Abstract
Purpose
The use of cyanoacrylate (CA)-based tissue adhesives for mesh fixation in abdominal hernia repair is increasing due to the fast action and bond strength of these glues. The aim of the present study was to assess tissue changes induced by different CA glues used for mesh fixation in an animal model.
Methods
Parietal defects were induced in the abdominal wall of 60 rats and repaired by polyvinylidene fluoride (PVDF) mesh fixation using different CA glues. At 1, 7, 15, and 30 days post-surgery, macroscopic and histopathological studies were performed to evaluate mesh adhesion, the presence of complications and the tissue response.
Results
All meshes were successfully fixed without signs of inflammatory reaction, displacement or detachment. In areas where CA adhesives were applied, the acute tissue response was limited and transient. At 7 days post-surgery, collagen fibril production around prosthetic materials was observed, and collagen maturation was achieved at 30 days post-surgery. Good mesh incorporation was detected with all three glues, but the application of Glubran-2 was associated with an early macrophagic response and the early production and maturation of collagen fibrils.
Conclusions
Our study confirmed that CA tissue adhesives induced the good incorporation of prosthetic mesh within host tissue with a low incidence of complications and reduced acute tissue reaction. At 30 days post-surgery no signs of mesh disinsertion or migration were observed, the prosthetic mesh adhesion was due to the presence of a dense mature connective tissue rich in type I collagen fibres.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the field of abdominal surgery, prosthetic hernia repair is one of the most common procedures. Mesh reinforcement is more effective than suture repair with a low percentage of hernia recurrence [1]. Since the occurrence of chronic pain remains a major complication after this surgical procedure, the use of less traumatic mesh fixation techniques, such as recently introduced tissue adhesives, has shown striking results. In several controlled trials, the use of surgical tissue was related to less postoperative pain, shorter operating time and lower incidence of postoperative complications [2]. Biologic materials such as fibrin derivatives and synthetic glues, i.e., cyanoacrylates (CAs), are the main surgical tissue adhesives used [3]. However, for the continuous improvement of CA glues, preclinical studies are necessary to clarify tissue changes and repair processes related to glue application. In particular, there are a reduced number of studies regarding tissue reactions with respect to meshes fixed intraperitoneally with glue [4, 5]
The aim of this study was to compare tissue changes and repair processes induced by three different CA formulations in an animal model of intraperitoneal abdominal hernia repair.
Materials and methods
Animals and experimental design
In this preclinical study, tissue samples from 60 male Wistar rats (Rattus norvegicus) weighing between 297 and 336 g (mean 313 ± 10 g) were used. The implants were performed at the Center of Experimental Biomedicine, CNR-National Research Council Pisa (Italy).
Twenty rats were randomly assigned to each of the three groups treated with the different CA glues. The day before implantation, each animal was placed in a single cage and subjected to antibiotic therapy (oral Baytril® 2.5% sol., 1 ml/litre water). Prior to surgery, each animal was administered 7.5 mg/kg xylazine intraperitoneally and 10 mg tramadol as a pre-operative analgesic. Anaesthesia was induced with 3% isoflurane in a mixture of air and oxygen administered within an induction chamber, while for maintenance, 1.5% isoflurane in oxygen was administered by a face mask.
A U-shaped laparotomy was performed in the cranial third of the abdomen, and abdominal wall was flipped caudally, exposing the peritoneum and allowing access to the implant site [6]. Two 2 × 2 cm PVDF meshes per animal were placed on the peritoneum in the lateral position and 6 µl of different CA formulations was applied on the four corners and in the centre of the prosthetic material, with a total volume of 30 μl for prosthesis. At 3 min after application, the flap was flipped cranially, and the abdominal wall was surgically closed. Each animal was placed on a heated cushion until complete awakening, after which the animal was transferred into its cage. In the first 3 days, 10 mg/kg tramadol was administered orally in the drinking water, and antibiotic therapy was continued for 7 days. The animals were checked daily for signs of adverse effects.
At 1, 7, 15, and 30 days post-surgery, five animals from each group were sacrificed by anaesthetic overdose (isoflurane) and submitted to a macroscopic examination that included the evaluation of mesh displacement, integration, deformation and retraction, signs of inflammation and the presence of seroma or visceral adhesions. The adhesions were rated according to a quantitative and qualitative scoring system based on the published literature [7]. The flap of the abdominal wall involved in the implant was excised in toto and immediately placed into 10% buffered formalin fixative.
Prosthetic materials and fixation devices
As reinforcement for the experimental abdominal hernias, a square portion of a macroporous polyvinylidene fluoride (PVDF) mesh (Dynamesh-Endolap®; FEG Textiltechnik mbH, Achen, Germany) measuring two centimetres on each side was used. The Cas adhesives used for the mesh fixation were Ifabond® (n-hexyl-CA; IFA Medical, France), Glubran®2 (n-butyl-2 CA plus MS; GEM S.r.l., Italy) and Histoacryl® (n-Butyl-2 CA; Braun Corporation, Germany).
