Abstract
Introduction
Ventral hernias are a common problem in a general surgery and hernioplasty is an integral part of a general surgeon’s practice. The use of prosthetic material has drastically reduced the risk of recurrence, but has introduced additional potential complications such as surgical wound infections, adhesion formation, graft rejection, etc. The development of a wound infection in a hernia that is repaired with a prosthetic material is a grave complication, often requiring removal of the prosthesis. This experimental study examined efficacy of completely absorbable, hydrophilic, PGA–TMC (polyglycolic acid–trimethylene carbonate) prosthesis impregnated with antibiotic for reduction of infectious complications.
Methods
Antibiotic-impregnated PGA–TMC prostheses were placed intraperitoneally in 90 Wistar white rats that were randomized and distributed into four groups. Group 0 (23 rats): there were placed PGA–TMC prosthesis without antibiotic impregnation (control group). Group 1 (25 rats): meshes were placed and infected later with 1 × 108 UFC of S. aureus/1 ml/2 cm2 (Staphylococcus aureus ATCC 6538 American Type Culture Collection, Rockville, MD). Group 2 (21 rats): cefazolin-impregnated prostheses were placed (1 g × 100 ml, at the rate of 1 ml/cm2 of prosthesis) and were subsequently infected with the same bacterial inoculate. Group 3 (21 rats): cefazolin-impregnated prostheses with double quantity of cefazolin and infected. A week later these animals were killed and specimens were extracted for bacterial quantification and histological studies.
Results
Evident decrease of bacterial colonization was observed in series 2 and 3 [the ones impregnated with cefazolin, in comparison with the group 1 (infected without previous antibiotic impregnation)] with statistically significant results (p < 0.00). Results were really positive when the antibiotic solution had been applied to the mesh. There have been formed adherences to the prosthesis when placing it in contact with intraabdominal viscera. However, cefazolin impregnation of the mesh has reduced an adhesion formation, mostly when the infection reached a minimum, inhibiting the inflammatory answer to the infection in a prosthetic material.
Conclusion
Impregnation of the absorbable hydrophilic prosthesis PGA–TMC with cefazolin prevents the infection of the prosthesis placed in infected localization. Therefore, we think this option should be considered as a new and useful alternative in case of contaminated and dirty surgical fields or when a replacement of the prosthesis is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hernia disease has an important economic impact. Despite being considered a trivial disease, it represents a huge social cost.
The goal of surgeons is to be able to offer their patients the best treatment. In hernia surgery, one of the main concerns to be considered is surgical wound infection, particularly those related to the prosthetic materials. In fact, nosocomial infections are a growing problem that concerns all European healthcare systems [1].
An increasing number of hernia repairs are performed each year in Spain. According to the Spanish Society of Epidemiology, the rate of surgical infections in Spain is 4.6 %, it means 100,000 new cases per year that increase the average hospital stay from 7 to 10 days. Severe cases of infections are commonly related to the use of surgical implants such as vascular or orthopaedic prostheses [2].
About 75 % of Spanish hospitals have created units of infection prevention and control in order to decrease the rate of surgical infections [2, 3].
Infection is difficult to be treated when the wound becomes colonized. In fact, this complication occurs in Spain, with the similar frequency than in the rest of Western Europe [1, 2]. Surgeons are aware of this problem, being implemented different measures to minimize the impact of infections. However, our experience shows that it is very difficult to solve this problem. As a consequence of inadequate use of antibiotics, we have created bacterial resistance. Surgical infections have a serious clinical and socioeconomic impact; we have to consider that the cost of infectious complications management in digestive surgery has triplicated the total direct cost of the surgery. Furthermore, the economic impact, patients’ quality of life and waiting lists are usually affected [1, 3].
The ideal prosthesis has not been developed yet, but new totally or partially absorbable materials, such as PGA–TMC, are being launched to the market in order to be used for abdominal wall surgery, especially in contaminated fields [4, 5].
