Tagawa K, Noguchi S, Ishihara T, Nonaka I, Arahata K (2001) Selective deficiency of alpha-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology 57:115–121
Takeda S, Kondo M, Sasaki J, Kurahashi H, Kano H, Arai K, Misaki K, Fukui T, Kobayashi K, Tachikawa M, Imamura M, Nakamura Y, Shimizu T, Murakami T, Sunada Y, Fujikado T, Matsumura K, Terashima T, Toda T (2003) Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum Mol Genet 12:1449–1459
Willer T, Prados B, Falcon-Perez JM, Renner-Muller I, Przemeck GK, Lommel M, Coloma A, Valero MC, de Angelis MH, Tanner W, Wolf E, Strahl S, Cruces J (2004) Targeted disruption of the Walker-Warburg syndrome gene Pomt1 in mouse results in embryonic lethality. Proc Natl Acad Sci USA 101:14126–1431
Liu J, Ball SL, Yang Y, Mei P, Zhang L, Shi H, Kaminski HJ, Lemmon VP, Hu H (2006) A genetic model for muscle-eye-brain disease in mice lacking protein O-mannose 1,2-N-acetylglucosaminyltransferase (POMGnT1). Mech Dev 123:228–240
Stoltenburg-Didinger G, Steinbrecher A (2003) Morphogenesis of type II lissencephaly: neuropathology, genetics and pathomechanisms. Neuroembryology 2:32–39
Manya H, Sakai K, Kobayashi K, Taniguchi K, Kawakita M, Toda T, Endo T (2003) Loss-of-function of an N-acetylglucosaminyltransferase, POMGnT1, in muscle–eye–brain disease. Biochem Biophys Res Commun 306:93–97
Biancheri R, Bertini E, Falace A, Pedemonte M, Rossi A, D’Amico A, Scapolan S, Bergamino L, Petrini S, Cassandrini D, Broda P, Manfredi M, Zara F, Santorelli FM, Minetti C, Bruno C (2006) POMGnT1 mutations in congenital muscular dystrophy: genotype–phenotype correlation and expanded clinical spectrum. Arch Neurol 63:1491–1495
Matsumoto H, Hayashi YK, Kim DS, Ogawa M, Murakami T, Noguchi S, Nonaka I, Nakazawa T, Matsuo T, Futagami S, Campbell KP, Nishino I (2005) Congenital muscular dystrophy with glycosylation defects of alpha-dystroglycan in Japan. Neuromuscul Disord 15:342–348
Vajsar J, Zhang W, Dobyns WB, Biggar D, Holden KR, Hawkins C, Ray P, Olney AH, Burson CM, Srivastava AK, Schachter H (2006) Carriers and patients with muscle-eye-brain disease can be rapidly diagnosed by enzymatic analysis of fibroblasts and lymphoblasts. Neuromuscul Disord 16:132–136
Balci B, Morris-Rosendahl DJ, Celebi A, Talim B, Topaloglu H, Dincer P (2007) Prenatal diagnosis of muscle–eye–brain disease. Prenat Diagn 27:51–54
Acknowledgments
We thank the families for their participation in this study, B.H.F. Weber for his support and scientific advice, and C. Mai and F. Koehler for excellent technical assistance. This work was supported in part by the “Regensburger Forschungsförderung der Medizinischen Fakultät” (ReForM; University of Regensburg, Germany), the Joint German-Israeli Research Program of the Federal Ministry of Education and Research (BMBF) and the Ministry of Science and Technology (MOST) “Towards neural stem cell based therapies: the impact of neuronal migration” (BMBF no. 01GA0510). MCW and HL are members of the German Muscular Dystrophy Network (MD-NET; 01GM0601; www.md-net.org) funded by the German Ministry of Education and Research (BMBF, Bonn, Germany). MD-NET is a partner of TREAT-NMD (EC, 6th FP, proposal no. 036825; www.treat-nmd.eu).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Summary of novel POMGnT1 mutations.
ESM
POMGnT1 missense mutations at codon 442: wild-type sequence (a), patient 2a homozygous Arg442Cys (b), and patient 7 homozygous Arg442His (c). MspI restriction fragment length polymorphism and sequence demonstrating the presence of the POMGnT1 mutation c.1540−2A>G in homozygous state in patient 5 and in heterozygous state in both parents but not in the control (d). Patient 6, homozygous for the frameshift mutation c.1350_1354delCTGGG in exon 16 of the POMGnT1 gene; (wild-type in upper lanes, e) (PDF 269 kb)
Rights and permissions
About this article
Cite this article
Hehr, U., Uyanik, G., Gross, C. et al. Novel POMGnT1 mutations define broader phenotypic spectrum of muscle–eye–brain disease. Neurogenetics 8, 279–288 (2007). https://doi.org/10.1007/s10048-007-0096-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-007-0096-y