Morphological studies
Histopathology
Tissue samples were routinely processed for paraffin embedding. 5-µm serial sections were dewaxed, rehydrated and stained with haematoxylin and eosin (H–E) for routine examination, Masson’s trichrome stain to evaluate connective tissue, Perls’ Prussian blue stain to detect haemosiderin deposits, and Picro-Sirius red stain to determine the collagen type contents. Slides were examined by three pathologists (FP, FM and A.P.) in a blinded fashion.
Immunohistochemistry and morphometrical studies
For immunohistochemistry, tissue sections mounted on treated glass slides (Superfrost Plus; Menzel-Glaser, Germany) were used. Heat-induced epitope retrieval with citrate buffer pH 6 was used. Immunohistochemical labelling was performed manually and non-specific antigen binding was blocked by incubation with UltraVision Protein Block (TA-125-PBQ; Thermo Scientific, Cheshire, UK). A panel of primary antibodies was applied to serial sections and incubated overnight at 4 °C. For macrophages, an anti-iba1 rabbit polyclonal serum (Wako, Neuss. Germany; diluted 1:300) was used, for T-lymphocytes an anti-CD3 rabbit polyclonal antibody (Dako, Glostrup, Denmark; diluted 1:200) was used, and for B-lymphocytes an anti-Cd79a mouse monoclonal antibody (Exbio, Praha, Czech Rep.; diluted 1:100) was used. Antibody binding was detected by a biotinylated goat polyvalent secondary antibody (TP-125-BN; ThermoScientific, Cheshire, UK), streptavidin peroxidase (TS-125-HR; ThermoScientific, Cheshire, UK) and DAB chromogen (SK-4105; ImmPact DAB, Vector, Burlingame, CA) as indicated by the manufacturer’s instructions, and the slides were counterstained with haematoxylin. Substitution of the primary antibody with an unrelated matched primary antibody was used to provide a negative control. Serial sections of a rat lymph node were used as a positive control.
Bright field images were acquired at ×40 magnification with a Leica Microsystem DFC490 digital camera. Counting was performed using a semiautomatic analysis system (LASV 4.3, Leica) in six 15,000 μm2 random fields of three different areas of the interaction between the mesh fibres, the abdominal wall and glue application. These sampled areas were used to evaluate the thickness of the tissue reaction, the thickness of the connective tissue production, the percentage of type I and type III connective fibrils, new blood vessels and the composition of cell populations involved in the inflammatory reaction.
Statistical analysis
Statistical analysis was performed using the statistical package SPSS Advanced Statistics 21.0 (SPSS Inc., Chicago, IL, USA). ANOVA was used to compare the different parameters observed at different times and when different CA glues were used. Post-hoc analysis was performed with the Bonferroni Test. Statistical significance was based on a 5% (0.05) significance level.
Results
Macroscopic study
Animals of different groups did not exhibit symptoms related to a painful state. All rats presented a slight decrease in body weight at 7 days post-surgery and promptly recovered at 15 days, followed by a weight increase at 30 days post-implantation. No weight differences were observed among the three groups.
All the meshes fixed with the different adhesives appeared well secured without signs of inflammatory reaction and displacement or detachment. In all groups the prosthetic meshes well adhered to the parietal wall surface and a good host tissue ingrowth appeared already after 7 days and at 30 days post-implantation appeared strongly fixed. The adhesive strength of these synthetic glues promoted for mesh fixation has been largely demonstrated in previous biomechanical studies [8].
At 7 days post-surgery, seromas were observed in 2/5 rats in the Histoacryl group, 1/5 animals in the Glubran-2 group and in 0/5 subjects in the Ifabond group. These alterations were characterized by a liquid collection (about 2 ml) into virtual cavity created by the surgical dissection of the subcutaneous tissue from the muscular plane of the abdominal wall. After 15 days post-surgery, only 1/5 rats in the Histoacryl group showed post-surgery seroma.
At 7 days post-implantation, small adherences between the prosthetic materials and the bowels of the abdominal cavity were observed in the treated subjects, particularly in the rats treated with the Ifabond glue (3/5 vs 2/5 in the Glubran®2 and Histoacryl group; score 3). In animals sacrificed at 15 days post-surgery, visceral adherences were reduced, with only 1/5 rats detected in the Glubran®2 and Histoacryl groups (score 4). At 30 days post-surgery, the presence of adherences was similar in rats treated with the three different CA glues (1/5 subjects; score 4/5).
Tissue reaction around the mesh prosthetic material
Morphologic studies of implants
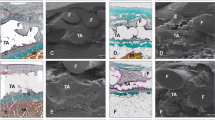
At 1 day post-implantation, no tissue reactions were observed around the mesh. At 7 days after surgery, mesh fibres were integrated within the abdominal host tissue in all three groups of rats. At this time, scar tissue rich in inflammatory cells, including macrophage cells, eosinophilic granulocytes, mast cells, rare neutrophils and giant cells together with a loose fibrillary collagen network accompanied by small blood vessels, was present around the prosthetic materials (Fig. 1a–c). This tissue reaction was also evident around the mesh filaments in areas not placed in the immediate proximity of the glue application areas. At 15 days post-surgery, there was an increase in inflammatory infiltrates and connective tissue (Fig. 1d–f). At 30 days, the host tissue reaction was decreased, and only a thin halo of inflammatory cells surrounded by a dense connective tissue was observed around the mesh fibres (Fig. 1g–i). The infiltration of adipose tissue was also observed at the periphery of the connective tissue.