The use of absorbable materials, such as PGA–TMC, has already been experimentally tested in inguinal hernia repair, allowing prosthesis to be impregnated or embedded with a solution of antibiotics or antiseptics.
Current trends in hernia repair are focussed on the development of new prosthetic materials which do not need traumatic fixation, and include substances designed to stimulate proper healing and to decrease the rate of infection [5, 6].
We have developed an experimental study in order to decrease infection and control bacterial colonization at the site where the prosthesis is implanted. We have impregnated a totally absorbable mesh with an antibiotic solution, which would be progressively released at the surgical site.
Method
Material
Ninety Wistar white rats weighing 200–350 g were used as experimental animals. Each animal was kept in an individual cage. The study was conducted with the approval of the local ethics committee for experimental studies.
The prosthetic material used during the study was a 2 × 2 cm and 0.10 cm thick absorbable mesh made of 67 % trimethylene carbonate polyglycolate and 33 % (PGA–TMC). Each mesh was impregnated with 4 ml of cefazolin (1 ml/cm2 of mesh). Meshes were fixed using sutures made of polypropylene (Prolene 2.0).
The surgical procedure of mesh implantation was performed using a general anaesthesia, intraperitoneal application of Ketamine (20 mg/kg).
The inoculum of bacteria used was 1 × 108 UFC of S. aureus/1 ml/2 cm2 (S. aureus ATCC 6538 American Type Culture Collection, Rockville, MD).
Method
-
1.
Preliminary “in vitro” studies prior to animal testing:
-
The first step was to determine the absorption capacity of the material, being established in 1 ml/cm2 (4 ml of cefazolin each mesh).
-
The second “in vitro” test was conducted to determine the ability of the antibiotic-impregnated prosthesis to inhibit microbial growth. The mesh was placed in a Petri dish and infected with the inoculum. No infection was detected in the mesh.
-
The third one was conducted using a contaminated Petri dish with a culture of S. aureus. 24 h later an antibiotic-impregnated mesh was placed in it. There was an inhibition of the bacterial growth around the mesh, and in the mesh.
-
-
2.
Surgery: under general anaesthesia, animals underwent a midline laparotomy placing intraperitoneally 2 × 2 cm mesh, it was fixed to the fascia using a nonabsorbable suture. All animals were killed 7 days later, analysing macroscopic findings and removing the anterior abdominal wall for microbiological and histological study in order to check microbial growth and the pathological changes developed during the healing process in the different groups (Fig. 1).
-
3.
Groups: animals were distributed into four randomized groups:
-
Group 0 (23 animals), PGA–TMC prosthesis was implanted as a control group.
-
Group 1 (25 animals), PGA–TMC prosthesis with the bacterial inoculum was placed.
-
Group 2 (21 animals), antibiotic-impregnated PGA–TMC prosthesis with cefazolin and the bacterial inoculum were placed.
-
Group 3 (21 animals), antibiotic-impregnated PGA–TMC prosthesis with double concentration of cefazolin and the bacterial inoculum were implanted.
-
-
4.
Macroscopic and histological study of implants:
-
Macroscopic findings of the skin. Wound and meshes were analysed, identifying the rate of adhesions to the mesh. The scale used was: [7] grade 0, no adhesion; grade 1 (soft), extremely labile adhesions; grade 2 (medium), adhesions more firm, but removable with blunt dissection; grade 3 (hard), firm adherence removable only with sharp instruments.
-
Histological study: hematoxylin–eosin was used to examine the specimens. Two sections were made in each piece.
-
-
5.
Microbiology: the procedure to quantitate bacterial culture was performed by placing the specimens in a glass homogenizer with 1 ml of saline and seeding them subsequently on the blood agar. The plates were incubated at 37 °C for 24 h, whereupon the number of colony grown units per gram of tissue was assessed.
-
6.
Statistical analysis of samples: statistical analysis was performed using SPSS 14.0 software. For the analysis of the samples we used the nonparametric test: Wilcoxon, Kruskal–Wallis test for independent samples and Friedman test for paired samples. Statistical significance was p < 0.05.
