Abstract
Background
Tissue sealants have been proposed as an alternative to permanent fixation devices in hernia repair with the aim of reducing perforation-associated complications and chronic pain. Sealants can be divided into three main categories: synthetic glues (e.g., cyanoacrylate based), biologic products (e.g., fibrin sealant), and genetically engineered polymer protein glues. The beneficial effects of fibrin sealant have been reported in both experimental and clinical hernia repair. However, data on cyanoacrylate glues for mesh sealing are limited.
Methods
In 20 Sprague-Dawley rats, two hernia defects (1.5 cm in diameter) per animal were created bilaterally in the midline of the abdominal wall. The peritoneum was spared. The lesions were left untreated for 10 days to achieve a chronic condition. Defects then were covered with TI-Mesh xl (2 × 2 cm), which was glued with Glubran-II. The time points of sacrifice were 17 days, 28 days, and 3 months. At autopsy, meshes were biomechanically tested, and histology was performed.
Results
Tissue integration of the meshes was impaired at all time points by impenetrable glue plaques. At application sites, the elasticity of the abdominal wall was significantly reduced because of nonresorbed, rigid glue residues.
Conclusions
Mesh fixation by Glubran-II impairs tissue integration, elicits inflammation, and unfavorably alters the biomechanics of macroporous mesh and the abdominal wall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mesh reinforcement, currently the clinical standard in hernia repair, provides convincing results in terms of recurrence [13, 19]. Although the design of lightweight macroporous meshes have led to improved patient comfort and reduced rates of infections, severe complications have been reported with the use of perforating fixation devices [3, 10, 18]. Sutures, anchors, tacks, and staples all have been linked to iatrogenic tissue trauma and to the development of chronic pain, defined as pain persisting more than 6 months after the operation [6].
A frequent cause of chronic pain is the misplacement of these devices (permanent or bioresorbable), especially into the so-called “triangle of pain” or “triangle of doom” (the anatomic areas below the lateral iliopubic tract and the area inferior to the internal inguinal ring) in transabdominal preperitoneal laparoscopic repair (TAPP) or the entrapment of nerves during open procedures (e.g., Lichtenstein repair). Chronic pain often necessitates the removal of fixation devices and/or the mesh or triple neurectomy [1, 4]. These reinterventions are associated with substantial concomitant costs for society and invariably expose the patient to peri- and postoperative risks. According to recent literature, the incidence of chronic pain is as high as 30% for open and laparoscopic repair, indisputably representing one of the most important areas for improvement [9].
Various kinds of tissue sealants have been proposed for the fixation of hernia meshes with the aim of eliminating the aforementioned threats. These products can be divided into three main categories: synthetic glues (e.g., cyanoacrylate based), biologic products (e.g., fibrin sealant), and genetically engineered polymer protein glues [5].
Biological sealants such as fibrin sealant have a long history in surgery, where they are used primarily for sealing and hemostatic purposes. Their exceptional safety record is attributable to their complete biodegradability and physiologic mechanism of action. Both clinical and experimental trials demonstrate that fibrin sealant is a feasible option for mesh fixation in hernia repair [8, 11, 16].
On the other hand, polymer protein glues are not commonly used in general surgery. Synthetic glues such as Glubran-II (butyl-2-cyanoacrylate) are promoted for hernia mesh fixation. However, their surgical use is not widely accepted because of reported cytotoxicity and the lack of published studies outlining the potential side effects of cyanaocrylate glue for mesh sealing [7, 12, 15].
In our study, the influence of Glubran-II (GII; Dahlhausen, Cologne, Germany) on mesh integration was assessed together with the biomechanics of the abdominal wall and its biocompatibility. Fixation of TI-Mesh xl ((TMxl; GfE, Nuremberg, Germany) with fibrin sealant and staples was previously tested, and favorable results were published [16]. The controversial results from the application of cyanoacrylate-based glues in other surgical areas demanded thorough experimental investigation before their introduction to the clinical routine of hernia repair.
Methods
Male Sprague-Dawley rats weighing 400 to 450 g were obtained from the Institut fuer Labortierkunde und genetik der Medizinischen Fakultaet der Universitaet Wien (Himberg, Austria). All reagents used were of analytical grade. The study protocol was approved by the city government of Vienna. Before surgery, the rats were randomized to one of three groups and observed for 17 days, 28 days, and 3 months.
Creation of hernia
The rats were anesthetized with an intramuscular injection of Ketavet 100 mg/ml (ketamine-hydrochloride; Pharmacia, Erlangen, Germany ) and Rompun (xylazine-hydrochloride; Bayer, Leverkusen, Germany). Surgery was performed under sterile conditions.