Section of the abdominal wall of rats subjected to mesh fixation using Ifabond, Glubran®2 and Histoacryl glues. Interaction between prosthetic mesh fibres and host tissues at 7 (a, b, c), 15 (d, e, f) and 30 days post-surgery (g, h, i). An evident inflammatory reaction surrounded by loose connective tissue was present around mesh fibres at 7 and 15 days post-implantation. At 30 days post-implantation, the host tissue reaction was drastically reduced and delimited by dense connective tissue (haematoxylin–eosin stain; bar = 100 µm)
Collagen expression in implants
The composition of collagen fibrils in the connective tissue detected at the periphery of the mesh fibres was determined by Picro-Sirius red staining (Fig. 2a–i). At 7 days post-surgery, type III collagen fibrils were the main components of the proliferating connective tissue (Fig. 2a–c), while after 30 days, there was a strong increase in type I fibrils (Fig. 2g–i).
Section of abdominal wall of rats submitted to mesh fixation using Ifabond, Glubran®2 and Histoacryl glues. Collagen production within the fibrotic reaction around mesh fibres at 7 (a, b, c), 15 (d, e, f) and 30 days post-surgery (g, h, i). At 7 and 15 days post-implantation, the connective tissue mainly comprised type III collagen fibrils, while after 30 days, the percent of type I collagen fibrils strongly increased (Picro-Sirius red stain; bar = 50 µm)
Morphometric analysis
The results of the study performed to characterize the inflammatory and fibrotic reactions around the mesh prosthetic material, is presented in Fig. 3. At 1 day post-surgery, no changes were detected around the meshes, while 7 days post-surgery inflammatory infiltrates were observed around the prosthesis fixed with the three CA glues. At this time, the total amount of collagen fibrils was significantly higher around the mesh fixed with Glubran®2 than those observed around the mesh fixed with the other two CA glues (p < 0.011 vs Histoacryl and p < 0.0001 vs Ifabond) and around the mesh fixed with Histoacryl than that fixed with Ifabond (p < 0001). At 7 days post-surgery, the amount of type I collagen fibrils around the prosthetic material ranged from 16% around the mesh fixed with Glubran®2 and Ifabond CA glues to 22% of the total collagen fibrils for Histoacryl, without significant differences. At 15 days post-surgery, the inflammatory cell response around the mesh fibre slightly increased compared with that observed after 7 days of implantation and became more intense around the mesh fixed with Ifabond glue than that observed with the other two CA glues (p < 0.001). The amount of total collagen fibrils was similar in all three groups, and the percentage of type I collagen fibrils increased from 28 to 33% of the total collagen fibrils without significant differences in the three groups examined. At 30 days post-surgery, the amount of inflammatory infiltrates significantly decreased compared to that at 15 days post implantation and was significantly higher around the mesh fixed with Ifabond glue than that around those fixed with the other two CA glues (p < 0.0001 vs Histoacryl and p < 001 vs Glubran®2) and around the mesh fixed with Glubran®2 glue when compared with that fixed with Histoacryl glue (p < 0.001). The amount of total collagen fibrils around the mesh fixed with Ifabond glue was significantly higher than that around the prosthesis fixed with the other two CA glues (p < 0.005), and the percentage of type I collagen fibrils around the mesh strongly increased compared to that at 7 and 15 days post-surgery in all three groups, ranging from 51 to 65% and was statistically higher around the mesh fixed with Ifabond CA glue (p < 0.05 vs Glubran®2 and p < 0.012 vs Histoacryl).
Amount of inflammatory reaction and total and type I collagen fibrils were detected around the prosthetic mesh fixed with Ifabond, Glubran®2 and Histoacryl glues at different times post-implantation. a p < 0.0001 vs Ifabond and p < 0.0011 vs Histoacryl; b p < 0.0001 vs Ifabond; c p < 0.001 vs Glubran®2 and Histoacryl; d p < 0.0001 vs Histoacryl and p < 0.001 vs Glubran®2; e p < 0.001 vs Histoacryl; f p < 0.005 vs Glubran®2 and Histoacryl; g p < 0.012 vs Histoacryl and 0.05 vs Glubran®2)
Tissue reaction around the glue deposits
Morphologic studies
In the tissue sections, the CA deposits appeared as optically empty areas with irregular contours, around which the tissue reaction developed (Fig. 4). At 1 day post-surgery, there was evidence of inflammatory oedema around CA glue deposits, accompanied by limited regressive phenomena of the peripheral myocytes of the abdominal wall muscular layer and limited acute inflammatory infiltrates, predominantly consisting of mast cells, eosinophils and rare macrophages (Fig. 4a–c). At 7 days post-surgery, a cellular inflammatory infiltration delimited by loose connective tissue rich in neo-formed small blood vessels was evident around the glue deposits (Fig. 4d–f). At 15 days after surgery, a strong reduction in the inflammatory response together with a decrease in fibrotic band thickening accompanied by reduced vascularization was observed around the glue deposits (Fig. 4g–i). Finally, after 30 days post-surgery at the periphery of the CA glue residues, few inflammatory infiltrates associated with thin dense connective tissue and a lower number of blood vessels were detected (Fig. 4j–l).
