Abstract
Solid state ionics is one of the key research topics of the Institute of Solid State Physics, University of Latvia since its establishment. The research direction included topics ranging from electrochromic phenomena in transition metal oxides through gas sensors and electronic nose to materials for rechargeable battery electrodes and materials for hydrogen energy. By the late 1980s, the institute had become one of the biggest and most prolific solid state ionic centres in the USSR and Eastern Europe and continues to maintain its position among the regional leaders in the field. Regular regional conferences and workshops were organized and some of the published works can be ranked among the pioneering works in the world of science. This extensive historical review summarizes information on the development of solid state ionics, actual research and achievements from establishment of the institute to the present day. Currently many collaborations are ongoing with partners across Europe and beyond in research ranging from battery materials and smart windows for zero energy buildings to hydrogen production, maintaining and growing its strength as key national, regional, and international centre of research excellence in solid state ionics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Historical background (1968–2022)
1968–1989
The Semiconductor Material Department (SMD) in the Laboratory of Semiconductor Physics Problems (LSPP) of the University of Latvia (UL) was established on June 30, 1968. It was related to the development of the semiconductor industry in Soviet Latvia and the growing demand for specialists educated in the field of semiconductor physics and electronics. Doctoral student Andrejs Lūsis (1937–2017) was appointed as the head of the department. His defended doctoral thesis was related to studies of the conductivity switching effect in copper glasses [1]. In the newly founded department, he immediately attracted chemists (Irēna Millere (1928–2014), Zigrīda Purviņa), as their expertise was irreplaceable in the research and synthesis of electron- and ion-conducting materials.
A new stage in the research of ion processes and phenomena in solid matter began in 1972 with the research of the electrochromic effect in tungsten trioxide thin films [2,3,4] and devices based on it [5,6,7,8]. At the beginning of 1974, the first thin-film electrochromic elements-indicators, light switches, and filters were designed [5,6,7,8], which immediately aroused great interest from the USSR’s industrial complex and ensured large-scale and long-term special contract works.
The materials found by the researchers, which only had the necessary electronic and ionic conductivity and colouring-bleaching (decolouring) effect in the form of thin layers, had to be placed on top of each other in a certain order and form to obtain the required electrochromic or photoelectrochromic so-called Deb [9] solid state electrochromic and photoelectrochromic multilayer structure (Fig. 1). Physical vapour deposition sputtering technologies were developed by new members of the department (Juris Purāns, Tālivaldis Zamozdiks, Vilnis Bēts, Uldis Kanders, Armands Plāte) to deposit cathodic (WO3 and MoO3) and anodic (NiO, IrO2-x) thin films as well as transparent conducting indium-tin oxide (ITO) thin films. Photographer Raimo Lielbriedis performed macro-lithography works and made masks for different layers of electrochromic and photochromic thin layer systems. To demonstrate these systems in prototypes (Fig. 1), electronic equipment was needed, and here at the beginning of the research, were also own electronics engineers Ojārs Rode and Uldis Cekulis. In the course of the work, new ideas arose, and the institute’s specialists trained in patent knowledge helped to shape them into new patents and certificates [10, 11].
This led to a rapid expansion of the SMD in the future Institute of Solid State Physics of UL (ISSP UL), established in 1978. In 12 years, the SMD, under the leadership of Andrejs Lūsis, became the largest and most powerful scientific organization working with electrochromic materials and devices in the whole of the former Soviet Union. “The fundamental electronic properties (including optical) of a solid ion/electron conducting materials are determined by the character of the interaction between their ionic and electronic subsystems”—this is how A. Lūsis used to start his lectures and reports on the electrochemical nature of electrochromic solids (Fig. 2).
Twelve doctoral dissertations and 23 university diploma graduate works in physics and chemistry were defended, 14 patent applications (local—USSR; international—USA, Switzerland were issued, and more than 200 scientific research and popular science papers in Latvian, Russian, and English were published in this prosperity period (1979–1999). Andrejs Lūsis initiated cooperation with electrochemist Dr. Chem Gunārs Slaidiņš (1934–1980) (Department of Physical Chemistry of the Faculty of Chemistry of the University of Latvia) and his co-researcher Sigurds Takeris (1943–2016) in matters of hydrogen diffusion in palladium and metal oxides and Aldis Zekunde in research of solid electrolytes. Since 1977, Jānis Kleperis and Sigurds Takeris were actively involved in the research of hydrogen diffusion in metals [12].
In 1979, thanks to A. Lūsis, good connections with Professor E.A. Ukshe (1928–1993) from the Institute of Problems of Chemical Physics of the Russian Academy of Sciences (Chernogolovka, Moscow region), cooperation expanded from the Latvian SSR to the USSR and a new scientific direction—solid state ionics (SSI)—was identified. The content of this new direction was formulated according to the ideas put forward in Japan and America. Solid state ionics is a synthesis of solid state physics and electrochemistry and is analogous to solid state electronics. Andrejs Lūsis involved talented students from the Faculty of Chemistry—Gunārs Bajārs, Guntars Petrovskis (Vaivars), brothers Aigars Vītiņš and Ģirts Vītiņš, Jānis Pitkevičs in research, and added talented electronic engineers Jānis Žuks, Normunds Bergs, and others to the group of designing ionic devices for variety of applications.
The ISSP UL became the organiser of annual All-Union international conferences “Solid State Ionics—SSI” in Riga (1979–1989) (Fig. 3).
As the first example of cooperation with Western countries in the field of solid state ionics, G. Bajārs' internship at the Institute of Technical Electrochemistry should be mentioned (Technical University of Vienna) under the supervision of Professor Manfred W. Breiter (1925–2005), which resulted in a joint publication [13]
1990–1999
It was a complicated transition period from Soviet Province to independent European state and starting 2004—a member of the European Union. Many researchers left the institute because they were deprived of state funding, and there were few opportunities to apply for and win projects and grants. At the same time, however, new opportunities for international collaboration and research exchange now became more available. A. Lūsis stayed in his place and tried to ensure the stay of his researchers and engineers, too. Research activities changed from military application-oriented works to more basic fundamental research in solid state ionics (ionic processes on surfaces and two or three phase interfaces as well as electrode processes) and sensorics [14,15,16].
The Semiconductor Materials Department was divided into 3 laboratories—Solid State Ionics (A. Lūsis), Gas Sensors and Sensor Systems (from 2012 Hydrogen Energy Materials—J. Kleperis) and Thin Film and EXAFS (J. Purāns). Investigations of stability (degradation) and life time of electrochromic coatings and display devices [17] as well as new electrochromic materials (Andris Āzens) and polymer-composite solid electrolytes for electrochromic coatings of smart windows (G. Vaivars) in cooperation with University of Uppsala (Prof. C.-G. Granqvist) [18, 19] continued. Instrumentation for physico-chemical monitoring of environmental pollution was developed, and studies on pollution by X-ray fluorescence method were initiated in cooperation with the Chalmers University of Technology and the Royal Agriculture and Veterinary University of Denmark. Furthermore, new investigations on metal hydrides and lithium intercalation electrodes based on transition metal oxides for batteries in cooperation with Stockholm University, Technical University of Denmark, Warsaw Technological University, and Central Battery Laboratory in Poznan were initiated (J. Kleperis, Ģ. Vītiņš, G. Vaivars). Wide cooperation with Synchrotron radiation facilities in Italy and France for the investigation of transition metal oxides by EXAFS spectroscopy (J. Purāns, A. Kuzmins) was also developed [20].
2000–2009
To integrate the adaptation and use of hydrogen in society, the production and usage of it must become viable in competition with the fossil fuels that still dominate the energy economy today. Hydrogen can be produced through the electrolysis of water, various chemical processes involving fossil fuels and natural gas, and biological processes of plants and animals. Water electrolysis is the most desirable way to produce hydrogen due to the water availability. If electricity for water splitting is produced from renewable energy sources, it is also environmentally friendly and sustainable. In addition to production, safe, cheap, and accessible hydrogen storage systems are advisable. A detailed study was performed to determine processes on electrodes during electrolysis, define the kinetics of hydrogen evolution on new materials, and investigate mechanisms of hydrogen evolution and absorption, formation, and decomposition of the hydride phases [21]. Experiments on hydrogen spill-over effect on a glass substrate, Pd, and WO3 were carried out by laureate of the Nordic-Baltic doctoral scholarship Līga Grīnberga in studies at the RISØ centre in Denmark under the guidance of Dr. Finn Willy Poulsen and Dr. Allan Schroeder Pedersen [22]. The research aimed to investigate the possibility of creating another metal/alloy with catalytic properties able to split hydrogen molecules and facilitate the spillover [23] of atomic hydrogen onto non-metallic surfaces [24].
Research of the water electrolysis process in the Hydrogen Energy Materials laboratory of the ISSP UL under the leadership of J. Kleperis became active in the first decade of the twenty-first century. A big boost to hydrogen energy technology research was cooperation with the Latvian Hydrogen Association founded in 2005. It coincided with the time when the hydrogen energy materials laboratory was established at the institute, which was involved in the research of the first state research program in energy.
2010–2022
After the first decade of the 2000s, directions on hydrogen storage research at ISSP UL adapted to global trends, research capacity, and available funding conditions. Investigations of metal hydride alloys deviated to store hydrogen produced by anaerobic bacterial fermentation [25]. The choice of materials for hydrogen storage was determined by their wide availability, lightweight, and ability to modify their properties quickly and cheaply. Therefore, the research and improvement of nanoporous natural zeolite properties [26] for the storing of gaseous hydrogen at non-cryogenic temperatures developed. Other topics covered research on complex multilayer carbon structures and modified graphene sheet stacks for hydrogen binding and usage in green energetics. Since green energy became a necessity, not only a scientific passion, research at the institute expanded in the directions of theoretical studies [27] on hydrogen storage capacity and grain boundary wetting phenomenon research of high entropy alloys [28].
The development of the hydrogen economy highlighted another crucial research topic—hydrogen production. Fossil fuels are still the most used source of hydrogen production. However, water splitting would be one of the friendliest ways to obtain hydrogen, especially if using such a widely available energy source as the Sun. Unfortunately, there are many problems to solve before this technology becomes economically suitable. The photocatalytic water splitting concept lies in the materials producing chemical reactions by light absorption. These materials, called photocatalysts, are complex materials that should provide the right band structure, suitable bulk and surface properties, and, preferably, work in visible light.
ISSP UL scientists started experimental [29] and theoretical studies [30] of photocatalytic materials using traditional semiconductor materials such as TiO2 [31] and ZnO, modifying them with various dopants, computational studies of SrTiO3 nanoparticles and nanostructures, and, lately, the first-principles calculations to predict the photocatalytic activity of novel two-dimensional carbon allotrope–graphyne [32].
To expand the possible application of the researched materials and methods to meet the European Green Deal call, investigations for the use of photocatalysis in CO2 reduction [33] measures have been initiated in recent years. Moreover, research in the Li-ion and Na-ion batteries was developed. With some exploratory studies of materials for Li-ion batteries taking place in the early 2000s (Ģ. Vītiņš, A. Vītiņš, G. Vaivars), the research subject was restarted in 2010s in close cooperation between two laboratories (Hydrogen Energy Materials and Solid State Ionics), with the research of thin films [34,35,36] and reduced graphene oxide composites [37, 38] as electrodes for Li-ion batteries. In 2014 Gunārs Bajārs became the head of the Solid State Ionics laboratory, and battery materials research was gradually concentrated at this laboratory. However, part of the battery material research was still carried out in the laboratory of Hydrogen Energy Materials, and cooperation with the newly established Laboratory of Chemical Technologies (the head G. Vaivars) was developed. During this time, the scope of practical research expanded as cooperation with industry was initiated.