Results
No recurrences were found in any of the groups. Although meshes were absorbed in nearly 50 % of the original size, the remaining fragments of the prosthesis showed no shrinkage.
Macroscopic findings and histological study (Table 1; Fig. 2)
-
Group 0
-
Two animals died (2/23) in the first six postoperative hours due to problems related to the general anaesthesia.
-
Five others showed macroscopic signs of active infection, tested with positive microbiological cultures. (5/21 alive animals).
-
10 out of those 21 rats showed intraperitoneal adhesions to the mesh (3/10 showed firm adhesions).
-
Macroscopic view of the skin, the mesh, and the pathology of the explant. Group 0 normal skin, no infection and normal inflammatory cell. Group 1 infected wound, infection of the mesh, strong cluster of bacteria and inflammatory response. Group 2 normal skin, no infection and correct pathology. Group 3 normal skin, normal mesh and normal inflammatory response
Histology: the inflammatory reaction to the mesh did not show special inflammatory parameters as of cells distribution. A foreign body reaction with high macrophage component without abscesses or necrosis was present in the specimens.
-
Group 1: In group 1 (contaminated mesh), among the results are:
-
Three animals died in the first four postoperative hours due to problems related to the general anaesthesia. (3 animals/25).
-
All meshes showed macroscopic sings of infection (22 animals).
-
This group showed adhesions in all cases (22 animals), being 12 of them qualified as grade 3.
-
Histology: there was a severe reaction to the mesh with a global infection, abscesses and necrosis in the specimens.
-
Group 2
-
None of the animals died during the study.
-
A clear reduction of the rate of infected meshes was found. Infection was only detected in four animals (even less than in group 0, control group).
-
Intraperitoneal adhesions to the mesh were found in 12 animals (12/21), with similar distribution in the quality of them (mild, medium or strong).
-
Macroscopic findings; we can observe a trend towards reduction of mesh infection in this group and as well a decrease in adhesion to the mesh.
Histology: there was a reduction in the inflammatory parameters (necrosis, abscesses, macrophage, etc.) and a partial reabsorption of the mesh.
-
Group 3
-
None of the animals died during the study.
-
There was no macroscopic mesh infection in rats of this group.
-
Only three rats had developed adhesions (3/21), mild in two cases and strong in the other.
-
Histological findings show a normal distribution of the inflammatory cells (very similar to the Group 0), with normal incorporation of the biomaterial to the tissues of the rats.
Infection
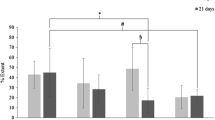
The amount of bacteria (number of bacteria CFU × 104/g of tissue) has been expressed in correction with decimal logarithms (number of grown colonies)/ml. Infection is considered if the number of bacteria (CFU × 104) in the specimens is larger than 80–100/g of tissue (Fig. 3).
-
Group 0: average: 2.3988. Median (50 percentile): 0.
-
Group 1: average: 6.2918 (213,4737 without the logarithmic correction). Median (50 percentile): 6.2695.
-
Group 2: average: 4.0889 (91,2000 without the logarithmic correction). Median (50 percentile): 5.5851.
-
Group 3: average: 3.3426 median (39,6500 without the logarithmic correction). Median (50 percentile): 5.1034.
The statistical analysis of the result shows:
-
Group 0 vs group 1: there are significant differences between these two groups (p < 0'000).
-
Group 0 vs group 2: there are also significant differences (p < 0.05).
-
Group 0 vs group 3: no statistical difference was found (p > 0.1).
-
Group 1 vs group 2: there is statistical difference (p < 0.000) between both groups.
-
Group 1 vs group 3: there is statistical difference (p < 0'000).
-
Group 2 vs group 3: when we compared the antibiotic-impregnated mesh and the one with double amount of antibiotics no significance was found.
Adhesions to the mesh (Table 1)
-
Group 0: 10/21 (47.61 %) animals with adhesions (2 mild, 2 medium, 6 severe).