Section of the abdominal wall of rats subjected to mesh fixation by Ifabond, Glubran®2 and Histoacryl glues. Tissue response around glue deposits at different time points post-surgery. a, b, c Acute inflammatory response at 1 day post-surgery characterized by oedema and scanty cellular infiltrates at 1 day post-surgery; d, e, f at 7 days post-surgery, a cellular infiltration delimited by loose connective tissue is evident; g, h, i at 15 days post-surgery, there was a reduction in the inflammatory infiltrates and of the fibrotic peripheral tissue; j, k, l at 30 days post-implantation the glue residues were lined by a reduced inflammatory response delimited by dense connective tissue
Morphometric analysis
The results of the morphometric analysis performed to characterize the inflammatory reaction and the fibrosis around the glue deposits are presented in Fig. 5.
Amount of inflammatory cell response, connective production and new blood vessel counts around CA glue deposits at different times post-implantation. a p < 0.001 vs Histoacryl and p < 0.05 vs Glubran®2; b p < 0.001 vs Histoacryl; c p < 0.001 vs Glubran®2 and Histoacryl; d p < 0.001 vs Histoacryl and p < 0.0001 vs Ifabond; e p < 0.0001 vs Ifabond; f p < 0.0001 vs Glubran®2 and Histoacryl; g p < 0.001 vs Glubran®2 and Histoacryl; h p < 0.0001 vs Ifabond and Glubran®2; i p < 0.001 vs Glubran®2 and Histoacryl; j p < 0.001 vs Glubran®2 and Histoacryl
At 1 day after surgery, the inflammatory reaction around Histoacryl deposits was significantly lower than that induced by Glubran®2 and Ifabond adhesives (p < 0.001). At this time, the inflammatory infiltrates around the Glubran®2 glue deposits were lower than those induced by Ifabond glue (p < 0.05).
At 7 days post-surgery, the inflammatory reaction around the glue deposits increased and was statistically higher around Ifabond glue than those observed around Glubran®2 and Histoacryl deposits (p < 0.001). At this time, a fibrotic reaction became evident and was again significantly lower at the periphery of Ifabond deposits than those observed around the other two glues (p < 0.001). The lower fibrotic reaction at the periphery of Ifabond deposits was also associated with the presence of reduced neo-angiogenesis (p < 0.001).
At 15 days post-surgery, the inflammatory infiltrates around the CA deposits tended to decrease without significant differences for the three glues. Due to the gradual maturation of the connective tissue, the thickness of the fibrotic reaction drastically reduced, remaining statistically higher around Ifabond deposits than those observed at the periphery of Glubran®2 and Histoacryl deposits (p < 0.001). At this time, there was also a drastic reduction in neo-angiogenesis. The number of blood vessels remained higher at the periphery of the Histoacryl glue than those revealed at the periphery of Glubran®2 and Ifabond deposits (p < 0.0001).
Thirty days post-surgery, the inflammatory reaction around the deposits of the three different glues showed a further reduction, without significant differences. At the periphery of the CA glue deposits, dense connective tissue with a reduced number of blood vessels was present. This fibrotic reaction, as well as the presence of blood vessels, was still more evident around Ifabond deposits than those detected around Glubran®2 and Histoacryl deposits (p < 0.001 and p < 0.001, respectively).
Inflammatory cell presence around glue deposits.
Morphometric analysis was performed on H–E-stained sections and for T- and B-lymphocytes and macrophages on immunohistochemically labelled sections (Fig. 6). The results are presented in Fig. 7. At 1 day post-surgery, the mast cell and eosinophil numbers in inflammatory infiltrates were higher around Ifabond and Histoacryl glue deposits. The mast cell counts at the periphery of Ifabond were higher than those around the other two CA glues (p < 0.0001). The eosinophil numbers in inflammatory infiltrates around Histoacryl deposits were higher than those observed at the periphery of Ifabond (p < 0.001) and Glubran®2 (p < 0.0001) deposits. The number of eosinophils at the periphery of Ifabond glue was higher than that detected around Glubran®2 deposits (p < 0.001). At the periphery of these glue deposits, an early onset of macrophages was evident (p < 0.001). Seven days post-surgery at the periphery of glue deposits, the mast cell counts decreased but were still more present in the infiltrates observed at the periphery of Ifabond glue than around Glubran®2 and Histoacryl glues (p < 0.001). The number of macrophages strongly increased in the inflammatory infiltrates around the three CA glues without significant differences. At the periphery of Glubran®2 glue, a reduced number of eosinophils was present (p < 0.001), while an increased number of giant cells was already present (p < 0.0001). No difference was observed at the periphery of glue deposits regarding the number of T- and B-lymphocytes. At 15 days post-surgery, a new increase in mast cells, which were more numerous at the periphery of the Ifabond (p < 0.01 vs Glubran®2 and p < 0.05 vs Histoacryl) and Histoacryl glue deposits (p < 0.01 vs Glubran®2), was observed. The eosinophil number remained elevated at the periphery of Ifabond glue deposits (p < 0.001 vs Histoacryl and Glubran®2). The macrophages slightly decreased at the periphery of all three glues, but macrophage counts remained higher at the periphery of Glubran®2 (vs Ifabond and Histoacryl; p < 0.001) and Histoacryl (vs Ifabond; p < 0.01). Giant cells increased at the periphery of Ifabond and Histoacryl deposits and were reduced at the periphery of Glubran®2 deposits (p < 0.0001). At 30 days post-surgery, there was a new decrease in the number of mast cells at the periphery of the three glue deposits, together with a reduction in the eosinophil counts. The presence of eosinophils remained higher at the periphery of Ifabond (p < 0.01 vs Histoacryl and p < 0.0001 vs Glubran®2) and Histoacryl compared to that of Glubran®2 (p < 0.01). A reduced number of macrophages and giant cells was observed at the periphery of Ifabond and Histoacryl residues, while a higher number of these cells were still present at the periphery of the Glubran®2 glue deposits (p < 0.001). No difference was detected for T- and B-lymphocyte counts.
