Abstract
Background and aims
A contaminated or infected surgical site is considered a contraindication for the use of the nonabsorbable alloplastic materials employed to repair abdominal wall defects. Therefore, the biocompatibility of new prosthetic materials was investigated.
Materials and methods
Meshes measuring 1.5×1.5 cm made of conventional and titanium-coated polypropylene, polyglycol, or porcine dermal collagen were implanted under the abdominal wall of 96 rats (eight groups of 12 animals each) employing the inlay technique. Implantation of all four materials was performed both under semisterile conditions and bacterial contamination of the mesh. The meshes were explanted after 28 days.
Results
All the materials implanted under semisterile conditions were incorporated into the abdominal wall with only few intraabdominal adhesions (mean adhesion scores: 1.0, 1.2, 1.0, 0.8 points, respectively, not significant). With the porcine dermal collagen, proliferation rate and the proportion of inflammatory cells were statistically lower (p<0.01). In the bacterial contamination group, all meshes were associated with a suppurating infection and strong adhesions between the bowel and mesh, which were most prominent in the case of dermal collagen (mean adhesion scores: 1.6, 1.7, 1.7, and 1.9 points, respectively, not significant). In this group, two animals died of peritonitis. In comparison with the other materials, the proliferation rate was significantly elevated (p=0.03). No significant differences were seen between the other materials employed.
Conclusion
Irrespective of the material employed, implantation of alloplastic meshes in an abdominal wall contaminated with bacteria, is associated with suppurating infections, in particular in the case of the membrane-like porcine dermal collagen. Nonabsorbable alloplastic meshes and dermal skin grafts should therefore not be used to repair infected abdominal wall defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous studies have shown that in hernia surgery, the recurrence rate can be dramatically reduced through the use of prosthetic materials, in particular alloplastic meshes [1–3]. However, infection of such implants is a dreaded complication, so that a contaminated wound is considered a relative contraindication for the use of such materials [4]. This has meant that many patients have been unable to benefit from the biomechanical properties of these meshes. In the presence of peritonitis, a stoma or intraoperative breaching of the colon, either no materials or absorbable materials (polyglycol meshes) are employed. The result has been a considerable prolongation of treatment, and subsequent interventions for definitive hernial repair. Against this background, the development of materials combining the mechanical properties of alloplastic meshes with good biocompatibility that allow implantation in contaminated or even infected wounds is most desirable. Because of their excellent tissue tolerability, titanium-coated implants are employed in particular in the fields of orthopedics and orthodontics. In the porcine model, it has been shown that the titanium coating of polypropylene meshes significantly reduces the inflammatory reaction in the tissue. In view of these particular properties, it has been postulated that titanium-coated alloplastic materials and biological materials (autodermal skin graft or porcine dermal collagen) might have advantages over conventional materials in infected tissue; studies to date have, however, failed to confirm this hypothesis [5, 6]. The present experimental study compares the biocompatibility of titanium-coated polypropylene meshes (tPPM) and porcine dermal collagen (PDC) with the commonly employed polypropylene meshes (PPM) and the absorbable polyglycol mesh (PGM) implanted into the bacterially infected abdominal wall.
Materials and methods
Surgical groups
The study was carried out in compliance with the German animal protection law. A total of 96 female Sprague–Dawley rats weighing between 250 and 300 g were divided into eight groups of 12 animals each. In groups 1 to 4, the procedure was performed under “clean” (nonsterile) conditions, with the following mesh materials being implanted: in group 1 a titanium-coated polypropylene mesh (TiMesh light®, GfE, Nuremberg, Germany), in group 2 a noncoated polypropylene mesh (Premilene®, Aesculap, Tuttlingen, Germany), in group 3 a polyglycol mesh (Vicryl®, Ethicon, Norderstedt, Germany), and in group 4 deproteinized, sterilized pig skin (porcine dermal collagen) Xenoderm®, GfE, Nuremberg, Germany). In groups 5 to 8, the meshes were contaminated with a standardized bacterial suspension before implantation.
Surgical procedure
After first rendering the animals unconscious with ether, anesthesia was applied by intramuscular injection of ketamine (100 mg/kg bw), together with atropine (0.1 mg/kg bw), and xylazine (10 mg/kg bw).The abdomen was then shaved and disinfected with alcohol, and a 3-cm-long median incision made. Thereafter, a mesh measuring 1.5×1.5 cm was placed in the right lower abdomen using the inlay technique, and fixed to the peritoneum with a continuous suture (Vicryl® 4.0). The muscle layer and skin were then closed each with a continuous suture (Vicryl® 4.0). Postoperatively, all animals received metamizole (0.8 mg/ml) in the drinking water to control pain. The daily amount of water drank varied between 15 and 35 ml. Water and a standard feed were proffered 6 h after the procedure. During the first 6 weeks, the animals were housed in individual cages.
