Abstract
A new composition of magnesium (Mg)-ion-conducting polymer electrolyte comprising poly(ethylene oxide) (PEO) complexed with Mg trifluoromethanesulfonate (Mg triflate or Mg(Tf)2) containing different amounts of a nonionic plastic crystal succinonitrile (SN) has been prepared and characterized. High polarity and rotational disorder of the SN molecules in the plastic-crystalline phase, supports the enhancement of ionic conductivity of the PEO-Mg(Tf)2 complex system, showing a maximum room temperature ionic conductivity of ∼6 × 10-4 S cm−1 observed with the addition of 50 wt.% of SN. X-ray diffraction, optical microscopy, and differential scanning calorimetry suggest a substantial structural modification, decrease in crystallinity, and various interactions in the polymer electrolyte components due to addition of SN. The cyclic voltammetry, impedance, and dc polarization studies confirm the Mg-ion conduction in the PEO complex. The electrochemical potential window of the electrolyte, observed from the linear sweep voltammetry, is determined to be ∼4.1 V. The performance characteristics of the SN-incorporated polymer electrolyte system indicate their potential applicability as electrolytes in ionic devices including Mg batteries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, development of compact, light-weight, cost-effective, and high-energy density batteries are in great demand [1]. Rechargeable lithium (Li) batteries have been studied for a long time [1–12] because of the high electrochemical reduction potential of Li (−3.04 V with respect to standard hydrogen electrode) and high volumetric capacity (2,062 mA h cm−3). But the passivation layer formation at the electrode–electrolyte interfaces and dendritic growth of Li in the electrolyte region are some of the problems which limit the shelf life and cyclic stability of Li batteries [1, 2]. Recently, the electrochemistry of sodium, zinc, and magnesium (Mg) has been investigated and found these metals as suitable alternatives of the Li anode for the development of the rechargeable batteries [13–20]. Particularly, the Mg-based rechargeable batteries are under the current investigations because of their performance capabilities close to the Li-based systems [17–20]. The ionic radius of Mg2+ ions (r Mg 2+ = 0.072 nm) is comparable to that of Li (r Li + = 0.076 nm). Apart from the fact that Mg metal is stable, abundant, and more cost effective than Li, it possesses high volumetric capacity of 3,833 mA h cm−3 and electrochemical reduction potential (−2.37 V with respect to standard hydrogen electrode) [20]. These properties of Mg metal are attractive enough to develop the Mg batteries in combination with the suitable electrolyte and cathode materials.
One of the key factors which decides the lifetime and cyclic performance of a battery is a suitable choice of electrolyte which provides the sufficient control at the interfaces and medium for the ions for high mobility. Batteries using liquid electrolytes have been known for a long time; however, they are faced with several problems including leakage, flammability and toxicity of the electrolyte, corrosion of the electrodes during multiple charge–discharge cycling, bulky battery design, etc. [21]. The ion-conducting polymers as electrolytes have potential to overcome these problems because of their solid-like nature, improved electrochemical and thermal stability, absence of leakage, miniaturization, and moldability for better transportability [22, 23].
Extensive studies have been carried on solid polymer electrolytes/complexes consisting of poly(ethylene oxide) (PEO) and alkali metal salts for solid-state batteries. PEO has the ability to coordinate effectively with salt cations and offers excellent dimensional stability in the form of free-standing films with good ionic conductivity [22, 23]. Most of the reported PEO-based electrolytes comprise Li salts which are useful electrolytes in Li batteries [2, 24]. Few PEO-Mg salts complexes have been reported which are predominantly anionic conductors with negligible Mg2+-ion conductivity because of the strong electrostatic interaction between Mg2+ ion and ether oxygen of PEO [22, 25]. Some efforts have been devoted to enhance Mg2+-ion transport in PEO/Mg salt complexes, to employ them in Mg batteries, by incorporating plasticizers like ethylene carbonate, propylene carbonate, etc. [26]. Recently, Kumar et al. [27] reported a substantial enhancement in Mg2+-ion transport in PEO/Mg triflate system due to the addition of an ionic liquid. The ionic liquid incorporation leads to the additional properties of polymer electrolytes including nonflammability, nonvolatility, and high thermal and electrochemical stability [27]. However, the main drawback of such ionic liquid-based polymer electrolytes is the complexities in ionic conduction owing to the mobility of component ions of the ionic liquids [28].