A midline skin incision was made along the linea alba, and subcutaneous fat tissue was bluntly detached from the underlying muscular wall. Subsequently, two lesions 1.5 cm in diameter were dissected with a scalpel in the right and left abdominal rectus muscles while the peritoneum was left intact as previously described [16]. The locations of the defects were defined at 1.5 cm below the rib cage and 1.5 cm lateral to the linea alba.
The skin incison was closed with nonresorbable suture material, and 1 ml of physiologic saline was administered subcutaneously to replace fluid loss during surgery. The animals were single caged and allowed to recover for 10 days. This interval ensured treatment of defects free of acute inflammatory response.
Treatment of hernia
The rats were reanesthesized 10 days after the creation of the defects, as described earlier. The skin incision was reopened, and the defects were examined macroscopically. The absence of defect closure and free accessibility to the peritoneum were confirmed in all the animals.
Next, TMxl was cut to 2 × 2 cm pieces and positioned over the hernias. All the implants were taken from one sample. All meshes were glued with 0.3 ml of GII (1 drop in each corner of the mesh). To avoid adhesions to the implant, the skin was lifted for 90 s to allow the glue to polymerize. Then the incision was closed with resorbable suture material, and 1 ml of physiologic saline was administered subcutaneously to replace fluid loss during surgery. The rats were single-caged and checked daily for signs of cutaneous infection, inflammation, and seroma formation.
Autopsy
The rats were killed in deep anesthesia on the day 17 (n = 4/8 defects total), on day 28 (n = 8/16 defects total), and at 3 months (n = 8/16 defects total) postoperatively by a 1-ml intravenous injection of anesthetic cocktail (Ketavet and Rompun), causing immediate cardiac arrest. All biomechanical tests were performed postmortem.
Macroscopic evaluation
The skin incision was reopened, and the macroscopical status of the implant site was assessed. If opaque glue residues were present (clearly distinguishable to the naked eye and gentle touch with a forceps), the defects were rated P for “present.” If no residues could be found macroscopically, the meshes were rated A for “absent.”
Biomechanical tests (described in chronological order)
Measurement of elastic deformation and elasticity
To elucidate the impact of GII on elastic deformation and elasticity of the abdominal wall, a high-resolution target laser and negative pressure transducer (BTC-2000; SRLI, Nashville, TN, USA) were used, which allow precise measurements of elastic deformation and rebound of tissue (elasticity) by laser detection. The area of interest (e.g., defect site with or without mesh) was placed under the opening (2 cm in diameter) of a glass chamber, and predefined cycles of suction and release were delivered. The center spot was marked by a red laser dot.
A suction force of 50 mmHg was applied in four repetitive cycles. Elastic deformation represented the deformation (measured in millimeters) during the applied suction force. Elasticity corresponded to the rebound of the deformated tissue to the original position.
We compared elastic deformation and elasticity of the abdominal wall at the sites where defects were consequently created in eight randomized rats with the situation at defect sites 28 days and 3 months after mesh positioning. The untreated abdominal wall served as the control condition because no other possible mesh-fixation combination can be considered the gold standard in incisional hernia repair. These time points were chosen because a slow degradation of GII was expected.
Burst strength
At autopsy, a large-bore needle (inset with 50-ml perfusor syringes; Braun, Melsungen, Germany) was inserted 1 cm subumbilically in the midline, and the abdomen was inflated with air to 80 mmHg. The pressure level was monitored and maintained for 1 min (Draeger Austria, Vienna, Austria). This test was performed postmortem to detect recurrence of hernia.
Tensile strength
After the burst strength test had been performed, the meshes were pulled with a force of 300 g (measured with a spring scale). This test assessed adhesive properties of the glue and showed areas in which the mesh was not firmly attached to the abdominal wall. After these tests had been performed, the meshes were harvested with surrounding muscular tissue and fixed in 10% buffered formalin (Merck, Vienna, Austria) for histologic processing.
Histology
After biomechanical testing, all the samples were embedded in paraffin, and 5-μm sections were stained with hematoxylin and eosin (H&E). Analysis was performed by an experienced pathologist (W.O.) who was not aware of the randomization protocol. Samples were screened for pathologic characteristics of inflammation, abscedation, and foreign body reaction elicited by residual glue. Findings were scored semiquantitatively as 0 (none), 1 (mild), 2 (moderate), or 3 (severe).
Statistical analysis
The differences between the groups were compared using the Kruskal-Wallis test. All p values less than 0.05 were considered significant.