Inflammatory cells in cellular infiltrates detected at the periphery of the three CA glues used at different time points post-surgery. A One day after mesh implantation: a p < 0.0001 vs Ifabond Histoacryl; b p < 0.001 vs Ifabond and Glubran®2; c p < 0.001 vs Glubran®2; d p < 0.001 vs Histoacryl and Ifabond. B Seven days after mesh implantation. a p < 0.001 vs Glubran®2 and Histoacryl b p < 0.0001 vs Ifabond and Histoacryl; c p < 0.001 vs Histoacryl and Ifabond. C Fifteen days after mesh implantation: a p < 0.01 vs Glubran®2 and p < 0.05 vs Histoacryl; b p < 0.01 vs Glubran®2; c p < 0.001 vs Glubran®2 and Histoacryl; d p < 0.001 vs Histoacryl; e p < 0.001 vs Ifabond and Histoacryl; f p < 0.01 vs Ifabond; g p < 0.0001 vs Ifabond and Histoacryl. D Thirty days after mesh implantation: a p < 0.0001 vs Glubran®2 and p < 0.01 vs Histoacryl; b p < 0.01 vs Glubran®2; c p < 0.001 vs Ifabond and Histoacryl; d p < 0.0001 vs Ifabond and Histoacryl; e p < 0.001 vs Histoacryl
Discussion
Post-surgical pain is one of the most serious complications of the extraperitoneal repair of abdominal hernia when conventional fixing methods (sutures, tacks or staples; absorbable or non-absorbable) are used to fix a polypropylene or polyester mesh. Because of the large number of nerve endings in this area, these traumatic fixation systems can sometimes trap neural structures, with negative impacts on the patient’s quality life [9]. Although it has not been definitively determined, post-hernioplasty neuralgia following the conventional fixation of prosthetic materials can reach 5–12% [10].
For this reason, synthetic adhesives have been considered an interesting alternative for the fixation of prosthetic materials in the extraperitoneal repair of the abdominal wall. In fact, synthetic adhesives can drastically reduce these complications, ensuring a good seal of the prosthetic material [11]. However, for extraperitoneal fixation, there are enough data [12, 13], and less is known about intraperitoneal fixation, especially regarding the tissue reactions induced by the different fixing methods.
In our study, performed in a rat model by inducing a defect in the abdominal wall and applying the prosthetic material on its inner side involving the peritoneum in the repair process as used in a previous study [6], an optimal fixation of the mesh with the three CA adhesives with a low number of complications, such as seroma and/or peritoneal adhesions, was macroscopically observed. This implant technique was different from that used by other authors, which provided the induction of a partial defect in the lateral abdominal wall to avoid the involvement of the peritoneum in the repair process [14].
Seroma and visceral adhesions are common complications that can occur after the intraperitoneal implantation of hernia repair materials. In particular, the persistent presence of seroma can prevent or delay the integration of the biomaterial within the host tissue and cause hernia recurrence. Seroma can arise for several causes, including surgical procedures, patient intrinsic characteristics and prosthetic materials [15]. In our study, despite the large number of implants, the presence of seromas was reduced, as previously observed in a similar animal model when implants were fixed with Glubran 2 [14]. In contrast, in other investigations, similar CA adhesives showed the onset of these complications [16].
Adhesion formations are further complications occasionally reported in experimental animal models when a traumatic sealant was used to fix prosthetic materials in intraperitoneal hernioplasty or for sealing colonic anastomoses [10, 17]. In our experimental study, we observed the presence of some visceral adhesions, independent of the CA adhesive used, particularly at 7 and 30 days post implantation. The early adhesion formation with the bowel of the abdominal cavity could depend on an adhesive bound to the surrounding tissue rather than host collagen formation characteristics of adhesion phenomena that occurred later during repair processes. The presence of these adhesions should be correctly interpreted, particularly considering the animal species used for the animal model. In fact, these complications were less frequently observed in larger animal models, such as rabbits [17] or swine [18], than in rats and other animal species with a small abdominal cavity and reduced distance between the implanted prosthetic material and abdominal viscera. Furthermore, the horizontal station of the animal used in the experimental study can facilitate the onset of these adhesions, in contrast to what can occur in humans.