Recently, some polymer-based electrolytes have been investigated using plastic-crystalline material-like succinonitrile (SN) which acts as solid plasticizer [29–34]. The SN is a nonionic plastic crystal which acts as a polar solvent having high dielectric constant (∼55 at 25 °C) [35]. The high dielectric constant value of SN ensures its ability to dissociate various salts for ionic conduction. Its plastic character before its melting point, due to the presence of impurity trans isomers, provides the vacancies for ion transport and makes the polymer network flexible which improves the electrical properties of polymer electrolytes. Polymer electrolytes containing the plastic crystal SN have attractive features including vanishingly small vapor pressure (hence the electrolyte evaporation can be avoided) and reduced flammability which reduces the safety concern.
In the present work, we report a novel composition of Mg2+-ion-conducting PEO-based polymer electrolyte complexed with Mg trifluoromethanesulfonate, i.e., Mg(Tf)2 incorporated with the plastic-crystalline SN as plasticizer in solid phase. Various techniques such as X-ray diffraction (XRD), optical microscopy, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), impedance analysis, and cyclic/linear sweep voltammetry have been used to characterize and optimize the polymer electrolytes. It has been observed that the polymer electrolyte PEO/Mg(Tf)2/SN is a Mg-ion-conducting, free-standing film, useful as a separator/electrolyte in Mg batteries.
Experimental
Preparation of materials
PEO (average molecular weight, ∼6 × 105), Mg trifluoromethane sulfonate Mg(Tf)2 (purity, 97 %), and SN (purity, 99 %) were obtained from Sigma-Aldrich. The PEO and salt Mg(Tf)2 were vacuum dried at temperatures of 30 and 100 °C, respectively. SN was further purified by recrystallization process. The polymer electrolyte films were prepared by “solution-cast” method. PEO and different amounts of SN (with respect to the weight of PEO) were separately dissolved in a common solvent acetonitrile by stirring them magnetically at room temperature for about 24 h. The appropriate amounts of Mg(Tf)2 were added to the SN solutions and stirred for ∼12 h to obtain homogeneous solutions. These solutions were then mixed with the PEO/acetonitrile solutions and stirred magnetically further for ∼12 h. The EO/Mg2+ ratio was kept at ∼20 for all the compositions. These solutions were then poured in Teflon Petri dishes, and the common solvent acetonitrile was allowed to evaporate slowly at room temperature for 4–5 days. After complete drying, free-standing polymer electrolyte films were peeled off from the dishes. Finally, these films were dried rigorously under vacuum (∼10−3 Torr) at room temperature for 2–3 days and stored in argon atmosphere to avoid adsorption of moisture. Binary mixtures of 5 mol% of Mg(Tf)2 in SN and PEO/SN (2:1( w/w) ratio) were also prepared using the above method for comparison. The composition of the polymer electrolytes will be referred to as PEO20·Mg(Tf)2 + x wt.% SN in rest of the text.
The circular discs of Mg electrodes (area, ∼1.2 cm2) were prepared by pressing the Mg powder (Aldrich) at 4 tons cm−2 to use them as reversible electrodes. These discs were polished with successive grades of emery papers for a smooth finishing then washed thoroughly in acetone and dried. The stainless steel (SS) sheets (Alfa Aesar, grade 304) were used as blocking electrodes.
Physical characterization
Thermal studies of the polymer electrolyte films were carried out by DSC and TGA. DSC measurements were performed under static nitrogen atmosphere using a calorimeter of TA Instruments (model, Q100). The samples were loaded in low-mass aluminum pans, and measurement temperatures were taken from −90 to 250 °C at a heating rate of 10 °C min−1. Each sample was first cooled from room temperature to −90 °C by a cooling rate of 3 °C min−1 and was equilibrated at this temperature for 10 min before recording the DSC plot in the heating cycle. TGA was carried out using a Perkin Elmer TGA system (TGA-7) in dry nitrogen atmosphere from room temperature to 480 °C at the same heating rate mentioned above.