-
Group 1: 19/22 (86.36 %) animals with adhesions (3 mild, 4 medium, 12 severe).
-
Group 2: 12/21 (57.14 %) animals with adhesions (3 mild, 4 medium, 5 severe).
-
Group 3: 3/21 (14.28 %) animals with adhesions (2 mild, 1 severe).
There was observed an important reaction to the mesh infection, what was shown by an increase of adhesion formation and its severity. Antibiotics protect from infection and therefore from adhesions in number and quality.
A statistical analysis of the adhesions shows that the difference between groups 0 and 1 was no statistically significant result (figures in the group 1 were smaller than the group 2, but with no significant differences in statistical tests). In the comparison between groups 2 and 1, and groups 3 and 1 there were statistically significant differences (p < 0.001), therefore, with decreasing mesh infection, also decreased the number of adhesions.
Discussion
The surgical site infections represent approximately 40 % of all nosocomial infections according to the study performed by the Institute for Healthcare Improvement (IHI). Nearly 3 % of patients undergoing surgery develop a surgical infection, which prolongs hospitalization and increases economic costs. These infections take on a special character when it comes to prosthetic surgery, and denote us that material acts as a vector for bacteria [8].
The magnitude of the problem of mesh-related infection has a high cost consequence; Schierholz and Beuth in 2001 analysed the cost of prostheses and graft infection in patients, which entails an expenditure of 11 million dollars annually [9].
Taylor et al. [10] recorded up to 20 % of late infections after hernia repair in a surveying study in British surgeons (analysis in vivo of the infection and colonization of bioprosthesis). Thus, the existence of bacteria does not imply infection. They conclude their work stating their preference for macroporous and absorbable mesh in order to prevent infection.
The infection of the abdominal wall prosthesis has been previously studied both in patients and experimental models similar to the one developed in our study.
In our experiment, the choice of the microorganism did not involve difficulties, since S. aureus is primarily responsible for nosocomial infections, especially in prosthetic surgery of the abdominal wall.
Falagas and Kasiakou [11] presented a literature review to get a better understanding of prosthetic infections in hernia surgery and their causes, finding that the organisms associated with cases of infection in the mesh are staphylococci, mainly S. aureus, and Streptococcus spp. (Including group B streptococci), Gram-negative bacteria (mainly Enterobacteriaceae), and finally, anaerobic bacteria (including Peptostreptococcus spp.).
Ott et al. [12] tested the resistance of current mesh infection comparing different materials (titanium-coated polypropylene, polyglycol and pigs subdermal collagen prosthesis) in rats (n = 96), contaminated with a mixture of bacteria: E. coli, Enterobacter, Bacteroides, and S. aureus. It confirms that colonization of the mesh is mainly caused by S. aureus. The used methodology is similar to our study, with the same measure and a similar bacterial inoculum. Bacteria producing biofilms (slime) cause more infections in prosthetic material (in this case staphylococci), adhering to the mesh and producing a persistent infection. All meshes with hard adhesions were infected, compared with non-contaminated hernioplasty in control group. This is a significant fact that we found a decrease of inflammatory cells when the absorbable mesh was used (p < 0.01). In this study there is no reference to any reduction of the infection, but it raises the possibility that the advance of coated absorbable prostheses and other materials may be a good way to fight against infections after having been performed a hernioplasty [12].
The dose of bacterial inoculum used in our study followed scientific literature recommendations, and is also suggested by Bellon, Klinge and other authors who have experimented on animals using S. aureus or other bacteria. [13–15].
The microbiological methodology for counting bacteria in the explants is the standard used in all studies of prosthetic infection.
Bellon et al. [14] examines macro- and microscopic characteristics of polypropylene and PTFE infected with S. epidermidis and S. aureus in rabbits and found that those bioprostheses infected with S. aureus showed denuded areas with exposed filaments in the polypropylene; in PTFE surface erosion of the prosthesis, necrosis and haemorrhage were observed.