Excellent macroscopic mesh fixation was achieved using a reduced amount of tissue adhesives that set the mesh to the host tissues without compromising the physiological wound healing process, as previously observed [16]. Using an excessive amount of adhesive could, in fact, provoke poor wound healing and severe inflammatory reactions [19].
To better investigate the tissue changes induced by the different CA glues used, we examined the earlier tissue modifications (1 and 7 days post-surgery) other than those detectable at 15 and 30 days after implantation as in previous studies [16, 19, 20]. Morphometric analysis was used to better characterize the tissue modifications induced both around prosthetic materials fixed with the different CA glues and at the periphery of the three different glue deposits. Although the tissue responses detected at the periphery of the prosthetic material fixed with the different glues tested and that observed directly at the periphery of the CA glue deposits were similar, the inflammatory response and the fibroplasia detected at the periphery of the glue deposits was statistically greater than those observed around the mesh fibres due to the direct action of the different CA glues on the host tissues.
Our study revealed the presence of an evident inflammatory reaction around the mesh filaments fixed using the different CA adhesives starting from 7 days post-implantation together with the production of collagen fibres. Starting from 15 days post-surgery, good tissue incorporation of the mesh within the tissue of the abdominal wall was detected in all subjects treated with the different CA glues, particularly for Glubran®2 glue, confirming that the use of this biocomponent glue (NBCA + MS) for mesh fixation in hernia repair provides satisfactory performance [14, 21,22,23]. As in previous studies, mesh incorporation and inflammatory reactions around the filaments also occurred in adhesive-free areas, as described by other authors [16, 19].
Morphometric analysis of the different types of collagen fibrils demonstrated that at 7 days post-surgery, the fibrotic reaction mainly comprised type III collagen fibres, while the type I collagen fibres in this phase were reduced. Type III collagen fibres are characteristic of extensible connective tissue and are common in fast-growing tissue, particularly in the early stage of wound repair, and are subsequently replaced by types I collagen fibrils that give a greater tensile strength to the connective tissue. The percentage of type I collagen fibrils strongly increased at 15 days post implantation to reach the maximum amount in rats sacrificed after 30 days (51–65%). The presence of collagen type III fibres in the early phase of mesh incorporation is essential for subsequent collagen I fibrillogenesis [24], and the high percentage of these fibrils around mesh filaments at 30 days post-implantation histologically confirmed the inclusion and increased tensile strength of the mesh implant observed macroscopically and is essential for tissue repair of the abdominal wall, an area constantly subjected to tensile forces.
Data obtained by morphometric analysis demonstrated an early increase in total collagen fibre production when Glubran®2 and Histoacryl glues were used to fix the mesh, with the highest level achieved for Glubran®2 CA adhesive. In contrast, a lower production of total collagen fibres was elicited by Ifabond glue, confirming previous studies that demonstrated lower levels of mRNA encoding collagen I and III compared to other CA fixative adhesive at the early healing stage [16]. Nevertheless, at 30 days post-surgery, the production of total collagen fibres and particularly the presence of type I collagen fibres at the periphery of mesh fibrils fixed with Ifabond CA glue was higher than that observed in the other groups. Our results confirmed previous studies that demonstrated a similar tensile strength at 14 and 90 days post-implantation for prosthetic materials, irrespective of the CA glue used [16].
The possible complications observed in the current use of CA glues are related to their polymerization and biodegradation features. To better investigate the tissue changes directly induced by the three different CA glues, we characterized the tissue response at the periphery of glue deposits at different times post-implantation.
The early tissue response can be related to the production of molecules of CA metabolism, which can cause the release of inflammation mediators [25]. The tissue changes due to the direct application of CA glues were detected early, only in rats sacrificed at 1 day post-implantation. The aftermath consisted of slight oedema and mild changes in the underlying muscular fibres.
After 1 week post-implantation, inflammatory infiltrates were evident together with the onset of a fibrotic reaction and the production of small blood vessels. Subsequently, these acute inflammatory changes progressively decreased at 15 and 30 days post-implantation, leaving a fibrotic reaction characterized by the presence of macrophages, giant cells and dense connective tissue. At 15 days post-implantation, a new increase in mast cells and macrophages was observed in all animal groups, most likely related to the beginning of glue degradation phenomena with the release of substances that reactivated the inflammatory processes. The increase in connective fibrils was accompanied by increasing neo-angiogenesis, which was clearly evident at 7 days post-surgery at the periphery of CA glue deposits (Glubran®2 and Histoacryl), inducing stronger fibroplasia. The reduction in the inflammatory reaction and the progressive differentiation of connective tissue at the periphery of glue deposits was associated with a reduction of the blood vessels that were still evident at the periphery of Ifabond deposits where the fibrotic tissue was more present at 30 days post-surgery.