Comparative changes in crystalline/amorphous textures in the electrolyte films were monitored by XRD at room temperature using an X-ray diffractometer (model, D8 DISCOVER, Brucker) at a scan rate of 2°/min in a Bragg angle (2θ) range from 10° to 60°. Morphological changes in the polymer electrolytes were observed using Nomarsky contrast optical microscope (model, MX61, Olympus, Japan).
Electrochemical impedance spectroscopic (EIS) studies were carried out to evaluate the ionic conductivity using LCR Hi-TESTER (model, 3522–50, HIOKI, Japan) in a frequency range from 1 Hz to 100 kHz with signal amplitude of 10 mV. The conductivity measurements were performed by sandwiching the electrolyte films between two symmetrical SS sheets. The temperature dependence of the ionic conductivity was recorded over the temperature range from 28 to 100 °C. The electrochemical studies were performed by cyclic voltammetry (CV) and linear sweep voltammetry (LSV) using an electrochemical analyzer (model, 608C, CH Instruments, USA). The dc polarization measurements were carried out for two cells with the symmetrical configuration using SS and Mg electrodes, namely, SS|electrolyte|SS and Mg|electrolyte|Mg. These cells were polarized by applying a dc voltage of 0.75 V.
Results and discussion
Thermal studies: DSC and TGA
DSC curves of pure PEO and SN, binary mixtures PEO/SN and SN/Mg(Tf)2, and PEO20·Mg(Tf)2 polymer complexes added with different amounts of SN (varying from 0 to 50 wt.%), recorded from −90 to 250 °C, are shown in Fig. 1. Pure PEO shows an endothermic melting peak at ∼67 °C (Fig. 1a). Pure SN shows a plastic-crystalline phase between −38 (the transition from normal to plastic-crystalline phase (T pc)) and 57 °C (melting point of SN (T m)) (Fig. 1b) [36]. The abrupt peaks observed for pure SN beyond 150 °C (Fig. 1b) are due to the fast evaporation of SN. On the addition of Mg(Tf)2, the melting peak of SN has been decreased to ∼33 °C (Fig. 1d), while T pc remains unaffected. The decrease in T m is due to the solvating ability of SN [37] which indicates the Mg–salt interaction with SN.
When SN is added to pure PEO (with a weight ratio of 1:2), only one endothermic peak is observed at a temperature of ∼60 °C. Normal to plastic-crystalline phase transition peak of SN at T pc ∼ −38 °C completely disappears (Fig. 1c). The melting peak of PEO (T m, ∼67 °C) reduces to ∼60 °C and possibly overlaps with the melting peak of SN, showing a broader peak (Fig. 1c). These changes indicate the possible donor–acceptor interaction of ether oxygen of PEO with N ≡ C group of SN [30]. Such interaction has also been evidenced on the basis of FTIR studies reported earlier [38–40].
When PEO is complexed with Mg(Tf)2, its melting peak is lowered to ∼59 °C (Fig. 1e), and as the SN concentration increases from 5 to 50 wt. %, the intensity of this peak decreases (Fig. 1f–j), which shows the decrease in crystalline nature of PEO. Different parameters, glass-transition temperature (T g), T m, ΔH m, and degree of crystallinity, evaluated from the melting peaks of PEO for all the compositions, are listed in Table 1. A substantial decrease in the degree of crystallinity due to the addition of SN in the PEO20·Mg(Tf)2 complex can be seen from Table 1. The decrease in crystallinity (hence increase in the amorphous fraction) in polymer electrolyte films has been observed due to the localization of the PEO chains in SN domain. The decrease in the crystalline nature of PEO-Mg salt complexes due to the addition of SN are discussed in XRD studies also in the following section.
An endothermic broad hump has also been observed between 65 and 170 °C for the PEO20·Mg(Tf)2 system which is assigned to the amorphous phase of PEO complex with Mg salt [27]. As the SN concentration increases in PEO20·Mg(Tf)2 complex, the intensity of this broad hump decreases and shifts towards the higher temperature range.