Another experiment by Klinge et al. was focussed on the mesh properties. This study compared two meshes, a monofilament and multifilament (polypropylene, polypropylene with polyglactin composite) each one in terms of infection with S. aureus in rats (n = 72). It was found that there was an increment in bacterial adherence to the multifilament mesh, without active infection (no significant difference in the macroscopic view of infection). It was associated with less inflammatory reaction and decreased response to foreign body in the multifilament mesh. These results explain that bacterial adhesion to foreign depends on polymer and its surface (lower in the macroporous and monofilament) [15].
Experimentally, bacterial adhesion can be reduced by metal salts and antibiotics (tried out with e-PTFE), also demonstrated with S. aureus [14].
Junge described the incorporation of antimicrobials to nonabsorbable bioprosthesis, the fluoropolymers (whose main representative is the PTFE). In a study of infection caused in a polyvinylidene fluoride mesh (PVDF, polyvinylidenfluoride) with gentamicin incorporated [16], different microorganisms were used in this experiment (S. aureus, E. coli, S. epidermidis) in 45 rats. It was concluded that the experience was right, and the addition of antibiotics to meshes helps to prevent infection both in vitro and in vivo, with no significant alterations of integration of the mesh itself (good biocompatibility, including less inflammatory response) [15, 16].
Agalar et al. [17] describes in his experimental article on polypropylene mesh infection in rats, an experience very similar to our study. The authors used S. epidermidis in 70 rats to contaminate a normal density polypropylene mesh. The purpose is to check whether the local application of antibiotic prevents infection and subsequent intraabdominal sepsis by spreading microorganisms. They applied intraperitoneally rifampicin and teicoplanin. Their results were positive, and supported that applied antibiotics prevented the infection of the meshes [17].
In our results, comparing the group 0 (no infection) with group 1 (infected mesh), the presence of infection suggests that despite working according to a meticulous aseptic technique, meshes are always in risk to be infected. Absorbable materials resist infection better, but they can also suffer from this infection [10, 11].
These results reinforce our idea about the advantages of using an active mesh to release antibiotics in the area where it is required, because the use of absorbable mesh is not enough to decrease the infection as shown in the analysis of van’t Riet et al. [18]. A subsequent comparison of results between groups with antibiotics (groups 2 and 3) with group 1 corroborates its effectiveness against infection.
We think the use of absorbable mesh for treatment of surgical site infection is valid, and the incorporation of antimicrobials is viable as has been demonstrated in our study and in literature; this experimental experience shows good outcomes, decreasing the rate of infection after having performed hernia repairs.
Conclusion
-
This experimental model is valid for the study of infections in bioprostheses for hernia surgery and its prevention. Model and technique used are easily reproducible.
-
PGA–TMC implants are absorbable, hydrophilic, maintain a constant size, and can be used successfully for the correction of the hernia defect, no recurrence in any case detected.
-
Bacterial colonies have evidently decreased in number in the groups 2 and 3, those values are statistically significant.
-
Usually, those implants with a low rate of bacterial colonization have a lesser amount of adhesions, as showed by statistically significant differences.
To sum up, we acknowledge the contribution of the antibiotics embedded in this new material for preventing infection of the prosthetic material we use in hernia repair. These results can be extrapolated to clinical practice, being especially important in emergency surgery with contaminated or dirty surgical fields, and reoperations as a result of surgical site infection.