On the 28th postoperative day, the animals were examined, and any signs of an infected wound or peritonitis were recorded. Animals with clinical signs of peritonitis were killed and a postmortem was performed. At the end of the period under observation, the animals were killed with CO2 gas. The abdominal wall was then disinfected and excised en bloc together with the underlying mesh. A photograph was taken of the intraabdominal adhesions. The excised specimens were submitted to a microbiological and histological work-up.
Bacterial suspension
As a contaminant for the mesh, a standardized bacterial suspension was prepared. This contained 2×107 Bacteroides, 2×106 Escherichia coli, Enterokokken, and 5×106 Staphylococcus aureus bacteria in a volume of 50 μl. After preparation, the suspension was deep-frozen at −40°C, thawed immediately before surgery and, in groups 5 to 8, 0.1 ml applied to the mesh.
Documentation of adhesions
The extent of the adhesions on the implanted alloplastic material was assessed by an independent examiner on the basis of the photographs, and given a semiquantitative adhesion score (AS) (0 point: no adhesions, 1 point: omentum adherent; 2 points: adherent intestinal coils).
Histological and microbiological examination
For the microbiological examination, the specimens were placed in a sterile culture medium and investigated in accordance with standard methods. As a marker of a persistent bacterial infection, only S. aureus was employed because this bacterium is not normally to be found on the rat pelt.
For the histological workup, the specimens were first fixed in 10% formalin, embedded in paraffin, and cut into 3 μm-thick sections. These were then stained with hematoxylin and eosin and elastica van Giesson. Apoptosis was determined with the in situ end labeling (ISEL) method, proliferation with the aid of a Ki67 antibody using established methods. The extent of the inflammation was calculated by quantification of the partial volume of inflammatory infiltrate (%PV) using the methods described by Klinge et al. [7]. The histological workup and quantitative evaluation were done by a pathologist who was blind to the type of mesh material involved.
Statistics
For data analysis the SPSS 13-0.1 for Windows program was used. The results are presented as mean values±standard deviations. Differences in continuous variables were tested with the ANOVA test, discontinuous variables with Fisher’s exact test. For pairwise comparisons, the Tukey method was used, which is adjusted for multiple testing. Statistical significance was assumed at a value of p<0.05.
Results
Clinical findings
In the case of clean mesh implantation (groups 1–4), no infection of the wound or postoperative mortality occurred in any of the groups. Adhesions to the implanted mesh were least pronounced with porcine dermal collagen and most prominent with polypropylene meshes (mean adhesion score 10 vs 14 points, not significant). In the contaminated abdominal wall arm (groups 5–8), two rats in the PDC group died of peritonitis (days 3 and 4), and this group also contained the most wound healing disorders (n=4) in comparison with tPPM, PPM, and PGM (n=2 in each). Adhesions between bowel and mesh were found in most of the animals, most pronounced in PDC, least pronounced in tPPM (mean adhesion score 23 vs 19 points, not significant) (Table 1).
Histological findings and immunostaining
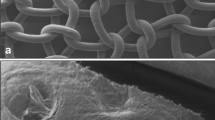
In the clean implantation arm, the histological analysis revealed regular incorporation (into the tissue) of all the materials employed (Fig. 1). Almost no inflammation and only few granulocytes were occasionally seen. We failed to observe foreign bodies in this study arm. In comparison with the other materials, PDC was shown histochemically to be associated with a significantly lower proliferation rate (Ki67) and a smaller partial volume of inflammation in the tissue. Furthermore, PDC was also associated with a lower apoptotic rate (ISEL), although the difference was not statistically significant. No significant differences were found between the other mesh materials. Under the microscope, all bacterially contaminated meshes revealed infection with signs of abscess formation. This was most pronounced in the case of PDC, and in some cases was associated with almost complete breakdown of the pigskin (Fig. 2). The proliferation rate was correspondingly elevated significantly (p=0.03), while the volume of inflammation and apoptotic rate revealed a trend towards higher figures in this group. Again, no significant difference was found between the other materials used (Table 2).
Microbiology
The microbiological investigation of the specimens revealed S. aureus in only two animals of the clean implantation arm (one with tPPM and one with PPM). In the bacterial contamination arm, however, S. aureus was cultivated in the majority of the animals (9 tPPM, 8 PPM, 10 PGM, and 6 PDC, p=not significant).