Results
Macroscopic evaluation
All 40 defects were rated P because glue residues were still present at autopsy. These areas were spared from transgression of underlying tissue and extended markedly beyond the punctual sites of the original application. This finding was consistent with the observation that GII dispersed immediately after its use because of its low viscosity. Two defect sites in different animals at each of the 28 days and in the 3-month group showed massive seroma formation and allowed no further biomechanical testing (Fig. 1).
Biomechanical tests
Elastic deformation and elasticity
Elastic deformation and elasticity were significantly impaired in GII-glued meshes. Elastic deformation was limited to about one-tenth of the physiologic level after 28 days, and to about one-fourth of the level after 3 months. Four defect sites in four animals (2 in the 28 days and 2 in the 3-month group) could not be measured because of seroma formation. Furthermore, elasticity also was significantly limited in GII-glued meshes (Table 1).
Burst strength
All treated defects withstood 80 mmHg of abdominal pressure applied for 1 min without recurrence of hernia.
Tensile strength
This test showed two features of GII. First, no mesh was fully detached by the 300-g pull. Second, all 40 meshes lost contact with the abdominal wall in areas that had been glued. Macroscopic examination during the test showed that the mesh was held at the implant site mainly by “anchors” of tissue that had formed between the glue residues (Fig. 2).
Histology
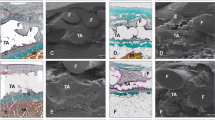
Histology showed signs of severe inflammation, with multiple micro-abscedation in all the samples. Tissue integration was perturbed by invasion of lymphocytes at all time points of observation, indicating a permanent stimulus. Besides the typical round aspect of transversally cut mesh fibers, glue residues appeared as sharp-edged irregular structures that formed accumulation areas of inflammatory response (Fig. 3). These histologic findings sharply contrasted with the good previous results for TMxl obtained with fibrin sealant.
Histology showed pronounced aggregation of inflammatory cells around Glubran-II residues at all time points of observation (star). This finding appeared in contrast to excellent histologic findings with the TI-Mesh xl (G&E, Nuremberg, Germany) in previous trials with staples or fibrin sealant (H&E staining, magnification, ×25).
Discussion
Mesh sealing has left the experimental stage. Recent studies have associated perforating fixation devices with severe complications and chronic pain while demonstrating benefits of mesh sealing in hernia repair [6, 10, 11, 15]. Most publications and ongoing clinical trials refer to fibrin sealant, whereas data on synthetic glues still are scarce.
We conducted this study to provide experimental data for fixation with GII and to allow comparison with previous results from stapling and fibrin sealing of the same implant. The major aim was to determine whether GII would alter tissue integration of TMxl, a macroporous lightweight mesh that has shown excellent properties with fibrin sealant and staples [16, 17]. It also was of interest whether GII would alter the biomechanical properties of the mesh and the abdominal wall. We hypothesized that increased rigidity and a low rate of glue degradation could minimize the benefits of the flexible, macroporous mesh.
Our results show that GII was not degraded 3 months postoperatively and could not be penetrated by cells. By inhibiting tissue integration, GII does not contribute to the desired features of mesh fixation, although sufficient adhesion force was initially achieved to secure the mesh in position. Tissue integration occurred only in areas of the mesh free of glue, and it was these areas that yielded tensile and burst endurance rather than GII. Histology confirmed the macroscopic findings with unanimous results of inflammatory response and formation of multiple abscesses around glue remnants. These observations were unknown in fibrin-sealed or stapled TMxl. These unfavorable histologic findings, which were sustained over the observation period, are in accordance with literature on the use of GII in experimental hernia repair [2]. In terms of biomechanics, GII residues frequently were torn from the underlying tissue in the pull test, impairing elastic deformation and elasticity. It is likely that the significant reduction of these parameters plays a role in the clinical setting because the 0.3 ml of GII used on a 2 × 2 cm TMxl matched the clinical proportions.
Another problematic aspect of cyanoacrylates is their impact on the health of the medical staff, which still is not sufficiently investigated. It must not be forgotten that substantially larger amounts of cyanoacrylate are required for mesh fixation than for wound closure or application in dentistry, in which health risks have been reported [12]. Matching our own experiences in the open, experimental setting, Novik et al. [14] pointed out that GII stuck to surgical instruments, impaired handling during laparoscopic hernia repair, and necessitated special chemical treatment for cleaning and resterilization.
In summary, we report that GII is not suitable for gluing a macroporous mesh in an animal model of ventral hernia. Inhibition of tissue integration of the implant combined with pronounced inflammatory response was elicited by the impenetrable and nondegrading compound. Furthermore, GII significantly reduced elastic deformation and elasticity of mesh and abdominal wall and impaired performance in biomechanical tests.