There was a merge of Hydrogen Energy Materials and Solid State Ionics laboratories, and a new laboratory of Materials for Energy Harvesting and Storage was established in 2017 under the leadership of J. Kleperis. In 2020, Gints Kučinskis became the head of the joint laboratory, and the research of battery materials at the institute took new and faster turns. Over the last years, the research has expanded into ageing phenomena on battery cells (collaboration with ZSW) [39, 40] and into the field of Na-ion batteries [41,42,43]. ISSP UL has currently initiated several European collaborations to further expand its battery material research.
Evaluating achievements in solid state ionics, ISSP UL was entrusted with organising the ISSFIT 9 symposium in 2010 in Riga (Fig. 4). Symposium papers were published in a specially dedicated issue of Solid State Ionics (eds. A. Lūsis, G. Bajārs). Besides presenting the latest scientific results, this event contributed to even closer cooperation of ISSP UL (E. Kotomin, A. Popov, V. Kuzovkov, Y. Zhukovskii, Y. Mastrikov, D. Bocharov, D. Gryaznov, J. Purāns, A. Kuzmins, G. Kučinskis) with Max -Planck Institute for Solid State Research, Germany (Director Joachim Maier) in the field of solid state physics and ionics.
The electrochemical nature of electrochromism
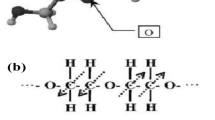
Research of electrochemical and physical characteristics of electrochromic phenomena made in 1972–1990 gave a chance to connect the electro-colouration efficiency of tungsten trioxide with processes and mechanisms of formation-induced colour centres from one side and with the electronic-atomic structure of them from the other side [16]. Research of structural features of tungsten-oxygen crystalline nanoparticles allowed connecting structural and optical properties of amorphous WO3 with nano-crystalline tungsten hydrates. Amorphous tungsten trioxide films, investigated by the Raman scattering method, are suggested to be composed of a spatial network of tightly bound (WO6)n·mH2O clusters with a large number of terminal oxygen W = O and W–O-W bonds between clusters. Jevgeņijs Gabrusenoks and Guntis Ramans [44] were the first tungsten oxide researchers to record the oscillation band at 950 cm−1 in the Raman spectrum of coloured a-WO3 for non-bridging oxygen in the bond W = O. This bond was observed in amorphous and polycrystalline WO3 film and disappeared after heating at 500 K.
The injected electrons in an amorphous tungsten trioxide film are localised in the tungsten 5d orbitals in an axially distorted octahedron, as is shown by Pēteris Cikmačs with ESR analysis [44, 45]. The optical absorption of a coloured amorphous tungsten trioxide film, as has previously been proposed, can be satisfactorily described by an intervalence charge-transfer transition between localised W5+ and W6+ states [45].
The a-WO3 films obtained in low vacuum (0.1 Pa) on substrates at RT on coloured state show the W5+ ESR signal at 120 K, with intensity proportional to the intensity of optical absorption of the broad asymmetrical band around 1.3 eV [46]. The same absorption band characterises the blue amorphous WO3 films obtained in high vacuum (10−2 Pa) or from the hot vaporizer boat (T > 1600 K), or on the substrates of high temperature (T > 500 K). The absorption maximum of the band around 1.3 eV shifts towards the smaller energy side if the ordering of the film structure takes place. For a coloured polycrystalline WO3 film, the absorption band’s maximum is located around 0.8 eV. The position of the fundamental absorption edge is also sensitive to the structure and coloration degree of the WO3 films. The colouring shifts the fundamental absorption edge to the higher energy side, but the crystallisation—to the smaller energy side. The increases of substrate temperature in the evaporation of WO3 film led to obtaining the blue WO3 films with high electrical conductivity, measured by Jānis Pinnis [4, 8] and high optical absorption at energies between 0.5 and 2.5 eV.
The first synchrotron radiation EXAFS experiments conducted by J. Purāns gives more detailed information about the relationships between the structures of amorphous (a) oxides WO3, MoO3 with octahedral framework. It was found that a-WO3, a-MoO3, a-V2O5 thin films, and glasses have strongly distorted but well defined, oxygen octahedra, which are joined by vertices (a-WO3, a-MoO3) or by vertices and edges (a-V2O5), and form a random network. At the same time, a-IrO2 and a-NiO have less distorted oxygen octahedra, which form the rutile type and rock salt–type nanocrystalline structures. The variation of the structure with the change of the metal valence is considered, and the possibility of small radius polaron detection by XAFS is discussed [20, 47,48,49].
Electrochromic systems
Researchers at ISSP UL have over 20 years of experience in the research of tungsten trioxide films in different multi-layer solid state ionic systems [5,6,7,8, 10, 11] (few examples in Fig. 5). The first were thin-layer systems on glass covered with transparent and electrically conductive indium oxide (with tin impurities)—ITO layer glass/ITO/WO3/SiOx/Au. The electrochemical reactions cover the main types of multi-phase interfaces of amorphous tungsten trioxide (a-WO3) film as cathode in electrochemical cell with solid proton conductor (SiOxOHy) and anode—the upper electrode semi-transparent Au, which absorbs moisture from the environment.
The relationship between the synthesis parameters of WO3 thin films and their chemical resistance and stability of properties has been clarified. These factors are important for understanding the causes of the degradation and ageing of electrochromic devices.
A new methodology analysing the functional parameters of electrochromic systems has been developed, which allows for determining the role and numerical value of the parameter for individual elements of the equivalent circuits of the electrochromic systems [50]. Two-step colouring mechanism of WO3 thin films was found, and the decrease of proton diffusion coefficient is obtained with the injection of charge x in protic electrolytes.
It optimises the design and manufacturing technology of electrochromic devices and links cathodic (WO3) and anodic (NiOx) electrochromic layers in one system. The world’s first electrochromic system composed of WO3 and Ni(OH)2 separated by antimonic acid hydrate (AAH) as a proton solid electrolyte was demonstrated by Lagzdons, Bajārs, and Lūsis in 1984 [51]. This is so-called complementary electrochromic system in which the colouring of both electrodes occurs simultaneously, and reaction (2) takes place at the same time as reaction (1).
The process is reversible and when the charge flows in opposite direction, the electrodes become uncoloured. Such a system with cathodic and anodic electrochromic layers made it possible to significantly increase the electrochromic efficiency and operating resources of devices [52, 53]. Original cyclic voltammetry technique, combined with light transmittance measurements, was developed by Ēvalds Pentjušs to obtain electro-optic characteristics of electrochromic electrodes [53, 54].
Electrical transfer and diffusion of ions and the irreversibility of ion and electron processes in heterojunctions are responsible for the degradation of ionic devices as it was shown by Ē. Pentjušs, G. Bajārs, and A. Lūsis [50,51,52,53,54]. These processes for electrochromic devices (ECD) determine the cycling capacity and lifetime. The basic problem here is how to match the electrochemical parameters (including chemical potential) of heterojunction. The experiments were carried out on ECD based on the system: (glass)- < ITO/WO3// a proton-conducting electrolyte//NiOx /ITO > -(glass). ECD’s cycling capacity and degradation processes are investigated by electro-optical and electro-chemical spectroscopy. The analysis of experimental data is based on the assumption that electrode reactions changed the composition of electrode and electrolyte materials and surface layers as well as the constitution of the heterojunction’s interface. The conclusions of these investigations and problem analyses are some considerations about the electrochemical battery model and cycling capacity of ECD [50, 51, 53]. That depends on the reversibility of solid state reactions on the electrode and ion insertion processes and the phase stability of electrode and electrolyte materials. The ions of sublattices of immobile ions of solid phases and other components of these phases have to be stable against chemical interactions to ensure a reversible diffusion and transfer of mobile ions during cycling [52, 54].
Gas sensors and e-nose
The hydrogen diffusion through heated above 230 °C palladium was first demonstrated by Thomas Graham [55]. Later it was proved that hydrogen passes through the crystal lattice of Pd in atomic form. When studying the electrochromic effect of WO3, we found that the colouring can be satisfactorily described by an intervalence charge-transfer transition between localised W5+ and W6+ states. J. Kleperis, S. Takeris, A. Lūsis, and J. Stradiņš suggested to estimate with differential cell the rate of hydrogen diffusion In Pd using the chemochromic effect (colour change of material induced by chemical reaction) in WO3 [12]. To do this, a WO3 layer was applied on one side of a Pd plate (thickness, 0.3 mm), the colour of which was controlled with a red laser, recording the intensity of the reflected light. A negative current pulse was applied to the palladium on the uncoated side, which generated hydrogen at the electrolyte/Pd interface that diffused into the palladium. After some time, the intensity of the reflected light began to decrease, which indicated that WO3 was getting coloured and atomic hydrogen had passed through the Pd plate. The chemochromic effect of the WO3/Pd sensor is determined by the chemical reaction at the interface of the adsorbed atomic hydrogen species coming from the Pd catalyst and reducing WO3 to HxWO3 as shown in Eq. (1).
The Pd catalyst reduces the activation energy of atomic hydrogen adsorption and brings about the adsorption of hydrogen to the interface with WO3 via the spillover effect [56]. The double injection/extraction of protons (hydrogen ions) and electrons involves a change in the tungsten charge state from W6+ (pale yellow) to W5+ (deep blue). The process is reversible if the sensor is re-exposed to the air [57]:
This colouring effect of WO3 deposited on a thin Pd film under the influence of atomic hydrogen released from the substrate has also been observed for an iron plate. This prompted to apply for the patent (in Soviet times, it was called an author’s certificate) [58].
But how can atomic hydrogen occur, for example, inside a steel pipe? It turns out that this happens in the process of corrosion. Academician Lidija Liepiņa worked on the research of metal corrosion processes in Institute of Inorganic Chemistry with whom SMD collaborated in later years in the synthesis and research of solid state electrolytes. A significant part of Liepiņa’s works is about the mechanism of reactions between metals and water [59]. During her research, the hydride theory (1955–1959) was formulated, which subsequently received further development. According to this theory, in the first stage of the reaction between the metal and water, unstable metal hydrides are formed, which later transform into hydroxides.
Ammonia-exchanged ceramic samples of beta-alumina have been obtained from plasma-dispersed powders. The ionic conductivity is slightly affected by ion exchange, but the surface conductivity for the ammonia-exchanged sample drastically changes in the presence of the water and ammonia vapours. The ammonia-doped xerogel of antimonic acid hydrate in the form of a thick film has been tested as a potentiometric ammonia sensor [60]. The chemical sensors for different gaseous (alcohol, acetone, ammonia, water vapour) detection at room temperature are developed using polycrystalline β-alumina and xerogel of antimonic acid hydrate (AAH). More success is achieved by producing ammonia-sensitive devices on β-alumina and AAH xerogel [14]. New-type resistive/capacitive gas sensitive structures were obtained using a specially prepared glass substrate covered by a thin conducting In2O3 layer, cut into comb-teeth-type electrodes by the help of a laser beam. Such laser-processed gap in the layer of conducting material was a prototype of an excellent humidity sensor, especially at high values of relative humidity (RH) [61]. Different additional coatings onto a laser-processed gap were examined for humidity sensing. Sol–gel, vacuum thermal evaporation, and laser evaporation methods were used to obtain thin layers of different materials [62, 63].
It is shown how chemically sensitive metal-oxide semiconductor field-effect transistors with a thin discontinuous platinum gate can be modified to detect NOx [64]. The temperature dependence of the NOx sensitivity suggests that after the ammonia pulse, there are two competing polarisation phenomena caused by the interaction between NOx and the sensing surface. The results indicate how thin sensing layers can be modified after fabrication to promote sensitivity towards specific molecules.