References
Zeller JL, Burke AE, Glass RM (2007) JAMA patient page. MRSA infections. JAMA 298(15):1826
Torrobal L, Rivero M, Otermin I, Gil A, Maraví-Poma E, García Irure JJ (2000) Antimicrobian resistance and antibiotics policy: MRSA, GISA and VRE. Anales Sis San Navarra 23(Suppl. 2):69–80
Rampling A, Wiseman S, Davis L, Hyett AP, Walbridge AN, Payne GC, Cornaby AJ (2001) Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect 49(2):109–116
Conference Consensus (1984) Clinical applications of biomaterials. JAMA 1984(249):1050
Carbonell AM, Kercher KW, Sing RF, Heniford BT (2005) Susceptibility of prosthetic biomaterials to infection. Surg Endosc 19(12):1670
Amid PK (1997) Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1:15
Martin-Cartes J, Morales-Conde S, Suarez-Grau J, Lopez-Bernal F, Bustos-Jimenez M, Cadet-Dussort H, Socas-Macias M, Alamo-Martinez J, Tutosaus-Gomez JD, Morales-Mendez S (2008) Use of hyaluronidase cream to prevent peritoneal adhesions in laparoscopic ventral hernia repair by means of intraperitoneal mesh fixation using spiral tacks. Surg Endosc 22(3):631–634
Culver DH, Horan TC, Gaynes RP, National Nosocomial Infections Surveillance Systems (NNIS) (1991) Surgical wound infections rates by wound class, operation, and risk index in U.S. hospitals. Am J Med 91(Suppl. 3B):152–157
Schierholz JM, Beuth J (2001) Implant infections: a haven for opportunistic bacteria. J Hosp Infect 49(2):87–93
Taylor EW, Duffy K, Lee K, Hill R, Noone A, Macintyre I, King PM, O’Dwyer PJ (2004) Surgical site infection after groin hernia repair. Br J Surg 91(1):105–111
Falagas ME, Kasiakou SK (2005) Mesh-related infections after hernia repair surgery. Clin Microbiol Infect 11(1):3–8
Ott R, Hartwig T, Tannapfel A, Blatz R, Rodloff AC, Madaj-Sterba P, Möbius Ch, Köckerling F (2007) Biocompatibility of bacterial contaminated prosthetic meshes and porcine dermal collagen used to repair abdominal wall defects. Langenbecks Arch Surg 392(4):473–478
Suarez Grau JM, De Toro Crespo M, Docobo Durantez F, Rubio Chaves C, Martin Cartes JA, Docobo Perez F (2007) Prevention of surgical infection using reabsorbable antibacterial suture (Vicryl Plus) versus reabsorbable conventional suture in hernioplasty. An experimental study in animals. Cir Esp 81(6):324–329
Bellon JM, Garcia-Carranza A, Garcia-Honduvilla N, Carrera-San Martin A, Bujan J (2004) Tissue integration and biomechanical behaviour of contaminated experimental polypropylene and expanded polytetrafluoroethylene implants. Br J Surg 91(4):489–494
Klinge U, Junge K, Spellerberg B, Piroth C, Klosterhalfen B, Schumpelick V (2002) Do multifilament alloplastic meshes increase the infection rate? Analysis of the polymeric surface, the bacteria adherence, and the in vivo consequences in a rat model. J Biomed Mater Res 63(6):765–771
Junge K, Rosch R, Klinge U, Krones C, Klosterhalfen B, Mertens PR, Lynen P, Kunz D, Preiss A, Peltroche-Llacsahuanga H, Schumpelick V (2005) Gentamicin supplementation of polyvinylidenfluoride mesh materials for infection prophylaxis. Biomaterials 26(7):787–793
Agalar C, Ozdogan M, Agalar F, Saygun O, Aydinuraz K, Akkus A, Ceken S, Akturk S (2006) A rat model of polypropylene graft infection caused by Staphylococcus epidermidis. ANZ J Surg. 76(5):387–391
van’t Riet M, de Vos van Steenwijk PJ, Bonjer HJ, Steyerberg EW, Jeekel J (2007) Mesh repair for postoperative wound dehiscence in the presence of infection: is absorbable mesh safer than non-absorbable mesh? Hernia 11(5):409–413
Conflict of interest
JMSG declares no conflict of interest. SMC declares no conflict of interest. VGG declares no conflict of interest. JMC declares no conflict of interest. FDD declares no conflict of interest. FJPD declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suárez-Grau, J.M., Morales-Conde, S., González Galán, V. et al. Antibiotic embedded absorbable prosthesis for prevention of surgical mesh infection: experimental study in rats. Hernia 19, 187–194 (2015). https://doi.org/10.1007/s10029-014-1334-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-014-1334-5