A step change, owing to the T g is distinctly observed in polymer electrolyte films containing 5–10 wt.% of SN at about −27 °C (with onset of T g at −32 °C). On increasing the content of SN, this step change shifts towards the lower temperature side (Fig. 1g–j). This down shifting of the T g shows the plasticizing effect of SN, responsible for increasing the flexibility of the polymeric films.
TGA curves for pure PEO and SN, and the PEO20·Mg(Tf)2 complexes added with different amounts of SN over the temperature range from room temperature to 480 °C are shown in Fig. 2. The host polymer PEO shows no weight loss indicating its thermal stability up to ∼340 °C. The SN, which is an adduct in the present polymer electrolyte system, is stable up to ∼100 °C and evaporates fully at 216 °C. The thermal stability of SN is observed up to 100 °C in the present condition of TGA measurement, however, the isothermal TGA study indicates the stability up to 80 °C only, as reported by Echeverri et al. [38]. The polymer electrolytes containing different amounts of SN show marginal weight loss of (∼4–7 wt.%) up to 100 °C, which may be due to the presence of moisture-sensitive salt Mg(Tf)2.
Structural studies: XRD and optical microscopy
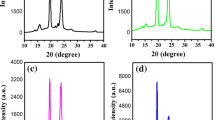
XRD and optical microscopic studies have been performed to investigate the possible changes in semi-crystalline nature of PEO20·Mg(Tf)2 complexes due to the addition of SN. Fig. 3 shows the XRD patterns of PEO (pure), PEO/SN blend, PEO20·Mg(Tf)2 complex, and PEO complexes containing different amounts of SN. The XRD peaks, observed at 2θ ∼19.0°, 23.1°, 26.1°, 26.9°, and 27.8° indicate the semi-crystalline nature of PEO. The addition of SN to PEO does not affect its semi-crystalline nature; however, no additional peak associated with SN is observed (Fig. 3b). It may be noted that the pure SN shows two characteristic peaks at ∼20° and 28°, as reported in literature [29, 41]. This indicates that the SN is fully dissolved in PEO [41]. A low-intensity broad hump appears between 15° and 30°, when Mg(Tf)2 is added in PEO to prepare the PEO complex (Fig. 3c). On the addition of SN in PEO-Mg(Tf)2 complex, the intensity of the broad peak increases with increasing content of SN. This indicates the enhancement of amorphous nature of the polymer electrolytes. It may be noted that even after the addition of substantial amount of SN, the sharp crystalline peaks of PEO still exist. This shows that the partial crystalline nature of the PEO persists even after the incorporation of SN. The enhancement in the amorphous nature increases the flexibility and hence the segmental motion of polymeric chains, which thereby increases the ion mobility and hence ionic conductivity. The enhancement in amorphous character (i.e., decrease in crystallinity) has also been observed in DSC studies, discussed in the previous section.
Gross morphological changes have been observed in PEO texture due to the addition of Mg salt and different amounts of SN. Figure 4 shows the optical micrographs of the pure PEO, PEO20·Mg(Tf)2 complex, and PEO20·Mg(Tf)2 complexes added with 5 and 30 wt.% SN. Pure PEO shows the spherulitic structure with dark boundaries which indicates its semi-crystalline nature. The spherulitic texture disappears and a completely different texture is observed when PEO is complexed with Mg salt, as shown in Fig. 4b. Regular arrays of polymer-complex strands are observed, separated by linear dark regions. On the addition of different amounts of SN, almost flat surfaces are observed, which indicates the interacting role of SN with PEO system. The dark islands shown in the micrographs (Fig. 4c, d) are possibly the regions where PEO could not find sufficient amount of SN for interaction. The spots are less in number and smaller in sizes when the SN content is more (Fig. 4d).