An electronic nose is a modular sensor system made for detecting gases and gas mixtures, aromas, and odours in an environment. It combines gas sensors for aroma detection and artificial intelligence to treat measured and stored reference data. Some fields of employment of electronic nose are quality control or process control in food, medical, perfume, chemical etc., technologies; raw material selection and inspection; manufacturing process monitoring; environmental monitoring. Our research proved that the electronic nose is applicable to distinguish between different sorts of textiles and to investigate the odour-absorption capability of different clothes [65,66,67]. Together with the Institute of Experimental Clinical Medicine of the University of Latvia, research was done to identify odour characteristics for lung cancer of lung disease patients by comparing breath patterns of specific lung diseases and by comparing such patterns for patients before surgical operation (removal of a sick part of the lung) and after that [68]. It has been shown that lung cancer can easily be discriminated from other lung diseases during a short (less than 1 min) breath sampling and analysis time. The volatiles produced by cancer make the most significant contribution to the responses of different e-nose sensors.
The 10th International Symposium on Olfaction and Electronic Nose (ISOEN`2003) took place in Riga (Latvia) on June 25–28, 2003 [69]. Ninety-three participants from 22 countries came together to discuss the main results in sensor and sensor array technologies, e-nose miniaturisation, odour description in electronic files and unified description language formation, and e-nose application in different fields, making emphasis on product adulteration and environmental pollution problems.
Hydrogen storage materials
The article by Vaivars et al. [70] on the AC impedance behaviour of hydrogen storage alloy electrodes for alkaline batteries represents the beginning of the ISSP UL researchers’ work on hydrogen storage. The article showed that the addition of copper or nickel powder as a current collector improved the electrochemical characteristics of the electrodes. Research interest and promising investigations of alloy electrodes resulted in considerable and most cited Kleperis et al. [71] review article on the electrochemical behaviour of specific alloy families (AB5, A2B, AB/AB2, and AB2) on the hydride stability, hydrogen storage capacity, and kinetics of hydrogen sorption–desorption on the interfaces of solid phase—gas, solid phase—electrolyte solution systems. The focus on metal hydride electrodes applicable in batteries was sharpened by researching the effect of the hydrogen sorption capability and electrochemical characterisation of specific AB5 alloy composites with oxides (WO3; SiO2, B2O3) and carbon [22]. The production of hydrogen by electrolysis and its storage in materials with the reduced or eliminated presence of noble metals prompted in-depth research in the direction of electrolytic hydrogen storage [21].
In the early 2000s, research into understanding aspects of hydrogen energy implementation was one of the freshly emerging hot topics worldwide. Research in the field of hydrogen storage was crucial and received significant attention from the transport sector. Safety aspects, selection of cheap, available materials with sufficient reserves, and the study of hydrogen storage/usage on demand systems operating close to normal temperature and pressure conditions were key research directions at ISSP UL. Available scientific infrastructure, capabilities, and experience of the institute’s scientists led to the development of new composite materials for hydrogen storage and investigations of physical and chemical characteristics. Research by Grīnberga et al. [23, 24, 72] showed that new composite material formed of AB5-type lanthanum nickel alloy with a silica-based glass showed increased hydrogen storage capability explained by spillover phenomena. This process ensures the dissociation of hydrogen molecules on the surface of the catalyst and spills over to the inert surface, causing an increase in the catalytic activity of an alloy and the presence of hydrogen atoms at the interface metal–glass, which enlarges an amount of adsorbed/absorbed hydrogen. Dimanta et al. [25] studied the other possibility of using metal hydride alloys to store hydrogen produced by anaerobic bacterial fermentation.
The need for hydrogen technologies became more urgent and worldwide research intensified by the great financial support of governments of developed countries, funding agencies, and industry. Gaseous and liquid hydrogen storage technologies took the lead; research on metal hydrides for hydrogen storage applications at ISSP UL was replaced by investigations on finding and characterising lightweight and widely available natural materials. Large-surface aluminosilicate compounds such as zeolites are not the first option for hydrogen storage due to their low-hydrogen sorption capacity above cryogenic temperatures. However, a well-known crystal structure with easy ion exchange possibilities turned zeolites into a tempting and easily tuneable porous media for hydrogen sorption. The research of Lesničenoks et al. [26, 73, 74] showed markedly high hydrogen adsorption capability of natural clinoptilolite activated with 1.25 wt% palladium.
In parallel, other promising material—graphene sheet stacks—was investigated for hydrogen storage and sensing. Graphene sheet stacks were obtained by exfoliating recycled graphite waste and simultaneously modified with metal dopants, thus significantly benefitting these studies and processes in general. Obtained graphene sheet stacks mainly formed open structures, having large active surface areas that were easy to modify with good semiconductor properties and low electrical resistance applicable for hydrogen sensing [75, 76].
Theoretical approaches to estimate the hydrogen storage capacity of novel materials using a few density functional theory calculations with the aid of a continuum model can provide a more accurate estimation of the hydrogen storage capacity were performed by Eglītis et al. [27, 77]. The use of the equation of state from virial expansion paves the way for determining the fugacity coefficients of the hydrogen fluid at arbitrary temperature and pressure having more physical meanings than the empirical values. Thereby, hydrogen storage capacity and the detailed thermodynamic information of a designed novel material are estimated with relatively high accuracy and low computing cost. Gained theoretical calculations are in good agreement with the experimental values.
A few decades ago, multicomponent alloys with a nearly equal concentration of components, also known as high entropy alloys (HEAs), were developed. These alloys rapidly became very important due to their unique properties and applicability in energy conversion and storage. To make these alloys even more appealing, cheap substrates with large surface areas were covered by HEAs using plasma cladding. At the end of cladding, the solid polycrystal appears, which contains few residual melts that completely or incompletely wet the grain boundaries. Kuzmin et al. in the review [28] analyses the grain boundaries wetting of HEAs, the microstructure of thick coatings, and changes of wetting, depending on HEAs thickness.
Photocatalytic materials
Due to the need for clean energy and since the solar radiation and water are widely available resources on the Earth, the splitting of water into hydrogen and oxygen became a significant research area worldwide. At ISSP UL, several groups of scientists began to study materials that are suitable as photocatalysts, both from an experimental and a theoretical point of view. Photocatalysts are typically semiconductors where photons excite an electron from the valence band to the conduction band. Such process causes reduction–oxidation reactions similar to electrolysis, where electrons produce hydrogen by reducing water molecules, while holes oxidise and form oxygen. The energy required to split water requires the band gap to be greater than 1.23 eV, which limits the choice of materials and challenges, finding ways to change material properties to obtain the desired photocatalytic response.
At the ISSP UL, Grigorjeva et al. [78] led the early experimental studies on photoluminescence and photocatalytic activity of ZnWO4 powders with grain size in the range of 20 nm–0 μm. This study tested the photocatalytic activity by degradation of methylene blue solution under UV light. A correlation between self-trapped exciton luminescence decay time and photocatalytic activity of ZnWO4 powders was explained to evaluate the possibility of the usage of visible light; the effect of excess iron on the structural, optical, and visible light photocatalytic activity of zinc ferrite samples was investigated [29].
Still, one of the most promising and widely studied photocatalysts is titanium dioxide (TiO2) due to its chemical stability and corresponding photocatalytic properties. For synthesis, various methods are used. However, for large-surface nanostructured TiO2, one of the dominant methods is the anodising process. Different electrolytes lead to different polymorphs of TiO2; however, uniform phase distribution on the surface is crucial for higher photocatalytic activity. Knoks et al. investigated the effect of two electrolytes on the distribution of TiO2 polymorph phases and the correlation with the optical band gap, charge density, and photocurrent values using Raman spectroscopy. The results revealed uniform, single-phase, and multi-phase TiO2, and non-uniform phase distribution, where the highest photocurrent and charge density values were displayed by showing the uniform single-phase samples [31]. To increase the photocatalytic activity of TiO2 nanotubes, various dopants were investigated, both experimentally and theoretically. Experimental studies and comparison of structural and photocatalytic properties between pristine TiO2 and TiO2/WO3 heterostructure were carried out. Results proved that the deposition of WO3 lowered the band gap and increased charge-carrier dynamics; however, it did not increase the measured photocurrent response [79].
Another method to obtain nanostructured TiO2 thin films with a large surface is electrophoretic deposition (EPD). Annealed TiO2 films obtained by sol-EPD are mainly formed in anatase structure with different morphology depending on the substrate material. UV light-induced potential measurements indicate that the morphology substantially impacts the photoactivity of TiO2 thin films [80]. EPD technique allows preparing composite coating structures with uniform distribution of components. Fe-TiO2 thin films obtained by the same sol-EPD method demonstrate photoactivity under visible light radiation due to doping with Fe3+ ions. Open circuit potential results show that increasing the film thickness and surface area improves the photoactivity of Fe-TiO2 [81]. Even the distribution of WO3 particles in the entire composite layer is also shown in composite coating structures of TiO2-WO3 structures obtained by EPD. Light absorption measurements were used for photocatalytic properties evaluation. It was found that the removal ratio of methylene blue depends on the TiO2-WO3 concentration ratio. The most effective photodegradation was determined for the sample that was electrophoretically deposited from the suspension with the molar content ratio TiO2-WO3 2:1 [82].
Meanwhile, experienced theoreticians of the ISSP UL led by Y.F. Zhukovskii started to apply quantum chemical simulations of titanium dioxide nanotubes. Full geometry optimisation for initial 2D TiO2 structures was performed to calculate the band gap width, energy positions of the valence band top, and the conduction band bottom as well as nanotube formation and strain energy [83]. Furthermore, Bocarov et al. theoretically estimated the photocatalytic suitability of single-wall fluorite-structured titania nanotube doped by Sc, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn atoms substituted for host Ti. Only Sc-doped TiO2 nanotubes showed clear evidence for a potential photocatalytic application for water splitting. Other dopants were unsuitable due to their defect-induced levels that, with very high probability, will lead to rapid electron–hole recombination [84]. Based on time-dependent density functional theory, S. Piskunov’s group performed the first-principle calculations to predict optical properties and transition states of pristine, N-, S- doped, and N + S-co-doped anatase TiO2 nanotubes. Results clearly demonstrated increased visible-light-driven photoresponse in N- and S-doped and the N + S-codoped TiO2 nanotubes, enhancing the efficiency of photocatalytic hydrogen production [85].
TiO2 is not the only material showing photocatalytic activity, therefore, quantum chemical simulations for doped ZnO nanowires were performed. The carbon-doped ZnO nanowires, the band gap of which was reduced to 2.1–2.2 eV, providing optimal 15–17% solar energy conversion efficiency, showed the highest photocatalytic suitability [86]. Excited state density functional theory calculations of two-dimensional nanocomposites MoS2 and WS2 placed on ZnO substrates were carried out to predict their validity for water-splitting applications and the influence of ZnO substrate thickness. Results showed that these monolayers placed on graphene-like ZnO substrate yield essential enhancement of the photoabsorption in the visible region of solar spectra [87]. Ab initio studies of strontium titanate nanotubes and nanocrystals for visible light-driven photocatalytic water splitting were performed, and the results made it possible to conclude the suitability of the selected materials for use in photocatalysis [30, 88].
Recent studies have been conducted to predict direct photocatalytic water splitting on a novel two-dimensional carbon allotrope–graphene. Studies show that this 2D material possesses a direct quasi-particle band gap of 3.06 eV, which is close to the value of the TiO2 rutile phase. Moreover, some of extended structures containing B and N dopants have band gaps in the range of 1.37–2.42 eV, perfectly fitting to the requirements for visible light-driven water splitting conditions [32].
Photocatalytic and electrocatalytic CO2 reformation processes are encouraging methods for lowering environmental impact. To reduce CO2 amount in the atmosphere, various technologies and materials at different levels of readiness are being elaborated. Knoks et al., in their review on electro-catalytic and photo-catalytic reformation of CO2, show reactions and efficiency of both processes based on existing established technological methods [33].