Discussion
For the repair of major abdominal wall defects, alloplastic meshes or other foreign materials frequently have to be employed when the fascia is not strong enough. Currently, various polypropylene meshes, more rarely expanded polytetrafluoroethylene patches (ePTFE), are most commonly employed. In noninflammed tissue, these alloplastic materials have excellent biomechanical properties. Numerous studies have shown that the recurrence risk for an incisional hernia repaired with these nonabsorbable materials in comparison with primary suture only can be significantly reduced [1–3]. As a dreaded complication, however, early postoperative infection of the mesh is considerably more likely. Over the long term, abundant adhesions to the bowel, possibly with the formation of a fistula leading to considerable morbidity and problems in the event of subsequent recurrence repairs, frequently occur [8]. Mainly on account of the risk of mesh infection, no polypropylene meshes or ePTFE materials are employed to repair infected or contaminated abdominal wall defects; autologous material (autodermal skin graft) or absorbable meshes (e.g., polyglycol) are used instead [9]. After the absorption of such meshes, however, a large hernia requiring a further intervention to achieve definitive repair is a regular occurrence. For this reason, the availability of materials with sufficient mechanical stability combined with better biocompatibility making them suitable for use in infected tissue would be most desirable.
In the porcine model, titanium-coated systems and meshes have been reported to possess superior biocompatibility to that of conventional materials; however, implantation was being carried out under sterile conditions [5]. In a comparison of tPPM with other meshes, Scheidbach et al. were able to show an appreciably reduced inflammatory reaction in the tissue, with the CD4/CD8 ratio, the apoptotic index, B-lymphocyte, macrophage, and monocyte activation all being significantly lower. On the basis of these data, it has been postulated that even in the presence of bacterial contamination, tPPM might be associated with a less marked inflammatory reaction.
In our rat model, no differences were found between tPPM, PPM, and PGM in terms of proliferation, apoptosis, or the percentage volume of inflammatory cells in the tissue (%PV), neither in the semisterile nor in the bacterial contamination arm, nor the clinical parameters (wound infection rate, peritonitis, and adhesion score) did any differences between the individual materials reveal. This is in accordance with the results of the experiments of Junge et al. [10], where no beneficial effect of titanium coating of propylene meshes was reported in a rat model. In the bacterial contamination arm, abscess-generating mesh infection was observed with all the materials used. It must, however, be noted that a very large number of bacteria were applied to the meshes, so that severe local peritonitis was induced. This means that our results cannot be unreservedly applied to the clinical situation because the mild contamination that might occur clinically after a brief breaching of the bowel was not investigated. In this situation, patients usually receive perioperative antibiotic prophylaxis, which may prevent mesh infection. Some investigators have reported that an incisional hernia can certainly be repaired with PPM when simultaneous resection of the colon or stoma is performed, with no increase in the incidence of mesh infection [11]. This suggests that currently valid restrictions of the indication for mesh implantation in contaminated surgical areas might possibly be too strict.
It is possible that as a determinative factor for the biocompatibility properties of prosthetic materials in the infected environment, pore size may be more important than other material parameters. Earlier studies reporting very high rates of mesh infections were done with heavyweight, small-pore, and/or multifilament meshes [12, 13]. Experimental or clinical data on the use of modern lightweight and large-pore meshes are currently not available. In comparison with microporous (e.g., ePTFE) or membranous materials (e.g., PDC), large-pore and monofilament meshes are more readily accessible to the actions of the immune system, so that control of local infection is better [7]. Bacterial biofilms, which develop in particular on membranous alloplastic materials, are considered to be the major pathogenetic factor for the adherence of bacteria to the foreign material and the development of persistent infections [14, 15].
Our observation that in the presence of bacterial contamination, PDC is associated with a particularly severe inflammatory reaction, is in accord with numerous other clinical and experimental reports in the literature. These have repeatedly noted that the infection rates associated with the use of membranous or microporous materials (ePTFE, PDC, and autodermal skin grafts) are greater than those seen with meshes [4, 13, 16–18].
Conclusion
In summary, titanium-coated polypropylene meshes have no particular advantages in infected tissue. In comparison with other materials employed, deproteinized porcine skin is associated with a particularly severe infection, and is therefore not suitable for use in infected tissue. The repair of infected or contaminated abdominal wall defects is still an unresolved problem. Our results show that the use of alloplastic material to repair an infected abdominal wall defect should as far as possible be avoided. Potential benefits may be provided in the future by large-pore or antibacterial-coated meshes, which are currently investigated in animal experimental studies [19].