Ionic conductivity
The variation in room-temperature (∼26 °C) ionic conductivity of the polymer electrolyte with respect to the content of SN is depicted in Fig. 5. A gradual increase in conductivity is observed when SN is added to the PEO20·Mg(Tf)2 complex and get almost saturated beyond ∼20 wt.% of SN. An optimum conductivity of ∼6 × 10-4 S cm−1 has been achieved on the addition of ∼50 wt.% SN. On further addition of the SN beyond 50 wt.%, the electrolyte loses its dimensional stability, showing a glue-like nature. The initial increase in the ionic conductivity (by more than one order of magnitude) is associated with the ionic conduction in the structurally modified polymeric network of PEO due to the addition of the plastic-crystalline SN molecules. The following important points can be considered responsible for the enhancement in the ionic conductivity:
-
1.
The Mg salt gets slightly dissociated, when added to the PEO network of dielectric constant of ∼5 to form PEO-Mg salt complexes. The dissociated Mg2+ cations have tendency to coordinate with ether oxygen of PEO via Lewis acid–base interactions [22]. Such interactions/coordination are strong enough, leading to almost no contribution of Mg2+ ions in total ionic conductivity. As a result, most of the PEO-Mg salt complexes are reported to be anionic conductors [22, 25]
-
2.
The Gutmann donor number of the nitrile group of SN is lower (∼15) as compared with that for PEO (∼22) and hence the nitrile group of SN has also a tendency to coordinate with ether oxygen of PEO [30, 42]. The interaction of the nitrile group of SN with ether oxygen of PEO has also been evidenced from FTIR studies, as mentioned earlier [38–40]. Thus, the formation of some PEO–SN complexes is also possible in the present polymer electrolyte system PEO20·Mg(Tf)2/SN.
-
3.
In the new environment of PEO/SN network of high dielectric constant (the dielectric constant of SN being 55 [35, 42]), a further charge separation of the Mg salts would occur. As a result, Mg2+ cations (along with the Tf− anions) would become freer for conduction. Thus, the PEO/SN/Mg(Tf)2 polymer electrolytes become Mg2+ ion conducting.
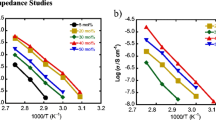
The temperature dependence of ionic conductivity of the PEO20·Mg(Tf)2 complexes added with different amounts of SN for the temperature range from 28 to 100 °C is presented in Fig. 6a. The “σ vs. 1/T” plot for the polymer electrolyte with 10 wt.% of SN depicts a typical behavior of PEO-based electrolytes. The material indicates steep increase in conductivity around melting temperature T m (67 °C) up to the temperature 70 °C, beyond that σ vs. 1/T plot shows a curved nature (Fig. 6a (a)). The curved nature of conductivity variations are observed for the larger range of temperature (from 28 to 100 °C) when a substantial amount of SN (30–50 wt.%) is added to PEO-Mg(Tf)2 system (Fig. 6a (b, c)). The curved variation of σ vs. 1/T plot indicates the non-Arrhenius Vogel–Tamman–Fulcher (VTF) behavior, expressed as:
where the parameter B is associated with the rate at which viscosity changes with temperature, A is a pre-exponential factor, i.e., the conductivity at infinitely high temperature and T 0 is the equilibrium glass transition temperature close to the T g value. The ln (σT 1/2) vs. 1/(T − T 0) plot for each polymer electrolyte has been drawn and found to be linear (Fig. 6b). These linear variations are fitted using nonlinear least square fitting to evaluate the parameters A, B, and T 0. These parameters are listed in Table 2. The polymer electrolytes with 30–50 wt.% of SN exhibit ionic conductivity more than 5 × 10-4 S cm−1 at room temperature and ∼5 × 10−3 S cm−1 at ∼100 °C, showing a good substitute of liquid electrolytes for the application in Mg batteries for a wider temperature span.
Electrochemical properties
The electrochemical stability window (i.e., working voltage range) for a typical composition of the polymer electrolyte film with 50 wt.% SN has been evaluated using LSV. The LSV response was recorded on the cell: SS|polymeric electrolyte|Mg using SS as a working electrode and Mg as both the counter and reference electrodes. It can be seen from Fig. 7 that the film has been electrochemically stable up to ∼4.1 V [43] which is a sufficient range for electrochemical applications, particularly for Mg batteries.