Solid electrolytes
Research of solid electrolytes at ISSP UL began in 1979, when after graduating from the Faculty of Chemistry, UL, G. Bajārs started working at the institute, where he continued the topic on silver solid electrolytes started in his diploma thesis (supervisor A. Zekunde). A patent was obtained for the development of an original method for obtaining glassy AgI–Ag2MoO4 in a wider concentration range with higher ion conductivity [89]. Since the main research topic of the department was related to electrochromic systems, which required a hydrogen ion conductor, studies of proton solid electrolytes were started. Transparent films of uranyl phosphate HUO2PO4·4H2O were created for use in electrochromic devices. However, the conductivity of these layers was not high enough [90].
Material that is much more promising turned out to be AAH, which possesses high-proton conductivity in the order of 10−3 S cm−1 at RH. The complementary electrochromic system WO3//AAH//Ni(OH)2 demonstrates high reversibility. The electrochemical reactions were determined, and moving of coloration fronts was demonstrated for the first time in wolfram and nickel oxides based electrochromic system [51]. Impedance measurements and frequency simulations of polycrystalline AAH cells were carried out in a wide frequency and temperature ranges in Vienna Technical University by Paul Linhardt. It was found that the bulk resistance of the solid electrolyte is mainly determined by the grain boundary resistance [13]. Original method for obtaining AAH obtaining AAH with high ionic conductivity and stability was elaborated and patented [91].
Around 1989, under supervision by G. Bajārs and A. Lūsis, Ģ. Vītiņš joined as a student from the Faculty of Chemistry of UL, and a research assistant participating in another strand of work in solid electrolytes, i.e., research of Na+-ion solid conductors, particularly Na-beta-alumina having its 2D-crystal structure, consisting of spinel-like slabs, which was of great interest as a solid electrolyte for Na//S batteries and potentially in sensor applications offering ion conductivities of order 10−2–10−3 S/cm at room temperature. The focus was largely in exploring conductivity single crystals synthesised in-house. The specially setup AC impedance measurements in wide temperature range gave the electrolyte conductivity values and activation energy, demonstrating strong anisotropy between the in-plane and perpendicular to slabs ion transport [92].
The well-established technique in investigation of solid ion conductors later was applied to other possible ion conductors with a 3D-network structure relating to NASICON-type materials, i.e., less-studied Na3Sc2(PO4)3, NaScP2O7, LiScP2O7, LiFeP2O7, which, however, had a lower conductivity in range10−5–10−6 S/cm at 300 °C. Synthesis work was done in collaboration with the Latvian Institute of Inorganic Chemistry [93, 94]. Later, in collaboration with Solid State Radiation Laboratory at the UL, Ģ. Vītiņš investigated Li-ion conductivity in the solid-state system Li2TiO3-Li1.33Ti1.67O4, where Li-ion vacancies were introduced, aiming for non-stoichiometric compounds and solid solution.
Li2-4xTi1+xO3 by reacting Li2TiO3 with up to 26 mol% of TiO2. The ion conductivity was increased by two orders from 10−7 to 10−5 S/cm at 300 °C. Li2TiO3 has been of interest in application in fusion reactors. These days, spinel-type Li1.33Ti1.67O4 or Li4Ti5O12 is also known as a lithium insertion electrode compound [95].
Furthermore, the proton-conducting solid electrolytes for electrochromic devices were developed under the leadership of G. Petrovskis (Vaivars) [91, 96]. Antimonic acid xerogels were synthesised using sol–gel method [97, 98] and used to prepare electrochromic display: < ITO/WO3/antimonic acid electrolyte/IrO2-x/ITO >. The response time for such a device is 0.5 s at switching potentials: 2 V for colouring and 0.7 V for bleaching. The testing of investigated devices lasted 5 years and number of cycles about 106–107 [97]. The particle size of antimonic acid was reduced to 20–40 nm to produce optically transparent films for electrochromic windows. At the same time, it was shown that it is possible to replace partly poisonous antimonic acid with aluminium oxide particles without lowering the proton conductivity [99]. The electrolyte was tested in laminated electrochromic devices, with W oxide and Ir oxide (or mixed Ti–Ce oxide) serving as the optically active layer, which corroded after 104 to 105 colour/bleach cycles. The device lifetime was slightly improved by use of electrolytes containing aluminium oxide [100]. Results show that the chemical and electrical properties of the electrolyte are governed by the acidity of the aluminium oxide. The neutral form of the electrolyte is suitable for use in electrochromic devices with WO3 and Ni(OH)2 electrodes, while the WO3 and Ni(OH)2 films are etched in contact with basic and acidic electrolytes, respectively.
High price of iridium oxide electrodes is limiting for large-area coatings, and nickel hydroxide/oxide was selected as a material of choice. The problem with the choice of electrolyte arises from the fact that WO3 is stable in an acidic environment, while Ni(OH)2 is stable in a basic one. For such electrode, the antimonic acid was too corrosive and for large-scale application, also, too poisonous. Suitable replacement was zirconium phosphate [101, 102], and it was tested in electrochromic devices in cooperation with Uppsala University (under supervision of Prof. C. Granqvist). Zirconium phosphate was dispersed in a polyvinyl acetate/glycerine mix to produce optically transparent solid gel. The conductivity as compared to the antimonic acid composite decreased, but the cycling capacity of EC element slightly improved. The composite was stable in a dry atmosphere (RH < 1%) and at temperatures up to 140 °C [102]. Composite formation conditions adjusted in such a way to exfoliate the zirconium phosphate–layered structure and to produce nanoparticles with a phosphorised surface [103, 104]. The conductivity was increased by one order up to 1 mS/cm). The reaction rate of phosphorisation was regulated by adding acetic acid, and the observed particle size was in the range 40–60 nm. The work was done in cooperation with University of Western Cape (RSA, Prof. V. Linkov). The phosphorised zirconium oxide membranes were tested in a methanol fuel cell by using standard platinum catalyst inks [105]. Glass composite fibre supports (with a pore diameter of 240 nm) [105, 106] or zirconium oxide microporous membranes [107] were used as a support or substrate membrane. The membrane was impregnated with a zirconium oxide sol, dried and treated with phosphoric acid. The efficiency equivalent to more than 50% of the Nafion efficiency was obtained [105, 106].
Polymer membranes and ionic liquids
From 2005, the research focus was changed to the polymer membranes, because G. Vaivars during his postdoctoral research at University Western Cape was working on polymer membrane synthesis for hydrogen and methanol fuel cells. Methodical approaches at ISSP UL at the same time included application of micro Raman spectroscopy to industrial fuel cell membranes (Nafion NRE-212, Fumapem® F-14100, and Fumasep® FAA) [108].
The proton-conducting membrane was prepared by cross-linking highly sulphonated and sulphinated poly(etheretherketone) (SsPEEK) [109] and poly(oxa-p-phenylene-3,3-phthalido-p-phenylene-oxa-p-phenyleneoxy-phenylene) (SsPEEK-WC) [110]. The best membranes with conductivity 0.03 S/cm at 20 °C and methanol permeability 1.3 · 10−7 cm2 were used for direct methanol fuel cell tests. Mechanical stability of SPEEK-WC membrane improved by covalent-ionic cross-linking [111] and SPEEK by two-step cross-linking [112] applications; the alkali-doped poly(2,5-benzimidazole) membrane was synthesised. The thermal and chemical stability of alkali-doped membrane in the alkali media was up to 100 °C [113].
Ionic liquid-based membranes were designed for application in electrochemical devices. The Nafion 112 membrane impregnated with various biodegradable hydroxyl ammonium-based ionic liquids (the investigated anions—formates, acetates, and lactates). All studied composite Nafion 112 membranes impregnated with different hydroxyl ammonium ionic liquids showed similar behaviour. The conductivity decrease was not observed in a temperature range from 30 to 90 °C in ambient environment due to the potential water loss [114]. The hydrogen sulphate anion increased conductivity by one-order-higher values as compared to phosphate or tosylate anion [115].
Sulphonated polyetheretherketone (SPEEK) membranes have been found a suitable matrix for composite formation. The mechanical stability of SPEEK membranes with ionic liquids is decreasing at higher temperatures [116]. The mechanical stability may be improved by forming composite with inorganic oxide nanoparticles. It was shown that similar effect may be achieved by adding activated carbon particles [117]. The organo-montmorillonite modified by polyionenes was studied as a potential additive for composite formation [118].
The methodology to improve the membrane conductivity measurements was investigated using sulphonated polyetheretherketone membranes. The differential method by measuring the polymer membrane in-between two NAFION membranes was found to provide repeatable results and not to damage the investigated membrane [119]. The IR spectra modelling for SPEEK membranes was used to reveal which structural changes may be identified by using IR spectroscopy. It was shown that IR spectra may be useful to identify the polymer cross-linking.
The through-plane measurements of SPEEK membranes were done by Hodakovska [120]: 12 mS/cm at RT and 23 mS/cm at 80 °C. The thermo-gravimetric analysis has shown a good thermal stability in the range from RT to 200–220 °C and two characteristic regions of weight loss—7.4% at ~ 140 °C (reversible water loss) and 10.3% at 200–220 °C (due to polymer degradation when cross-linked polymer chains permanently break down and their SO3H-groups are lost). Working with polymer composites [121, 122], she found that for composite polymer membrane sample, the changes in both modes are much more significant: for height it reaches 600 nm, but for phase – 20°. If surface potential of composite polymer sample clearly identifies some areas as SPEKK polymer, in topography and phase images, these places are identified as PVDF polymer only. This can be explained with the fact that polymers dissipate charge not only from surface but also little in depth. Even with such small potential contrast, the surface potential mode of AFM can be used to detect distinct polymer structures not only on surface but also in a volume under the surface.
Proton-conducting membranes, which have been commonly used in fuel cells, have raised interest for the application in harsh environments involving ionising radiation. NAFION and SPEEK membranes studied in dose range from 50 to 500 kGy, and SPEEK mechanical and chemical stability was increased due to the cross-linking. At the same time for NAFION, the destruction of the backbone was observed with the release of fluorine anion [123].
Materials for Li-ion and Na-ion batteries
Following the Sony’s successful commercialisation of Li-ion batteries in 1991, research of materials for Li-ion batteries intensified both in academia and industry. ISSP UL followed the trend, as the group had been working with electrochemical systems, charge and mass transport, and intercalation reactions since its establishment. The work on battery materials at ISSP UL has taken place since 1980s. Early research in the battery field was done in cooperation with Greifswald University, studying iodine and lithium batteries. For iodine batteries, the N-alkyl urotropinium polyiodides were used as an iodine ion electrolyte liquid at room temperature [124, 125]. The topic of battery materials was revisited in 1990s by Vītiņš et al. in collaboration with the Technical University of Denmark. They studied lithium intercalation into layered LiMnO2 [126] and performed several subsequent studies on other materials [127], with Ģ. Vītiņš later continuing his career at the University of Southampton [128,129,130].
The topic of battery materials was re-established at ISSP UL by J. Kleperis in collaboration with G. Bajārs in the 2010s, as thin films of LiFePO4 were studied. These were deposited by magnetron sputtering [35], optimising the deposition parameters [34] and studying the effects the reduced thickness and coarse morphology have on the electrochemical properties [36]. The work resulted in collaboration with Vilnius University (Lithuania) and National Cheng Kung University (Taiwan) [131]. Subsequently, the work of LiFePO4 branched out into studying bulk material and reduced graphene oxide as an electron-conducting additive [132], also resulting in a highly cited review article on the topic [133].