Analysis of the inflammatory infiltrate composition detected at the periphery of the deposits of the examined CA glues at different times post-implantation allowed us to detect some significant differences. The tissue changes induced by Glubran®2 glue were characterized by an early macrophagic response, as observed in previous studies [19]. The presence of these mononuclear cells is fundamental for the fibrous organization of scar tissues. In fact, inflammatory monocytes and tissue-resident macrophages are key regulators of tissue repair and fibrosis because these cells are an important source of chemokines, matrix metalloproteinases and other inflammatory mediators that drive the cellular response after tissue injury [26].
In conclusion, our preclinical study confirmed that CA tissue adhesives can be a good alternative for mesh fixation in abdominal hernia repair by the good incorporation of prosthetic mesh within host tissue and a low incidence of minor complications. Histopathological investigations demonstrated that the reaction induced by CA glue is acute, limited and detectable only at 1 day after glue implantation. After 15 days, a secondary inflammatory response was detected, probably related to the beginning of the degradation phenomena of glue deposits, confirming that the foreign body reaction induced by the glue deposits did not compromise the healing process. Finally, the morphometric analysis of collagen fibril production demonstrated that the absence of mesh dislocation or shrinkage and the good tissue integration of the prosthetic materials observed at 30 days post-surgery is confirmed by the presence of a dense mature connective tissue rich in type I collagen fibres.
Change history
05 February 2020
Unfortunately, during the revision of the manuscript the title has been incorrectly published
References
Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, Ijzermans JN, Boelhouwer RU, de Vries BC, Salu MK, Wereldsma JC, Bruijninckx CM, Jeekel J (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343:392–398. https://doi.org/10.1056/nejm200008103430603
Kim-Fuchs C, Angst E, Vorburger S, Helbling C, Candinas D, Schlumpf R (2012) Prospective randomized trial comparing sutured with sutureless mesh fixation for Lichtenstein hernia repair: long-term results. Hernia 16:21–27. https://doi.org/10.1007/s10029-011-0856-3
Cuschieri A (2001) Tissue adhesives in endosurgery. Semin Laparosc Surg 8:63–68. https://doi.org/10.1053/slas.2001.24087
Ladurner R, Drosse I, Bürklein D, Plitz W, Barbaryka G, Kirchhoff C, Kirchhoff S, Mutschler W, Schieker M, Mussack T (2011) Cyanoacrylate glue for intra-abdominal mesh fixation of polypropylene-polyvinylidene fluoride meshes in a rabbit model. J Surg Res 167(2):e157–e162. https://doi.org/10.1016/j.jss.2009.11.710
Bellón JM, Fernández-Gutiérrez M, Rodríguez M, Sotomayor S, Pérez-Köhler B, Kuhnhardt A, Pascual G, San Román J (2017) Bioassay of cyanoacrylate tissue adhesives used for intraperitoneal mesh fixation. J Biomed Mater Res B Appl Biomater 105(2):312–319. https://doi.org/10.1002/jbm.b.33558
Gruber-Blum S, Petter-Puchner AH, Brand J, Fortelny RH, Walder N, Oehlinger W, Koenig F, Redl H (2011) Comparison of three separate antiadhesive barriers for intraperitoneal onlay mesh hernia repair in an experimental model. Br J Surg 98:442–449. https://doi.org/10.1002/bjs.7334
Hollinsky C, Kolbe T, Walter I, Joachim A, Sandberg S, Koch T, Rulicke T, Tuchmann A (2010) Tensile strength and adhesion formation of mesh fixation systems used in laparoscopic incisional hernia repair. Surg Endosc 24:1318–1324. https://doi.org/10.1007/s00464-009-0767-x
Bellon JM, Fernandez-Gutierrez M, Rodriguez M, Perez-Lopez P, Perez-Kohler B, Kuhnhardt A, Pascual G, Roman JS (2017) Behavior of a new long-chain cyanoacrylate tissue adhesive used for mesh fixation in hernia repair. J Surg Res 208:68–83. https://doi.org/10.1016/j.jss.2016.09.021
Koehler RH, Begos D, Berger D, Carey S, LeBlanc K, Park A, Ramshaw B, Smoot R, Voeller G (2003) Minimal adhesions to ePTFE mesh after laparoscopic ventral incisional hernia repair: reoperative findings in 65 cases. JSLS 7:335–340. https://doi.org/10.1055/s-2003-41365
Suarez-Grau JM, Morales-Conde S, Martin-Cartes JA, Chaves CR, Jimenez MB, Ramirez FP, Docobo-Durantez F, Mendez SM (2009) Mesh fixation with sutures versus fibrin sealant in hernioplasty with re-absorbable prosthesis (polyglycolic acid and trimethylene carbonate). Experimental study in animals. Cir Esp 86:242–248. https://doi.org/10.1016/j.ciresp.2009.05.004
Colvin HS, Rao A, Cavali M, Campanelli G, Amin AI (2013) Glue versus suture fixation of mesh during open repair of inguinal hernias: a systematic review and meta-analysis. World J Surg 37:2282–2292. https://doi.org/10.1007/s00268-013-2140-4