To confirm the Mg-ion conduction in the polymer electrolyte system, the EIS, CV, and dc polarization studies have been performed on symmetrical cells: SS|electrolyte film|SS (cell-I) and Mg|electrolyte film|Mg (cell-II) with a typical composition of the polymer electrolyte PEO20·Mg(Tf)2 + 50 wt.% SN. The polymeric film was in contact with a SS in cell-I as a blocking electrode, whereas the pellets of Mg powder were used as reversible (nonblocking) electrodes in cell-II. The comparative EIS plots for cell-I and cell-II, recorded at room temperature are depicted in Fig. 8(a). The impedance response of cell-I shows a steep rise of imaginary impedance (Z″) with respect to real impedance (Z′) in the lower frequency range, indicating the ion blocking nature of SS electrodes. A well-defined semicircular curve is observed for cell-II, indicating the reversibility of Mg/Mg2+couple at the electrolyte–electrode interfaces. This confirms the Mg2+ ion conduction in the polymer electrolyte film.
The conduction of Mg2+ ions in the polymer electrolyte film is further confirmed from CV measurements. Figure 8b shows the CV curves for cell-I (with SS electrodes) and cell-II (with Mg electrodes) at the scan rate of 5 mV s−1. The cathodic and anodic current peaks are distinctly observed for cell-II, whereas no such features are present in the case of cell-I in the same potential range. These peaks correspond to equilibrium attained by Mg2+ ions at the interfaces following the reversible reaction:
that is, the cathodic deposition and anodic oxidation of Mg take place at the electrode–electrolyte interfaces.
In order to further confirm the Mg2+ ion conduction in the polymer electrolyte film, the polarization experiments have been performed on the cells: cell-I (SS/SS) and cell-II (Mg/Mg). The polarization (current vs. time) curves for the cells are shown in Fig. 9. For the cell-I (with SS electrodes), the polarization current decreases rapidly, and it approaches to a value close to zero. The ionic transport number (t ion), has been evaluated to be >0.99 [44]. The value of t ion and the variation of polarization curve indicate the predominant ionic conduction in the electrolyte. For the cell-II (with the reversible Mg electrodes), the current steeply decreases initially and a steady-state value is reached after a sufficient time of polarization. The steady state is obtained due to constant supply of Mg2+ ions from positive electrode and their dissolution in the negative Mg electrode. This further confirms the conduction of Mg2+ ions through the polymer electrolyte film.
Conclusions
A PEO-based Mg2+-ion-conducting polymer electrolyte complexed with Mg triflate containing a nonionic plastic-crystalline solid-phase plasticizer SN has been prepared and characterized. The semi-crystalline nature of the electrolyte materials with predominant amorphous character is evidenced from XRD, optical microscopy and DSC studies. The free-standing film of PEO complex with ∼50 wt.% of SN offers optimum ionic conductivity of ∼6 × 10−4 S cm−1 at room temperature with thermal stability of up to 100 °C. The electrochemical stability window, estimated from LSV, is found to be ∼4.1 V, which is sufficient for many electrochemical applications. The Mg2+-ion conduction is evidenced from CV, impedance, and dc polarization studies. The structurally modified environment of PEO/SN (with high dielectric constant) is responsible for further dissociation of the Mg salt and hence for the enhanced ionic conductivity including Mg2+-ion conduction. The Mg-ion-conducting polymeric systems are useful from the application point of view as electrolytes/separators in Mg batteries and other ionic devices.