First reported in 1997 [134], LiFePO4 had gained substantial momentum in the 2000s. LiFePO4 is currently among the most studied battery materials in academic literature. It subsequently gained traction with the battery industry (LiFePO4 is presently considered for budget-oriented electric vehicles) and with the researchers studying fundamental questions of mass transport and phase transitions in Li-ion batteries [135,136,137]. Following the ISSP UL research group’s interest in reduced graphene oxide [133], electrophoretic deposition of reduced graphene oxide was subsequently explored [38]. It was eventually found that reduced graphene oxide has a stabilising effect on the cycle life of metal oxides (TiO2 and Fe2O3), and reduced graphene oxide composites with these have some potential as anodes for Li-ion batteries [37].
In parallel, returned from his residency in South Africa (University of Western Cape) G. Vaivars started studies on ionic liquids as electrolytes for lithium-ion batteries. The electrode impedance spectra of the cells with the ionic liquid “ECOENG™ 110” were interpreted in terms of equivalent circuits denoted as R1(QR2) and R1[Q(R2W)] for metallic electrodes and carbon electrodes, respectively [138]. Ionic liquids were used to form composite membranes based on NAFION and sulphonated poly(ether ether ketones). Attention was paid to estimating ionic liquids’ impact on membranes’ mechanical properties [139]. It was found that imidazolium dimethyl phosphates are an excellent environment to dissolve the lithium salts. Lithium nitrate’s temperature stability was measured from room to 150 °C [140].
Initiated by G. Bajars and G. Kucinskis, just returning from a post-doctoral research position at Max Planck Institute for Solid State Research (J. Maier’s group) and supported by J. Kleperis, research on Na-ion battery materials was started at ISSP UL in 2019. This was in part a consequence of the rising popularity of Na-ion battery technology, which is currently considered an emerging potential alternative to Li-ion batteries in grid-scale storage and budget EVs by many [141]. Now, research of polyanionic [41, 43] and layered transition metal compounds [42] for Na-ion batteries is ongoing at G. Kučinskis’ group, along with investigations in ionic-liquid-based electrolytes and composites for Na-ion batteries at G. Vaivars’s research group. Following the rapid expansion of Li-ion technology, ongoing parallel studies on the ageing of Li-ion batteries are currently taking place, following a collaboration with the Center for Solar and Hydrogen Research of Baden-Wurttemberg (Ulm, Germany), where Arrhenius-type relations and their importance were studied together with T. Waldman’s group [39, 40]. The cited references have been the first to experimentally follow how the two governing ageing modes in Li-ion battery cells (growth of solid electrolyte interface and lithium plating) intersect with changing C-rate, temperature, and other cell parameters.
Ab initio quantum mechanical electronic structure simulations have been applied by theoretician R. Eglītis to predict the working potential of the cathode materials. It was shown that, for Li2CoMn3O8 cathode material, a battery with an average voltage of around 5 V is possible. This cathode material is stable against interchange of positions of Mn and Co atoms as well as the choice of the position of the Li vacancy [142, 143]. Kotomin and co-workers have made many theoretical calculations of materials and processes for lithium-ion batteries to improve their electrochemical performance. The articles were published in prestigious, highly cited scientific journals [144,145,146].
The research in the direction of materials for Li-ion batteries is ongoing, with students (K. Kaprāns, L. Britāla, I. Ņesterova, and B. Krūze) and researchers (J. Hodakovska, G. Bajārs, G. Vaivars, G. Kučinskis) currently active in this direction, with many more national and international collaborations started in recent years. Productive collaborative relationships with the rapidly developing battery field have been established, including several start-ups and more experienced companies (Electrify, Sidrabe). This, along with the rapid growth of the European industry, is helping to develop a lively local ecosystem of battery-related activities.
Water electrolysis and some applications
In the field of water electrolysis, various solutions were tested—inside each other’s anode and cathode cylinders made of stainless steel with a gap of 1–3 mm, water plasma discharge with tungsten metal cathode and graphite anode, very short high-voltage pulse generators, and much more. It should note the excellent performance of the engineering and technical work performed by Mārtiņš Vanags, Vladimirs Ņemcevs, and Jānis Straumēns. The goal was to create a water electrolysis device that splits water efficiently, consumes little power, and is long-lasting [147,148,149,150,151,152]. Their application was to enrich intake air in internal combustion engine vehicles with HOH gases from water electrolysis to reduce the fuel consumption and exhaust hydrocarbon concentrations. The created water electrolysis systems were tested in their own vehicles and gave positive results but only on cars that correspond to Euro3 and lower emission classes. The advantage of electrolysers with cylindrical metal electrodes is that no alkaline electrolyte is required; instead tap water works very well, since the distance between the electrodes is only a few millimetres, and high voltage (low amperage) in very short pulses is applied. A water-heating boiler was also built on the basis of such an electrolyser [153]. The boiler structure contains a water vapour condensation device resulting from the combustion of electrolysis gases (HOH gas emits only water vapour when burned), in which the condensed water is returned to the electrolyser. The gas burner is surrounded by a heat exchanger, near which semiconductor thermoelectric batteries are placed for generating electricity from heat. This increases the efficiency of the boiler.
New experience was gained while working in the H2020 project on the output of the electrocatalysis equipment on the cathode of the electrolyser, reducing the CO2 collected in the biogas plant into ethylene [33]. The role of the ISSP UL in the project was to create an alternative structure for the cathode based on self-made modified multilayer graphene flakes obtained from recycled graphite and coated with catalyst metallic copper nanograins for the electrocatalytic reduction of CO2 to ethylene. Institute scientists performed ab initio calculations for graphene modified with clusters of copper atoms, as well as constructed an innovative electrochemical half-cell for monitoring the gaseous products of CO2 reduction with the FTIR spectroscopy method. Composites of SPEEK polymer with zirconium oxide nanoparticles and imidazolium-derived ionic liquid were developed to form a membrane that is compatible with catalyst materials and stimulates electrode CO2 adsorption [119]. Calculations showed that the adsorption of CO2 onto catalyst nanocrystals is typically the rate-determining step in the CO2 reduction reaction, and energies of adsorption at the Cu7/graphene nanostructure have been calculated [154]. The strong binding of CO2 to the Cu7 nanocluster at graphene was explained by the presence of < 111 > facets at the Cu7 nanocluster, which agrees with the recent experimental observations to improve the selectivity of CO2 electrochemical reduction in producing the CH2–CH2 intermediates. It was found that the size of the copper crystals and the uniformity of the coating can be controlled by the length and amplitude of the current pulses, which prefers the electrochemical deposition method to obtain good catalyst electrodes [155].
Summary
The research on ion processes and phenomena of the electrochromic effect in tungsten trioxide thin films at the University of Latvia began in 1972. After 2 years, the first thin-film electrochromic systems, such as indicators, light switches, and tuning filters, were designed. This immediately aroused great interest in the USSR’s industrial complex and ensured large-scale and long-term special contract works.
The fundamental research of optical, electronic, and structural properties of mixed ion/electron-conducting transition metal oxides started under the leadership of Andrejs Lūsis. In 1979, a new scientific direction—solid state ionics—was created, combining solid-state physics and solid-state electrochemistry. Cooperation with electrochemists of the University of Latvia in research of hydrogen diffusion in metals and metal oxides led to the patent for hydrogen detection method in metals. A new methodology for analysing the functional parameters of electrochromic systems was developed. The world’s first electrochromic system composed of WO3 and Ni(OH)2 separated by antimonic acid hydrate as a proton solid electrolyte was demonstrated in 1984, significantly increasing the electrochromic efficiency and operating resources of devices.
A new type of resistive gas-sensitive structure was obtained by laser beam cutting the conducting In2O3 layer on a glass substrate into a comb-teeth-type electrode, creating a prototype of an excellent humidity sensor, especially at high relative humidity values. Furthermore, practical research was done with a modular sensor system with artificial intelligence called electronic nose, proving that it is applicable both in the search for the sources of disturbing odours, the changes in the smell of different product groups during ageing, and in the diagnosis of diseases. Together with the Institute of Experimental Clinical Medicine of the University of Latvia, odour characteristics for lung cancer patients by comparing breath patterns of specific lung diseases were performed, showing that lung cancer can easily be discriminated from other lung diseases during a short breath sampling and analysis time.
In early 2000s, the significance of climate-neutral, environmentally friendly, and alternative ways of obtaining, storing, and using energy became one of the hottest topics of world economy and scientific research. ISSP UL researchers turned their focus on research of materials with enhanced surface capabilities to increase hydrogen storage capacity for known materials as AB5, zeolite, to obtain new composites using spillover phenomena and storage of bio-hydrogen. Wide range of experimental and theoretical studies on photocatalytic degradation of organic substances, water splitting, and CO2 reformation were performed to investigate both known catalysts such as TiO2 and ZnO as well as new materials such as graphene.
The underlying knowledge on electrochemistry has also helped ISSP UL to expand the scope of its research to electrode materials and electrolytes for Li-ion and Na-ion batteries, as well as more recently ageing of battery materials and cells, with preliminary research taking place in first two decades of the century and accelerating starting 2019. The research on solid state ionics at ISSP UL has experienced considerable growth and recognition as well as strenuous times due to a complex set of historical and economic factors. It has persisted due to (and often despite) diverse external transformations and, at times, only due to sheer, selfless persistence and efforts of the researchers involved. Nevertheless, the scientists representing and driving forward the direction of solid-state ionics at ISSP UL are fortunate to experience the times where sustainable energy generation and storage are becoming ever more important, providing yet another crucial application for this multi-faceted research direction. The key focus of the researchers remains development of materials and techniques for hydrogen production, storage, transport and use, as well as development of environmentally friendly carbon-based catalysts for H2 generation and sensing. ISSP UL is also leveraging its expertise in solid-state ionics to keep expanding the research of high-performance sustainable materials for Li-ion and Na-ion batteries and characterisation and prevention of ageing of battery cells, employing techniques for analysing interfaces, mass transport, and phase transitions.
Many collaborations are already initiated with partners across Europe and beyond in research, ranging from battery materials to hydrogen production. Going forward, the researchers at ISSP UL will keep actively contributing to the rapidly expanding and developing field of solid-state ionics, maintaining and growing its strength as key national, regional, and international centre of research excellence.