References
Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN, Boelhouwer RU, de Vries BC, Salu MK, Wereldsma JC, Bruijninckx CM, Jeekel J (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343(6):392–398
Korenkov M, Sauerland S, Arndt M, Bograd L, Neugebauer EA, Troidl H (2002) Randomized clinical trial of suture repair, polypropylene mesh or autodermal hernioplasty for incisional hernia. Br J Surg 89(1):50–56
Flum DR, Horvath K, Koepsell T (2003) Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg 237(1):129–135
Law NW, Ellis H (1991) A comparison of polypropylene mesh and expanded polytetrafluoroethylene patch for the repair of contaminated abdominal wall defects—an experimental study. Surgery 109(5):652–655
Scheidbach H, Tannapfel A, Schmidt U, Lippert H, Köckerling F (2004) Influence of titanium coating on the biocompatibility of a heavyweight polypropylene mesh. An animal experimental model. Eur Surg Res 36(5):313–317
Chaplin JM, Costantino PD, Wolpoe ME, Bederson JB, Griffey ES, Zhang WX (1999) Use of an acellular dermal allograft for dural replacement: an experimental study. Neurosurgery 45:320–327
Klinge U, Junge K, Spellerberg B, Piroth C, Klosterhalfen B, Schumpelick V (2002) Do multifilament alloplastic meshes increase the infection rate? Analysis of the polymeric surface, the bacteria adherence, and the in vivo consequences in a rat model. J Biomed Mater Res 63:765–771
van ‘t Riet M, de Vos van Steenwijk PJ, Bonthuis F, Marquet RL, Steyerberg EW, Jeekel J, Bonjer HJ (2003) Prevention of adhesion to prosthetic mesh: comparison of different barriers using an incisional hernia model. Ann Surg 237(1):123–128
Lopez Villalta GC, Furio-Bacete V, Ortiz Oshiro E, Lopez DO, Vaca Vaticon D, Fdez-Represa JA (1995) Experimentally contaminated reabsorbable meshes: their evolution in abdominal wall defects. Int Surg 80(3):223–226
Junge K, Rosch R, Klinge U, Saklak M, Klosterhalfen B, Peiper C, Schumpelick V (2005) Titanium coating of a polypropylene mesh for hernia repair: effect on biocompatibility. Hernia 9(2):115–119
Birolini C, Utiyama EM, Rodrigues AJ Jr, Birolini D (2000) Elective colonic operation and prosthetic repair of incisional hernia: does contamination contraindicate abdominal wall prosthesis use? J Am Coll Surg 191(4):366–372
Leber GE, Garb JL, Alexander AI, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133(4):378–382
Vrijland WW, Jeekel J, Steyerberg EW, Den Hoed PT, Bonjer HJ (2000) Intraperitoneal polypropylene mesh repair of incisional hernia is not associated with enterocutaneous fistula. Br J Surg 87(3):348–352
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1321
An YH, Friedman RJ (1998) Concise review of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res 43:338–348
Bellon JM, Garcia-Carranza A, Garcia-Honduvilla N, Carrera-San Martin A, Bujan J (2004) Tissue integration and biochemical behaviour of contaminated experimental polypropylene and expanded polytetrafluoroethylene implants. Br J Surg 91:489–494
Bellon JM, Contreras LA, Bujan J (2000) Ultrastructural alterations of polytetrafluoroethylene prostheses implanted in abdominal wall provoked by infection: clinical and experimental study. World J Surg 24:528–532
Bleichrodt RP, Simmermacher RK, van der Lei B, Schakenraad JM (1993) Expanded polytetrafluoroethylene patch versus polypropylene mesh for the repair of contaminated defects of the abdominal wall. Surg Gynecol Obstet 176(1):18–24
Junge K, Rosch R, Klinge U, Krones C, Klosterhalfen B, Mertens PR, Lynen P, Kunz D, Preiss A, Peltroche-Llacsahuanga H, Schumpelick V (2005) Gentamicin supplementation of polyvinylidenfluoride mesh materials for infection prophylaxis. Biomaterials 26(7):787–793
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ott, R., Hartwig, T., Tannapfel, A. et al. Biocompatibility of bacterial contaminated prosthetic meshes and porcine dermal collagen used to repair abdominal wall defects. Langenbecks Arch Surg 392, 473–478 (2007). https://doi.org/10.1007/s00423-006-0080-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-006-0080-2