Conclusion
On the basis of our experimental results, the use of Glubran-II cannot be recommended for gluing of macroporous meshes.
References
Amid PK (2004) Causes, prevention, and surgical treatment of postherniorrhaphy neuropathic inguinodynia: triple neurectomy with proximal end implantation. Hernia 8: 343–349
Birch DW, Park A (2001) Octylcyanoacrylate tissue adhesive as an alternative to mechanical fixation of expanded polytetrafluoroethylene prosthesis. Am Surg 67: 974–978
Cobb WS, Kercher KW, Heniford BT (2005) The argument for lightweight polypropylene mesh in hernia repair. Surg Innov 12: 63–69
Courtney CA, Duffy K, Serpell MG, O’Dwyer PJ (2002) Outcome of patients with severe chronic pain following repair of groin hernia. Br J Surg 89: 1310–1314
Cuschieri A (2001) Tissue adhesives in endosurgery. Semin Laparosc Surg 8: 63–68
Hidalgo M, Castillo MJ, Eymar JL, Hidalgo A (2005) Lichtenstein inguinal hernioplasty: sutures versus glue. Hernia 9: 242–244
Jourdan IC, Bailey ME (1998) Initial experience with the use of N-butyl-2-cyanoacrylate glue for the fixation of polypropylene mesh in laparoscopic hernia repair. Surg Laparosc Endosc 8: 291–293
Katkhouda N, Mavor E, Friedlander MH, Mason RJ, Kiyabu M, Grant SW, Achanta K, Kirkman EL, Narayanan K, Essani R (2001) Use of fibrin sealant for prosthetic mesh fixation in laparoscopic extraperitoneal inguinal hernia repair. Ann Surg 233: 18–25
Kumar S, Wilson RG, Nixon SJ, Macintyre IM (2002) Chronic pain after laparoscopic and open mesh repair of groin hernia. Br J Surg 89: 1476–1479
Ladurner R, Mussack T (2004) Small bowel perforation due to protruding spiral tackers: a rare complication in laparoscopic incisional hernia repair. Surg Endosc 18: 1001
Langrehr JM, Schmidt SC, Neuhaus P (2005) Initial experience with the use of fibrin sealant for the fixation of the prosthetic mesh in laparoscopic transabdominal preperitoneal hernia repair. Rozhl Chir 84: 399–402
Leggat PA, Kedjarune U, Smith DR (2004) Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health 42: 207–211
McCormack K, Scott NW, Go PM, Ross S, Grant AM (2003) Laparoscopic techniques versus open techniques for inguinal hernia repair. Cochrane Database Syst Rev CD001785
Novik B, Hagedorn S, Mork UB, Dahlin K, Skullman S, Dalenback J (2006) Fibrin glue for securing the mesh in laparoscopic totally extraperitoneal inguinal hernia repair: a study with a 40-month prospective follow-up period. Surg Endosc 20: 462–467
Nowobilski W, Dobosz M, Wojciechowicz T, Mionskowska L (2004) Lichtenstein inguinal hernioplasty using butyl-2-cyanoacrylate versus sutures: preliminary experience of a prospective randomized trial. Eur Surg Res 36: 367–370
Petter-Puchner A, Fortelny R, Mittermayr R, Öhinger W, Redl H (2005) Fibrin sealing vs stapling of hernia meshes in an onlay model in the rat. Hernia 9: 322–329
Scheidbach H, Tamme C, Tannapfel A, Lippert H, Kockerling F (2004) In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty: an experimental study in pigs. Surg Endosc 18: 211–220
Schmidbauer S, Ladurner R, Hallfeldt KK, Mussack T (2005) Heavy-weight versus low-weight polypropylene meshes for open sublay mesh repair of incisional hernia. Eur J Med Res 10: 247–253
Wake BL, McCormack K, Fraser C, Vale L, Perez J, Grant AM (2005) Transabdominal preperitoneal (TAPP) vs totally extraperitoneal (TEP) laparoscopic techniques for inguinal hernia repair. Cochrane Database Syst Rev CD004703
Acknowledgments
We thank Karl Glaser, MD, and Christopher May, MD, for reviewing the manuscript and Karl Kropik, Eng., for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. H. Fortelny and S. H. Petter-Puchner contributed equally to this manuscript
Rights and permissions
About this article
Cite this article
Fortelny, R.H., Petter-Puchner, A.H., Walder, N. et al. Cyanoacrylate tissue sealant impairs tissue integration of macroporous mesh in experimental hernia repair. Surg Endosc 21, 1781–1785 (2007). https://doi.org/10.1007/s00464-007-9243-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-007-9243-7