References
Lusis A (1975) Electrophysical properties of copper phosphate glasses. Doctoral Thesis. Peteris Stucka Latvian State University
Lagzdons JL, Kleperis J, Lusis A (1975) Tungsten-oxygen compounds and electronic absorption spectra of W-P-O glasses. Sci Artic Pataeris Stucka Latv State Univ 231:7
Kleperis J, Lusis A (1976) Termomodulation absorption spectra of tungsten trioxide and W-P-O glasses. In: Physics and chemistry of glass-forming systems. Latvian State University, Riga, p 10
Klavins J, Kleperis J, Lusis A, Pinnis J (1976) Electrochromic effect in thin WO3 layers - role of layer porosity. In: Physics and chemistry of glass-forming systems. Latvian State University, Riga, p 11
Lusis A, Kleperis J, Zamozdik T et al (1980) Electrochromic devices for recording and displaying video information. In: Application of methods of optical information processing and holography. Leningrad, p 4
Zamozdik T, Kleperis J (1981) Electrophysical properties of electrochromic elements. In: Oxide electrochromic materials. Latvian State University, Riga, p 11
Brishka AA, Zamozdik TV, Kleperis J et al (1981) Volt-ampere and volt-transmission curves for thin-film electrochromic elements of tungsten trioxide. Sov Physics Tech Phys 26:583–586
Lusis A, Klavins J, Kleperis J, Pinnis J (1982) Electrochemical processes in solid electrochromic systems. Sov Electrochem 18:1372–1376
Deb SK (1969) A novel electrophotographic system. Appl Opt 8:192. https://doi.org/10.1364/AO.8.S1.000192
Lusis A, Klavins J, Zamozdik T et al (1981) Method of producing solid electrochromic element, Patent No. 4251138
Rode O, Lusis A, Kleperis J et al (1979) Electrochrome element control device, United States Patent Office, Patent No. US4132465A
Kleperis JJ, Takeris SJ, Lusis AR, Stradins JP (1984) The investigation of hydrogen diffusion in palladium by chemichromic reaction. Phys Status Solidi 81:K121–K125. https://doi.org/10.1002/pssa.2210810247
Bajars GE, Linhardt P, Breiter MW (1990) Impedance and simulation studies of the cell electrode/hydrous antimonic acid/electrode. Electrochim Acta 35:1031–1036. https://doi.org/10.1016/0013-4686(90)90038-2
Kleperis J, Bajars G, Vaivars G et al (1992) Gaseous sensors based on solid proton conductors. Sensors Actuators A Phys 32:476–479. https://doi.org/10.1016/0924-4247(92)80031-W
Vaivars G, Pitkevičs J, Lusis A (1993) Sol-gel produced humidity sensor. Sensors Actuators B Chem 13:111–113. https://doi.org/10.1016/0925-4005(93)85337-A
Lusis A, Kleperis J, Pentjušs E (2003) Model of electrochromic and related phenomena in tungsten oxide thin films. J Solid State Electrochem 7:106–112. https://doi.org/10.1007/s10008-002-0315-2
Lusis AR, Pentjuss E, Bajars G et al (1997) Long-term testing results of WO3-based electrochromic cells. In: Silinsh EA, Medvids A, Lusis AR, Ozols AO (eds) Proceedings of SPIE - the international society for optical engineering, pp 234–238
Azens A, Pentjuss E, Gutarra A et al (1994) Electrochromism in oxyfluoride thin films. In: Proceedings of SPIE - the international society for optical engineering. pp 435–442
Vaivars G, Azens A, Kleperis J et al (1997) Proton conducting polymer electrolytes for electrochromic devices. In: Silinsh EA, Medvids A, Lusis AR, Ozols AO (eds) Proceedings of SPIE - the international society for optical engineering, pp 225–229
Balerna A, Bernieri E, Burattini E et al (1991) EXAFS studies of MeO3−x (Me = W, Mo, Re, Ir) crystalline and amorphous oxides. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers, Detect Assoc Equip 308:234–239. https://doi.org/10.1016/0168-9002(91)90636-5
Grinberga L, Hodakovska J, Kleperis J et al (2007) Electrochemical hydrogen storage and usage aspects: nickel electrode in acidic electrolyte. Russ J Electrochem 43:598–602. https://doi.org/10.1134/S1023193507050163
Grinberga L, Kleperis J, Vaivars G et al (2007) Investigations of the influence of different additives to the lanthanum rich mischmetal. In: Hydrogen materials science and chemistry of carbon nanomaterials. Springer Netherlands, Dordrecht, pp 279–286
Grinberga L, Kleperis J (2007) Development of new composite materials for hydrogen storage. The AB 5 type hydride alloy with silica glass support. J Phys Conf Ser 93:012024. https://doi.org/10.1088/1742-6596/93/1/012024
Grinberga L, Kleperis J, Bajars G et al (2008) Estimation of hydrogen transfer mechanisms in composite materials. Solid State Ionics 179:42–45. https://doi.org/10.1016/j.ssi.2007.12.051
Dimanta I, Kleperis J, Nakurte I et al (2016) Metal hydride alloys for storing hydrogen produced by anaerobic bacterial fermentation. Int J Hydrogen Energy 41:9394–9401. https://doi.org/10.1016/j.ijhydene.2016.04.064
Lesnicenoks P, Grinberga L, Kleperis J (2014) Gravimetric and spectroscopic studies of reversible hydrogen sorption on nanoporous clinoptilolite. Latv J Phys Tech Sci 51:35–41. https://doi.org/10.2478/lpts-2014-0017
Fu P, Wang J, Jia R et al (2017) Theoretical study on hydrogen storage capacity of expanded h-BN systems. Comput Mater Sci 139:335–340. https://doi.org/10.1016/j.commatsci.2017.08.015
Straumal B, Korneva A, Kuzmin A et al (2022) High entropy alloys for energy conversion and storage: a review of grain boundary wetting phenomena. Energies 15:7130. https://doi.org/10.3390/en15197130
Šutka A, Pärna R, Kleperis J et al (2014) Photocatalytic activity of non-stoichiometric ZnFe 2 O 4 under visible light irradiation. Phys Scr 89:044011. https://doi.org/10.1088/0031-8949/89/04/044011
Piskunov S, Lisovski O, Begens J et al (2015) C-, N-, S-, and Fe-doped TiO 2 and SrTiO3 nanotubes for visible-light-driven photocatalytic water splitting: prediction from first principles. J Phys Chem C 119:18686–18696. https://doi.org/10.1021/acs.jpcc.5b03691
Knoks A, Kleperis J, Grinberga L (2017) Raman spectral identification of phase distribution in anodic titanium dioxide coating. Proc Est Acad Sci 66:422. https://doi.org/10.3176/proc.2017.4.19
Yang D-C, Eglitis RI, Yi Z-J et al (2022) Overall direct photocatalytic water-splitting on C 2 mm -graphyne: a novel two-dimensional carbon allotrope. J Mater Chem C 10:10843–10852. https://doi.org/10.1039/D2TC02345H
Knoks A, Lesnicenoks P, Kleperis J et al (2019) Electro-catalytic and photo-catalytic reformation of CO 2 –reactions and efficiencies processes (review). IOP Conf Ser Mater Sci Eng 503:012009. https://doi.org/10.1088/1757-899X/503/1/012009
Bajars G, Kucinskis G, Smits J, Kleperis J (2011) Physical and electrochemical properties of LiFePO4/C thin films deposited by direct current and radiofrequency magnetron sputtering. Solid State Ionics 188:156–159. https://doi.org/10.1016/j.ssi.2010.10.022
Smits J, Kucinskis G, Bajars G, Kleperis J (2011) Structure and electrochemical characteristics of LiFePO4 as cathode material for lithium-ion batteries. Latv J Phys Tech Sci 48:27–31. https://doi.org/10.2478/v10047-011-0012-y
Bajars G, Kucinskis G, Smits J et al (2012) Characterization of LiFePO 4/C composite thin films using electrochemical impedance spectroscopy. IOP Conf Ser Mater Sci Eng 38:012019. https://doi.org/10.1088/1757-899X/38/1/012019
Kaprans K, Mateuss J, Dorondo A et al (2018) Electrophoretically deposited α-Fe2O3 and TiO2 composite anchored on rGO with excellent cycle performance as anode for lithium ion batteries. Solid State Ionics 319:1–6. https://doi.org/10.1016/j.ssi.2018.01.042
Kaprans K, Bajars G, Kucinskis G et al (2015) Electrophoretic nanocrystalline graphene film electrode for lithium ion battery. IOP Conf Ser Mater Sci Eng 77:012042. https://doi.org/10.1088/1757-899X/77/1/012042
Kucinskis G, Bozorgchenani M, Feinauer M et al (2022) Arrhenius plots for Li-ion battery ageing as a function of temperature, C-rate, and ageing state – an experimental study. J Power Sources 549:232129. https://doi.org/10.1016/j.jpowsour.2022.232129
Bozorgchenani M, Kucinskis G, Wohlfahrt-Mehrens M, Waldmann T (2022) Experimental confirmation of C-rate dependent minima shifts in Arrhenius plots of Li-ion battery aging. J Electrochem Soc 169:030509. https://doi.org/10.1149/1945-7111/ac580d
Kucinskis G, Nesterova I, Sarakovskis A et al (2022) Electrochemical performance of Na2FeP2O7/C cathode for sodium-ion batteries in electrolyte with fluoroethylene carbonate additive. J Alloys Compd 895:162656. https://doi.org/10.1016/j.jallcom.2021.162656
Kucinskis G, Kruze B, Korde P et al (2022) Enhanced electrochemical properties of Na0.67MnO2 cathode for Na-ion batteries prepared with novel tetrabutylammonium alginate binder. Batteries 8:6. https://doi.org/10.3390/batteries8010006
Kavaliukė V, Nesterova I, Kežionis A et al (2022) Combined conductivity and electrochemical impedance spectroscopy study of Na2FeP2O7 cathode material for sodium ion batteries. Solid State Ionics 385:116024. https://doi.org/10.1016/j.ssi.2022.116024
Ramans GM, Gabrusenoks JV, Lusis AR, Patmalnieks AA (1987) Structure of amorphous thin films of WO3 and MoO3. J Non Cryst Solids 90:637–640. https://doi.org/10.1016/S0022-3093(87)80504-5
Gabrusenoks J, Cikmach P, Lusis A et al (1984) Electrochromic colour centres in amorphous tungsten trioxide thin films☆. Solid State Ionics 14:25–30. https://doi.org/10.1016/0167-2738(84)90006-7
Kleperis JJ, Cikmach PD, Lusis AR (1984) Colour centres in amorphous tungsten trioxide thin films. Phys Status Solidi 83:291–297. https://doi.org/10.1002/pssa.2210830132
Bets V, Zamozdiks T, Lusis A et al (1987) The structure of nickel and indium oxide thin films from EXAFS data. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers, Detect Assoc Equip 261:173–174. https://doi.org/10.1016/0168-9002(87)90592-4
Bets V, Veispals A, Lusis A et al (1987) Studies of tungsten oxide electrochromic thin films and polycrystals by the EXAFS method. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers, Detect Assoc Equip 261:175–177. https://doi.org/10.1016/0168-9002(87)90593-6
Balerna A, Bernieri E, Burattini E et al (1991) XANES studies of MeO3-x (Me = W, Re, Ir) crystalline and amorphous oxides. Nucl Instruments Methods Phys Res Sect A Accel Spectrometers, Detect Assoc Equip 308:240–242. https://doi.org/10.1016/0168-9002(91)90637-6
Bajars G, Pitkevics J, Straumens J, Lusis A (1989) Electrochemical impedance of a tungsten (VI) oxide injection electrode in protic electrolytes. Sov Electrochem 25:749–752
Lagzdons J, Bajars G, Lusis A (1984) Modelling of the solid state electrochromic system WO3/HSbO3 x 2H2O/Ni(OH)2. Phys Status Solidi 84:K197–K200
Lusis AR (1997) Functional models of electrochromic devices: cycling capacity and degradation. In: Silinsh EA, Medvids A, Lusis AR, Ozols AO (eds) Proceedings of SPIE - the international society for optical engineering, pp 167–173