References
Scrosati B (2011) History of lithium batteries. J Solid State Electrochem 15:1623–1630
Minami T, Tatsumisago M, Iwakura C, Wakihara M, Kohjiya S, Tanaka I (eds) (2005) Solid state ionics for batteries. Springer, Tokyo
Scrosati B, Garche J (2010) Lithium batteries: status, prospects and future. J Power Sources 195:2419–2430
Yada C, Iriyama Y, Abe T, Kikuchi K, Ogumi Z (2009) A novel all-solid-state thin-film-type lithium-ion battery with in situ prepared positive and negative electrode materials. Electrochem Commun 11:413–416
Ding N, Feng X, Liu S, Xu J, Fang X, Lieberwirth I, Chen C (2009) High capacity and excellent cyclability of vanadium(IV) oxide in lithium battery applications. Electrochem Commun 11:538–541
Zeng RH, Li XP, Qiu YC, Li WS, Yi J, Lu DS, Tan CL, Xu MQ (2010) Synthesis and properties of a lithium-organic coordination compound as lithium-inserted material for lithium ion batteries. Electrochem Commun 12:1253–1256
Sakuda A, Kitaura H, Hayashi A, Tadanaga K, Tatsumisago M (2009) All-solid-state lithium secondary batteries with oxide-coated LiCoO2 electrode and Li2S–P2S5 electrolyte. J Power Sources 189:527–530
Liu XH, Saito T, Doi T, Okada S, Yamaki JI (2009) Electrochemical properties of rechargeable aqueous lithium ion batteries with an olivine-type cathode and a Nasicon-type anode. J Power Sources 189:706–710
Lee JY, Lee YM, Bhattacharya B, Nho Y-C, Park J-K (2010) Solid polymer electrolytes based on crosslinkable polyoctahedral silsesquioxanes (POSS) for room temperature lithium polymer batteries. J Solid State Electrochem 14:1445–1449
Hassoun J, Fernicola A, Navarra MA, Panero S, Scrosati B (2010) An advanced lithium-ion battery based on a nanostructured Sn–C anode and an electrochemically stable LiTFSi-Py24TFSI ionic liquid electrolyte. J Power Sources 195:574–579
Li J, Daniel C, Wood D (2011) Materials processing for lithium-ion batteries. J Power Sources 196:2452–2460
Appetecchi GB, Kim GT, Montanino M, Alessandrini F, Passerini S (2011) Room temperature lithium polymer batteries based on ionic liquids. J Power Sources 196:6703–6709
Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-Gonzalez J, Rojo T (2012) Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ Sci 5:5884–5901
Kumar D, Suleman M, Hashmi SA (2011) Studies on poly(vinylidene fluoride-co-hexafluoropropylene) based gel electrolyte nanocomposite for sodium–sulfur batteries. Solid State Ionics 202:45–53
Hiralal P, Imaizumi S, Unalen HE, Matsumoto H, Minagawa M, Rouvala M, Tanioka A, Amaratunga GAJ (2010) Nanomaterial-enhanced all-solid flexible zinc–carbon batteries. ACS Nano 4:2730–2734
Sellam, Hashmi SA (2012) Enhanced zinc ion transport in gel polymer electrolyte: effect of nano-sized ZnO dispersion. J Solid State Electrochem 16:3105–3114
Yoshimoto N, Yakushiji S, Ishikawa M, Morita M (2003) Rechargeable magnesium batteries with polymeric gel electrolytes containing magnesium salts. Electrochim Acta 48:2317–2322
Aurbach D, Gofer Y, Lu Z, Schechter A, Chusid O, Gizbar H, Cohen Y, Ashkenazi V, Moshkovich M, Turgeman R, Levi E (2001) A short review on the comparison between Li battery systems and rechargeable magnesium battery technology. J Power Sources 97–98:28–32
Pandey GP, Agrawal RC, Hashmi SA (2009) Magnesium ion-conducting gel polymer electrolytes dispersed with nanosized magnesium oxide. J Power Sources 190:563–572
Novak P, Imhof R, Haas O (1999) Magnesium insertion electrodes for rechargeable nonaqueous batteries—a competitive alternative to lithium? Electrochim Acta 45:351–367
Agrawal RC, Pandey GP (2008) Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview. J Phys D Appl Phys 41:223001
Gray FM (1991) Solid polymer electrolytes—fundamentals and technological applications. VCH, New York
MacCallum JR, Vincent CA (eds) (1987) Polymer electrolyte reviews-I. Elsevier, London