Gupta A, Mazari F, Samuel N, Balehandra S (2017) Mesh fixation techniques for laparoscopic inguinal hernia repair in adults. Cochrane Database Syst Rev 2017:CD008954. https://doi.org/10.1002/14651858.cd008954.pub2
Kongyuan W, Cuncun L, Long G, Bei P, Huan Y, Jinhui T, Nong C (2018) Different types of mesh fixation for laparoscopic repair of inguinal hernia: a protocol for systematic review and network meta-analysis with randomized controlled trials. Medicine (Baltimore) 97:e0423. https://doi.org/10.1097/md.0000000000010423
Losi P, Burchielli S, Spiller D, Finotti V, Kull S, Briganti E, Soldani G (2010) Cyanoacrylate surgical glue as an alternative to suture threads for mesh fixation in hernia repair. J Surg Res 163:e53–e58. https://doi.org/10.1016/j.jss.2010.05.003
Jin J, Schomisch S, Rosen MJ (2009) In vitro evaluation of the permeability of prosthetic meshes as the possible cause of postoperative seroma formation. Surg Innov 16:129–133. https://doi.org/10.1177/1553350609337128
Pascual G, Rodriguez M, Perez-Kohler B, Mesa-Ciller C, Fernandez-Gutierrez M, Roman JS, Bellon JM (2017) Host tissue response by the expression of collagen to cyanoacrylate adhesives used in implant fixation for abdominal hernia repair. J Mater Sci Mater Med 28:58. https://doi.org/10.1007/s10856-017-5869-8
Ladurner R, Drosse I, Seitz S, Plitz W, Barbaryka G, Siebeck M, Burklein D, Kirchhoff C, Buhman S, Mutschler W, Schieker M, Mussack T (2008) Tissue attachment strength and adhesion formation of intraabdominal fixed meshes with cyanoacrylat glues. Eur J Med Res 13:185–191
Clarke T, Katkhouda N, Mason RJ, Cheng BC, Algra J, Olasky J, Sohn HJ, Moazzez A, Balouch M (2011) Fibrin glue for intraperitoneal laparoscopic mesh fixation: a comparative study in a swine model. Surg Endosc 25:737–748. https://doi.org/10.1007/s00464-010-1244-2
Fortelny RH, Petter-Puchner AH, Walder N, Mittermayr R, Ohlinger W, Heinze A, Redl H (2007) Cyanoacrylate tissue sealant impairs tissue integration of macroporous mesh in experimental hernia repair. Surg Endosc 21:1781–1785. https://doi.org/10.1007/s00464-007-9243-7
Fernandez-Gutierrez M, Rodriguez-Mancheno M, Perez-Kohler B, Pascual G, Bellon JM, Roman JS (2016) Structural analysis and application of n-alkyl cyanoacrylate surgical adhesives to the fixation of meshes for hernia repair. Macromol Biosci 16:1803–1814. https://doi.org/10.1002/mabi.201600246
Testini M, Lissidini G, Poli E, Gurrado A, Lardo D, Piccinni G (2010) A single-surgeon randomized trial comparing sutures, N-butyl-2-cyanoacrylate and human fibrin glue for mesh fixation during primary inguinal hernia repair. Can J Surg 53:155–160
Kukleta JF, Freytag C, Weber M (2012) Efficiency and safety of mesh fixation in laparoscopic inguinal hernia repair using n-butyl cyanoacrylate: long-term biocompatibility in over 1,300 mesh fixations. Hernia 16:153–162. https://doi.org/10.1007/s10029-011-0887-9
Garcia-Vallejo L, Couto-Gonzalez I, Concheiro-Coello P, Brea-Garcia B, Taboada-Suarez A (2014) Cyanoacrylate surgical glue for mesh fixation in laparoscopic total extraperitoneal hernia repair. Surg Laparosc Endosc Percutan Tech 24:240–243. https://doi.org/10.1097/SLE.0b013e3182a2f008
Liu X, Wu H, Byrne M, Krane S, Jaenisch R (1997) Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci USA 94:1852–1856. https://doi.org/10.1073/pnas.94.5.1852
Cerda DG, Ballester AM, Aliena-Valero A, Caraben-Redano A, Lloris JM (2015) Use of cyanoacrylate adhesives in general surgery. Surg Today 45:939–956. https://doi.org/10.1007/s00595-014-1056-4
Wynn TA, Barron L (2010) Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 30:245–257. https://doi.org/10.1055/s-0030-1255354
Funding
Restricted grant provided by GEM Srl for data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Human and animal rights
All procedures performed in studies involving animals were in accordance with the international ethical standards. This article does not contain any studies with human participants performed by any of the authors.
Ethical approval
Permission to conduct this experimental study (Authorization n. 755/2015-PR) was granted by the Italian Ministry of Health, in accordance with Italian Laws DL.gs 26/2014 and Directive 2010/63/EU on the protection of animals used for scientific purposes.
Informed consent
No informed consent was necessary for the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: Due to Title update.
Rights and permissions
About this article
Cite this article
Poli, A., Parisi, F., Millanta, F. et al. Fixation of polyvinylidene fluoride (PVDF) mesh with cyanoacrylate-derived glues in a rat experimental model: histopathologic immunohistochemical and morphometric study. Hernia 24, 1263–1273 (2020). https://doi.org/10.1007/s10029-019-02078-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-019-02078-5