Bajars G, Pitkevics J, Lusis A et al (1989) Electrooptic characteristics of an electrochromic nickel-oxide electrode under potentiodynamic conditions. Sov Electrochem 25:292–298
Pentjuss E, Rodionov A, Kalendarev R et al (1997) Influence of treatment on stability of electrochromic WO3 film in acidic electrolyte. In: Silinsh EA, Medvids A, Lusis AR, Ozols AO (eds) Proceedings of SPIE - the international society for optical engineering, pp 230–233
Darling A, AMIMechE (1958) The diffusion of hydrogen through palladium. Platin Met Rev 2:16
Kleperis J, Lūsis A (1993) Hydrogen transfer problems at metal/proton electrolyte interfaces*. Zeitschrift für Phys Chemie 181:321–328. https://doi.org/10.1524/zpch.1993.181.Part_1_2.321
Lagzdons J, Lusis A, Kleperis J, Bajars G (1987) Electrochemical hydrogen sensor, patent No. 1440179
Kleperis J, Lusis A, Takeris S (1986) Hydrogen detection method in metals, patent No. 1267233
Liepina LK (1978) About the mechanism of hydrides in the reaction metal + water. In: Proceedings of the Latvian Academy of Sciences, chemical series, pp 152–1576
Kleperis J, Vaivars G, Bajārs G et al (1993) Solid proton conductors as room-temperature gas sensors. Sensors Actuators B Chem 13:269–271. https://doi.org/10.1016/0925-4005(93)85378-N
Kleperis J, Kundzins M, Vitins G et al (1995) Gas-sensitive gap formation by laser ablation in In2O3 layer: application as humidity sensor. Sensors Actuators B Chem 28:135–138
Vaivars G, Kleperis J, Zubkans J et al (1995) Application of sol-gel and laser evaporation methods to obtain thin gas sensitive films. In: International conference on solid-state sensors and actuators, and Eurosensors IX, pp 870–873
Vaivars G, Kleperis J, Zubkans J et al (1996) Influence of thin film coatings on the gas sensitivity properties of narrow laser cut gap in In2O3 on glass substrate. Sensors Actuators B Chem B Chem 33:173–177
Zubkans J, Spetz AL, Sundgren H et al (1995) In-situ modification of the NOx sensitivity of thin discontinuous platinum films as gates of chemical sensors. Thin Solid Films 268:140–143. https://doi.org/10.1016/0040-6090(95)06863-5
Kleperis J, Grinberga L, Paegle K, Lusis A (2000) Two possible applicators of electronic nose: beer producer and customs inspector. In: Proceedings of 7th International Symposium Olfaction & Electronic Nose 2000, pp 97–101
Kleperis J, Grinberga L, Lusis A (2001) Electronic nose: what it is and application examples. Latv J Phys Tech Sci 5:57–66
Kleperis J, Grinberga L, Lusis A (2002) Quick authenticity testing of food and goods. Is it real with e/z – nose? In: Proceedings of the Ninth International Symposium on Olfaction and Electronic Nose, pp 89–94
Ogorodnik V, Kleperis J, Taivans I et al (2008) Electronic nose for identification of lung diseases. Latv J Phys Tech Sci 45:60–67. https://doi.org/10.2478/v10047-008-0026-2
Kleperis J, Grinberga L, D’Amico A (2003) Special issue papers: ISOEN 2003. In: Special Issue of Sensors and Actuators, vol. B106, issue 1, 2005. Riga, p 186
Vaivars G, Kleperis J, Mlynarek G et al (1999) AC impedance behavior of the Ti4Ni2Oy and Ti3.5Zr0.5Ni2Oy type metal hydride electrodes. Ionics (Kiel) 5:292–298. https://doi.org/10.1007/BF02375853
Kleperis J, Wójcik G, Czerwinski A et al (2001) Electrochemical behavior of metal hydrides. J Solid State Electrochem 5:229–249. https://doi.org/10.1007/s100080000149
Grinberga L, Kleperis J (2008) Hydrogen sorption properties of metal hydride and glass phase. In: NATO Science for Peace and Security Series C: Environmental Security, pp 543–548
Lesnicenoks P, Sivars A, Grinberga L, Kleperis J (2012) Hydrogen adsorption in zeolite studied with Sievert and thermogravimetric methods. IOP Conf Ser Mater Sci Eng 38:012060. https://doi.org/10.1088/1757-899X/38/1/012060
Kleperis J, Lesnicenoks P, Grinberga L et al (2013) Zeolite as material for hydrogen storage in transport applications. Latv J Phys Tech Sci 50:59–64. https://doi.org/10.2478/lpts-2013-0020
Lesnicenoks P, Zemitis J, Grinberga L et al (2017) Modified graphene sheet stacks for hydrogen binding. Mater Sci. https://doi.org/10.5755/j01.ms.23.1.13729
Lesnicenoks P, Berzina A, Lukoševičš I et al (2017) Complex multilayer carbon structures for green energetics. Proc Est Acad Sci 66:403. https://doi.org/10.3176/proc.2017.4.26
Fu P, Jia R, Kong C-P et al (2015) From determination of the fugacity coefficients to estimation of hydrogen storage capacity: a convenient theoretical method. Int J Hydrogen Energy 40:10908–10917. https://doi.org/10.1016/j.ijhydene.2015.07.005
Gigorjeva L, Millers D, Grabis J, Jankoviča D (2011) Photoluminescence and photocatalytic activity of zinc tungstate powders. Open Phys 9:510–514. https://doi.org/10.2478/s11534-010-0122-9
Knoks A, Kleperis J, Bajars G et al (2021) WO3 as additive for efficient photocatalyst binary system TiO2/WO3. Latv J Phys Tech Sci 58:24–34. https://doi.org/10.2478/lpts-2021-0043
Liepina I, Bajars G, Gabrusenoks J et al (2012) Preparation and photoactivity of electrophoretic TiO2 coating film. IOP Conf Ser Mater Sci Eng 38:012059. https://doi.org/10.1088/1757-899X/38/1/012059
Liepina I, Bajars G, Lusis A et al (2013) Preparation and characterization of nanostructured Fe-TiO 2 thin films produced by electrophoretic deposition. IOP Conf Ser Mater Sci Eng 49:012060. https://doi.org/10.1088/1757-899X/49/1/012060
Liepina I, Bajars G, Rublans M et al (2015) Structure and photocatalytic properties of TiO2 -WO3 composites prepared by electrophoretic deposition. IOP Conf Ser Mater Sci Eng 77:012039. https://doi.org/10.1088/1757-899X/77/1/012039
Lisovski O, Piskunov S, Zhukovskii YF, Bocharov D (2017) Quantum chemical simulations of titanium dioxide nanotubes used for photocatalytic water splitting. J Surf Investig X-ray, Synchrotron Neutron Tech 11:78–86. https://doi.org/10.1134/S1027451016050335
Bocharov D, Piskunov S, Zhukovskii YF et al (2017) First principles modeling of 3d-metal doped three-layer fluorite-structured TiO2 (4,4) nanotube to be used for photocatalytic hydrogen production. Vacuum 146:562–569. https://doi.org/10.1016/j.vacuum.2017.05.002
Lin Y-P, Isakoviča I, Gopejenko A et al (2021) Time-dependent density functional theory calculations of N- and S-doped TiO2 nanotube for water-splitting applications. Nanomaterials 11:2900. https://doi.org/10.3390/nano11112900
Zhukovskii YF, Piskunov S, Lisovski O et al (2016) Quantum chemical simulations of doped ZnO nanowires for photocatalytic hydrogen generation. Phys status solidi 253:2120–2128. https://doi.org/10.1002/pssb.201600452
Lin Y-P, Polyakov B, Butanovs E et al (2021) Excited states calculations of MoS2@ZnO and WS2@ZnO two-dimensional nanocomposites for water-splitting applications. Energies 15:150. https://doi.org/10.3390/en15010150
Sokolov M, Mastrikov YA, Zvejnieks G et al (2021) Water splitting on multifaceted SrTiO3 nanocrystals: computational study. Catalysts 11:1326. https://doi.org/10.3390/catal11111326
Bajars G, Lagzdons J, Lusis A, Veispals A (1986) Glassy solid electrolyte for solid-state ion devices, USSR Patent Office, Patent No. 1400359
Auksyalis S, Bajars G, Pitre K et al (1988) Electrical properties of HUO2PO4 × 4H2O polycrystals (HUP) in the frequency range 107–7.2 × 1010 Hz. Lith Phys Collect 28:757–762
Lagzdons L, Petrovskis G, Bajars G, Lagzdons J (1986) Antimonic acid hydrate solid proton electrolyte synthesis method, USSR Patent, Patent No. 13331117
Vitins G, Bajars G, Vaivars G et al (1994) Conductivity and volume impedance of Na-beta/beta’’-alumina. Latv J Chem 1:78
Kanepe Z, Vitins A, Vitins G et al (1996) Conductivity studies of Na3Sc2(PO4)3, NaScP2O7, and LiScP2O7 ceramic samples by means of impedance technique. Latv J Chem 3–4:31
Vītiņš Ģ, Kaņepe Z, Vītiņš A et al (2000) Structural and conductivity studies in LiFeP 2 O 7, LiScP 2 O 7, and NaScP 2 O 7. J Solid State Electrochem 4:146–152. https://doi.org/10.1007/s100080050012
Vītiņš Ģ, Ķizāne G, Lūsis A, Tīliks J (2002) Electrical conductivity studies in the system Li2TiO3-Li1.33Ti1.67O4. J Solid State Electrochem 6:311–319. https://doi.org/10.1007/s100080100239
Petrovskis G, Lusis A, Fullbier H, Lobitz P (1991) Verfahren zur Herstellung von Protonenfestelektrolyten (method for preparing proton-conducting solid electrolytes), DD291865, Patent No
Vaivars G, Kleperis J, Lusis A (1993) Antimonic acid hydrate xerogels as proton electrolytes. Solid State Ionics 61:317–321
Vaivars G, Kleperis J, Lusis A (1992) Investigation of antimonic acid hydrates produced by the sol-gel method. Sov Electrochem 28:1176–1180
Vaivars G, Kleperis J, Azens A et al (1997) Proton conducting composite electrolytes based on antimonic acid. Solid State Ionics 97:365–368. https://doi.org/10.1016/S0167-2738(97)00049-0
Granqvist C, Azens A, Hjelm A et al (1998) Recent advances in electrochromics for smart windows applications. Sol Energy 63:199–216. https://doi.org/10.1016/S0038-092X(98)00074-7
Vaivars G, Azens A, Granqvist CG (1999) Proton conducting polymer composites for electrochromic devices. Solid State Ionics 119:269–273. https://doi.org/10.1016/S0167-2738(98)00513-X
Vaivars G (2000) Polymeric nanocomposites with proton conducting hydrated particles for electrooptical systems. Mater Sci 6:68–72
Vaivars G, Furlani M, Mellander B-E, Granqvist CG (2003) Proton-conducting zirconium phosphate/poly(vinyl acetate)/glycerine gel electrolytes. J Solid State Electrochem 7:724–728. https://doi.org/10.1007/s10008-003-0391-y
Vaivars G, Shan J, Gericke G, Linkov V (2005) Phosphorized zirconium oxide nanoparticles. Appl Organomet Chem 19:1096–1100. https://doi.org/10.1002/aoc.978
Vaivars G, Mokrani T, Maxakatho N et al (2003) Inorganic direct methanol fuel cell. J Energy South Africa 47–50
Vaivars G, Maxakato NW, Mokrani T et al (2004) Zirconium phosphate based inorganic direct methanol fuel cell. Mater Sci 10:162–165
Vaivars G, Mokrani T, Hendricks N, Linkov V (2004) Inorganic membranes based on zirconium phosphate for fuel cells. J Solid State Electrochem 8:882–885. https://doi.org/10.1007/s10008-004-0529-6
Chikvaidze G, Gabrusenoks J, Kleperis J, Vaivars G (2007) Application of micro Raman spectroscopy to industrial FC membranes. J Phys Conf Ser 93:012026. https://doi.org/10.1088/1742-6596/93/1/012026
Luo H, Vaivars G, Mathe M et al (2009) Proton conducting membrane prepared by cross-linking highly sulfonated peek for PEMFC application. In: ASME 2009 7th International Conference on Fuel Cell Science, Engineering and Technology. ASMEDC, pp 723–728
Luo H, Vaivars G, Mathe M (2009) Cross-linked PEEK-WC proton exchange membrane for fuel cell. Int J Hydrogen Energy 34:8616–8621. https://doi.org/10.1016/j.ijhydene.2009.08.024