Fergus JW (2010) Ceramic and polymeric solid electrolytes for lithium-ion batteries. J Power Sources 195:4554–4569
Vincent CA (1995) Ion transport in polymer electrolytes. Electrochim Acta 40:2035–2040
Kumar GG, Munichandraiah N (2000) Effect of plasticizers on magnesium-poly(ethyleneoxide) polymer electrolyte. J Electroanal Chem 495:42–50
Kumar Y, Hashmi SA, Pandey GP (2011) Ionic liquid mediated magnesium ion conduction in poly(ethylene oxide) based polymer electrolyte. Electrochim Acta 56:3864–3873
Yoshizawa M, Ohno H (2005) Zwitterionic Liquid/Polymer Gels. In: Ohno H (ed) Electrochemical aspects of ionic liquids. Wiley, New Jersey, pp 331–336
Patel M, Chandrappa KG, Bhattacharyya AJ (2010) Increasing ionic conductivity of polymer–sodium salt complex by addition of a non-ionic plastic crystal. Solid State Ionics 181:844–848
Yue R, Niu Y, Wang Z, Douglas JF, Zhu X, Chen E (2009) Suppression of crystallization in a plastic crystal electrolyte (SN/LiClO4) by a polymeric additive (polyethylene oxide) for battery applications. Polymer 50:1288–1296
Fan LZ, Maier J (2006) Composite effects in poly(ethylene oxide)–succinonitrile based all-solid electrolytes. Electrochem Commun 8:1753–1756
Gupta RK, Kim HM, Rhee HW (2011) Poly(ethylene oxide): succinonitrile—a polymeric matrix for fast-ion conducting redox-couple solid electrolytes. J Phys D Appl Phys 44:205106
Patel M, Bhattacharya AJ (2008) Plastic-polymer composite electrolytes: novel soft matter electrolyte for rechargeable lithium batteries. Electrochem Commun 10:1912–1915
Patel M, Chandrappa KG, Bhattacharyya AJ (2008) Increasing ionic conductivity and mechanical strength of a plastic electrolyte by inclusion of a polymer. Electrochim Acta 54:209–215
MacFarlane DR (2001) Plastic crystal electrolyte materials: new perspectives on solid state ionics. M Adv Mater 13:957–966
Timmermans E (1961) Plastic crystals: a historical review. J Phys Chem Solids 18:1–8
Fan LZ, Wang XL, Long F (2009) All-solid-state polymer electrolyte with plastic crystal materials for rechargeable lithium-ion battery. J Power Sources 189:775–778
Echeverri M, Kim N, Kyu T (2012) Ionic conductivity in relation to ternary phase diagram of poly(ethylene oxide), succinonitrile, and lithium bis(trifluoromethane)sulfonamide blends. Macromolecules 45:6068–6077
Mosier-Boss PA (2005) Spectroscopic studies of the interaction between crown ethers and organic nitriles. Spectrochim Acta A 61:527–534
Gupta RK, Rhee H-W (2012) Effect of succinonitrile on electrical, structural, optical, and thermal properties of [poly(ethylene oxide)-succinonitrile]/LiI–I2 redox-couple solid polymer electrolyte. Electrochim Acta 76:159–164
Wu X-L, Xin S, Seo H-H, Kim J, Guo Y-G, Lee J-S (2011) Enhanced Li+ conductivity in PEO-LiBOB polymer electrolytes by using succinonitrile as a plasticizer. Solid State Ionics 186:1–6
Alarco PJ, Lebdeh YA, Abouimrane A, Armand M (2004) The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat Mater 3:476–481
Xu K, Ding MS, Jow TR (2001) A better quantification of electrochemical stability limits for electrolytes in double layer capacitors. Electrochim Acta 46:1823–1827
Hashmi SA, Chandra S (1995) Experimental investigations on a sodium-ion-conducting polymer electrolyte based on poly(ethylene oxide) complexed with NaPF6. J Mater Sci Eng B 34:18–26
Acknowledgments
The authors thankfully acknowledge the financial support received from the University of Delhi (under the scheme to strengthen R&D Doctoral Research Program by providing funds to University Faculty, 11–17 Research Fund).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, J., Hashmi, S.A. Magnesium ion transport in poly(ethylene oxide)-based polymer electrolyte containing plastic-crystalline succinonitrile. J Solid State Electrochem 17, 2283–2291 (2013). https://doi.org/10.1007/s10008-013-2104-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2104-5