Luo H, Vaivars G, Mathe M (2010) Covalent-ionically cross-linked polyetheretherketone proton exchange membrane for direct methanol fuel cell. J Power Sources 195:5197–5200. https://doi.org/10.1016/j.jpowsour.2010.03.023
Luo H, Vaivars G, Mathe M (2012) Double cross-linked polyetheretherketone proton exchange membrane for fuel cell. Int J Hydrogen Energy 37:6148–6152. https://doi.org/10.1016/j.ijhydene.2011.05.115
Luo H, Vaivars G, Agboola B et al (2012) Anion exchange membrane based on alkali doped poly(2,5-benzimidazole) for fuel cell. Solid State Ionics 208:52–55. https://doi.org/10.1016/j.ssi.2011.11.029
Garaev V, Kleperis J, Pavlovica S, Vaivars G (2012) Properties of the Nafion membrane impregnated with hydroxyl ammonium based ionic liquids. IOP Conf Ser Mater Sci Eng 38:012064. https://doi.org/10.1088/1757-899X/38/1/012064
Lasmane L, Ausekle E, Vaivars G, Priksane A (2013) Acidic ionic liquids as composite forming additives for ion-conducting materials. IOP Conf Ser Mater Sci Eng 49:012039. https://doi.org/10.1088/1757-899X/49/1/012039
Sprugis E, Vaivars G, Merijs Meri R (2019) A study of mechanical properties of polymer composite membranes with various ionic liquids at elevated temperatures. Mater Sci. https://doi.org/10.5755/j01.ms.25.1.18933
Fedorenko D, Vaivars G (2020) Composite membranes of sulfonated poly(ether ether ketone) with active carbon: composite preparation and investigation of their properties for potential application for CO2 electrochemical reduction. Mater Sci 26:444–450. https://doi.org/10.5755/j01.ms.26.4.24000
Sukhyi MP, Tomilo VI, Sukhyi KM et al (2020) Organo-montmorillonite modified by polyionenes for polymer composites. Chem Technol Appl Subst 3:187–190. https://doi.org/10.23939/ctas2020.02.187
Fedorenko D, Vaivars G (2019) Different approaches in sulfonated poly (ether ether ketone) conductivity measurements. IOP Conf Ser Mater Sci Eng 503:012030. https://doi.org/10.1088/1757-899X/503/1/012030
Hodakovska J, Kleperis J (2008) Sulfonated poly(ether-ether-ketone) polymer membranes for fuel cells. Latv J Phys Tech Sci 45:55–60. https://doi.org/10.2478/v10047-008-0029-z
Hodakovska J, Kleperis J (2011) Surface relief, phase and surface potential investigations of composite polymer membranes using AFM. IOP Conf Ser Mater Sci Eng 23:012017. https://doi.org/10.1088/1757-899X/23/1/012017
Hodakovska J, Kleperis J (2016) Sulfonated poly(ether-ether-ketone) and Nafion composite membrane with aluminium oxide additive for fuel cell applications. Polym Sci Ser A 58:167–171. https://doi.org/10.1134/S0965545X16020103
Pajuste E, Reinholds I, Vaivars G et al (2022) Evaluation of radiation stability of electron beam irradiated Nafion® and sulfonated poly(ether ether ketone) membranes. Polym Degrad Stab 200:109970. https://doi.org/10.1016/j.polymdegradstab.2022.109970
Stegemann H, Jabs G, Mittag H et al (1987) N-Alkylurotropiniumpolyiodide - Darstellung und Untersuchung der elektrischen und magnetischen Eigenschaften. Z Anorg Allg Chem 555:183–191. https://doi.org/10.1002/zaac.19875551220
Mittag H, Cikmach P, Fullbier H et al (1989) Investigation of the electrical and magnetic properties of N-alkylurotropinium polyiodides. Elektrokhymia 25:802–804
Vitins G, West K (1997) Lithium intercalation into layered LiMnO2. J Electrochem Soc 144:2587–2592. https://doi.org/10.1149/1.1837869
West K, Vitins G, Koksbang R (2000) Synthesis and host properties of tetragonal Li2Mn2O4 and Li2Co0.4Mn1.6O4. Electrochim Acta 45:3141–3149. https://doi.org/10.1016/S0013-4686(00)00395-9
Vitins G, Raekelboom E, Weller M, Owen J (2003) Li2CuO2 as an additive for capacity enhancement of lithium ion cells. J Power Sources 119–121:938–942. https://doi.org/10.1016/S0378-7753(03)00236-2
Spong AD, Vitins G, Owen JR (2005) A solution–precursor synthesis of carbon-coated LiFePO[sub 4] for Li-ion cells. J Electrochem Soc 152:A2376. https://doi.org/10.1149/1.2120427
Roberts MR, Spong AD, Vitins G, Owen JR (2007) High throughput screening of the effect of carbon coating in LiFePO[sub 4] electrodes. J Electrochem Soc 154:A921. https://doi.org/10.1149/1.2763968
Fung K-Z, Tsai S-Y, Ni C-T et al (2014) Fabrication of Li 4 Ti 5 O 12 spinel thin films for Li mirobattery applications. ECS Meet Abstr MA2014–02:431–431. https://doi.org/10.1149/MA2014-02/5/431
Kucinskis G, Bajars G, Bikova K et al (2019) Microstructural influence on electrochemical properties of LiFePO4/C/reduced graphene oxide composite cathode. Russ J Electrochem 55:517–523. https://doi.org/10.1134/S1023193519060120
Kucinskis G, Bajars G, Kleperis J (2013) Graphene in lithium ion battery cathode materials: a review. J Power Sources 240:66–79. https://doi.org/10.1016/j.jpowsour.2013.03.160
Padhi AK, Nanjundaswarny K, Goodenough J (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188. https://doi.org/10.1149/1.1837571
Amin R, Maier J, Balaya P et al (2008) Ionic and electronic transport in single crystalline LiFePO4 grown by optical floating zone technique. Solid State Ionics 179:1683–1687. https://doi.org/10.1016/j.ssi.2008.01.079
Ohmer N, Fenk B, Samuelis D et al (2015) Phase evolution in single-crystalline LiFePO4 followed by in situ scanning X-ray microscopy of a micrometre-sized battery. Nat Commun 6:6045. https://doi.org/10.1038/ncomms7045
Lim J, Li Y, Alsem DH et al (2016) Origin and hysteresis of lithium compositional spatiodynamics within battery primary particles. Science 353:566–571. https://doi.org/10.1126/science.aaf4914
Katkevics J, Viksna A, Zicmanis A, Vaivars G (2011) Electrical impedance spectroscopy of ionic liquid 1-ethyl-3-methylimidazolium methanesulfonate (ECOENG™ 110). Solid State Ionics 188:114–117. https://doi.org/10.1016/j.ssi.2010.11.013
Garaev V, Pavlovica S, Reinholds I, Vaivars G (2013) Mechanical properties and XRD of Nafion modified by 2-hydroxyethylammonium ionic liquids. IOP Conf Ser Mater Sci Eng 49:012058. https://doi.org/10.1088/1757-899X/49/1/012058
Zicmanis A, Brica S, Vaivars G et al (2019) Ionic liquids and their modification with lithium salts - synthesis and studies. In: Nanostructured composite materials for energy storage and conversion. LU Akademiskais apgads, pp 54–68
Tapia-Ruiz N, Armstrong AR, Alptekin H et al (2021) 2021 roadmap for sodium-ion batteries. J Phys Energy 3:031503. https://doi.org/10.1088/2515-7655/ac01ef
Eglitis RI, Borstel G (2005) Towards a practical rechargeable 5 V Li ion battery. Phys Status Solidi 202:R13–R15. https://doi.org/10.1002/pssa.200409083
Eglitis RI (2015) Theoretical prediction of the 5 V rechargeable Li ion battery using Li2CoMn3O8 as a cathode. Phys Scr 90:094012. https://doi.org/10.1088/0031-8949/90/9/094012
Zhukovskii YF, Balaya P, Kotomin EA, Maier J (2006) Evidence for interfacial-storage anomaly in nanocomposites for lithium batteries from first-principles simulations. Phys Rev Lett 96:058302. https://doi.org/10.1103/PhysRevLett.96.058302
Balaya P, Bhattacharyya AJ, Jamnik J et al (2006) Nano-ionics in the context of lithium batteries. J Power Sources 159:171–178. https://doi.org/10.1016/j.jpowsour.2006.04.115
Zhukovskii YF, Kotomin EA, Balaya P, Maier J (2008) Enhanced interfacial lithium storage in nanocomposites of transition metals with LiF and Li2O: comparison of DFT calculations and experimental studies. Solid State Sci 10:491–495. https://doi.org/10.1016/j.solidstatesciences.2007.12.030
Vanags M, Kleperis J, Bajars G, Lusis A (2007) Water electrolysis using electrodes with modified surface/volume. J Phys Conf Ser 93:012025. https://doi.org/10.1088/1742-6596/93/1/012025
Vanags M, Kleperis J, Bajars G (2011) Electrolyses model development for metal/electrolyte interface: testing with microrespiration sensors. Int J Hydrogen Energy 36:1316–1320. https://doi.org/10.1016/j.ijhydene.2010.07.100
Vanags M, Kleperis J, Bajars G (2011) Separation of charging and charge transition currents with inductive voltage pulses. Latv J Phys Tech Sci. https://doi.org/10.2478/v10047-011-0020-y
Vanags M, Kleperis J, Bajars G (2012) Water electrolysis with inductive voltage pulses. In: Electrolysis. InTech, pp 19–44
Vanags M, Aizpurietis P, Bajars G et al (2012) Water electrolysis with DC pulses and plasma discharge. Int Sci J Altern Energy Ecol 9:21–27
Aizpurietis P, Vanags M, Kleperis J, Bajars G (2013) Ni–Al protective coating of steel electrodes in Dc electrolysis for hydrogen production / Ni–Al Pārklājuma Ietekme Uz Tērauda Elektrodiem Līdzstrāvas Elektrolīzē Ūdeņraža Ražošanai. Latv J Phys Tech Sci 50:53–59. https://doi.org/10.2478/lpts-2013-0012
Vanags M, Nemcevs V, Kleperis J (2008) Water-powered heat and electricity supply system, Latvian Patent Office, Patent No. lv 13710
Lisovski O, Piskunov S, Bocharov D et al (2022) CO2 and CH2 adsorption on copper-decorated graphene: predictions from first principle calculations. Crystals 12:194. https://doi.org/10.3390/cryst12020194
Lesnicenoks P, Knoks A, Piskunov S et al (2022) N-graphene sheet stacks/Cu electrocatalyst for CO2 reduction to ethylene. Electrochem 3:229–238. https://doi.org/10.3390/electrochem3020015
Acknowledgements
G.B. and G.K. acknowledge funding from the Latvian Council of Science (lzp-2020/1–0425) for their contributions in the chapter on Li-ion battery research. Institute of Solid-State Physics, University of Latvia as the Centre of Excellence has received funding from the European Union’s Horizon 2020 Framework Program H2020-WIDESPREAD-01–2016-2017-TeamingPhase2 under grant agreement No. 739508, project CAMART2.
Author information
Authors and Affiliations
Contributions
All authors contributed to the article conception and design. The main authors of original drafts of chapters are: Chapter 1, J. Kleperis, G. Bajārs, and J. Purāns; Chapter 2, J. Kleperis and J. Purāns; Chapter 3, G. Bajārs and J. Kleperis; Chapter 4, J. Kleperis and L. Grīnberga; Chapter 5, L. Grīnberga, and J. Kleperis; Chapter 6, L. Grīnberga and G. Bajārs; Chapter 7, G. Vaivars, G. Bajārs, and Ģ. Vītiņš; Chapter 8, G. Vaivars and J. Kleperis; Chapter 9, G. Kučinskis, Ģ. Vītiņš, and G. Bajārs; Chapter 10, J. Kleperis and G. Bajārs); review and editing, G. Kučinskis and L. Grīnberga; supervision, G. Bajārs and J. Kleperis). All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kleperis, J., Bajārs, G., Grīnberga, L. et al. From electrochromic phenomena to energy harvesting and storage—an overview of solid state ionics research at the Institute of Solid State Physics, University of Latvia. J Solid State Electrochem 27, 1641–1660 (2023). https://doi.org/10.1007/s10008-023-05419-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05419-8