Abstract
A simple, fast, and effective method of fabricating electrochemical sensors using composite pencil graphite (CPG) lead modified with carboxylic multiwalled carbon nanotube (c-MWCNT) via electrophoretic deposition (EPD) has been developed. The EPD of c-MWCNT film on the CPG electrode (CPGE) was carried out at a constant applied potential of 25 V and deposition time of 1 min. The electrochemical performances of the modified CPGE, i.e., c-MWCNT/CPGE, in Fe(CN) 4−/3−6 has been studied by cyclic voltammetry and electrochemical impedance spectroscopy (EIS). The c-MWCNT/CPGE has better current density, onset potentials, and charge transfer resistances than the CPGE. The c-MWCNT/CPGE has been successfully used to analyze hyperin in dry fruits of Acanthopanax sessiliflorus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ever since its introduction, carbon nanotubes (CNTs) has been utilized in various applications in modern analytical sciences [1, 2]. This is largely due to their unique properties, e.g., field emission, electronic, mechanical, and chemical which are useful in the fabrication of many analytical probes [3–5]. One of the most significant applications is in the fabrication of nanosize electrochemical sensors [6–9]. Most of these nanosensors are prepared via coating of thin films of CNTs homogeneously dispersed on the substrate electrodes. These electrodes have the characteristic of the coating material, i.e., CNTs used such as excellent electrocatalytic effects, increase in electrode surface area, and antifouling capability. They have also shown good reproducibility and stability in many solvents and high sensitivity toward many inorganic and organic compounds [10–15].
The CNTs surface modification of substrate electrodes generally involves at least two preliminary steps viz. pretreatment and solute dispersion of CNTs prior to coating on the electrodes [16–25]. But this procedure is difficult to obtain uniform CNT films as well as thicknesses. Thus, the direct current electrophoresis method is proposed in this study to improve these drawbacks.
Electrophoretic depositions (EPDs) of CNTs onto various conductive substrates [26–32] have been used for different applications. However, there is no report as yet that these devices are being used as electrochemical sensors.
Hyperin or quercetin 3-galactoside is a flavonoid compound obtainable from plants. It is useful as antioxidants, anti-inflammatories, suppressing cough, antidiuretics, lowering blood pressure, and cholesterol levels [33, 34]. The Acanthopanax sessiliflorus, a plant mostly found in northeast region of China, has long been used in Chinese drug preparation to cure cardiovascular diseases and interestingly with few side effects. As A. sessiliflorus is rich in flavonoids like hyperin, rutin, and quercetin, these active compounds must have contributed to the positive medicinal effects [35]. Thus, the analysis of any of these active ingredients in A. sessiliflorus is relevant to monitor the quality of medicinal products on the shelves ready for public consumption. The determinations of hyperin using HPLC have been proposed [36, 37]. However, this method is laborious and not quite economical as samples are destroyed. Since most flavonoid compounds are electroactive, hence, hyperin should be feasible to be detected by electrochemical method.
The present study reports on the fabrication and electrochemical characterization of cMWCNT/CGPE and its possible application as sensor for hyperin.
Experimental
Materials
The CPG lead 6H, 4H, 2H, HB, 2B, 4B, 6B, and 8B were of Mars Lumograph 100 (Staedtler, Germany). The MWCNT (8–15 nm OD, 3–5 nm ID, 50-μm length, purity >95 %) was purchased from Chengdu Organic Chemicals, Chinese Academy of Sciences, China. Araldite@ epoxy resin from Ciba-Geigy, Switzerland; dimethyl sulfoxide (DMSO) and methanol from Fisher Scientific, UK; tetrahydrofuran (THF) from Merck, Germany; acetone, ethanol, acetonitrile (MeCN) and dimethylformamide (DMF) from BDH, UK; and hyperin from Sigma, USA were all of analytical grade used as delivery. Water was ultra pure (18.2 MΩ cm) from Direct-Q3 of Millipore, USA. The dry fruits of A. sessiliflorus were obtained from Liaoning Province, China.
Stock solutions of hyperin were prepared in methanol and stored at −20 °C. A small amount was taken and left to equilibrate at room temperature before analysis. The stock solution was diluted with 0.1 M phosphate buffer (pH 6.9) prior to use.
The dry fruits of A. sessiliflorus obtained from local farm in the northeast region of China were pulverized in a disintegrator and then sieved through 100 mesh (powder: 44.42 %, seed: 16.05 %, other: 39.53 %). The powder was then kept and sealed at 4 °C in brown bottles. A 10 g of the powder was soaked in 100 ml methanol for 24 h in the dark to extract hyperin. The extract obtained was then filtered and later subjected for the experiment.
Instrumentations
Electrochemical measurements were carried out on Potentiostat/Galvanostat Model 273A complete with the Power Suite program (EG&G, Princeton Applied Research, USA). A conventional three-electrode electrochemical cell comprising a platinum wire as auxiliary electrode, a carboxylic multiwalled carbon nanotube (c-MWCNTs)/CPG electrode (CPGE) as working electrode, and an Ag/AgCl (sat. NaCl) as reference electrode were used. Electrochemical impedance spectroscopy (EIS) measurements were carried out using frequency response analyser FRD 100 (EG&G, Princeton Applied Research) at frequency range of 100 kHz to 100 mHz and alternating current voltage amplitude 5 mV. The result of EIS was analysed using the ZSimpWin 3.22 software. The morphologies of the electrodes were characterized by LEO SUPRA 55VD ultra high resolution analytical field emission scanning electron microscopy (FESEM) of ZEISS, Germany.
Preparation of MWCNT suspensions for EPD
The MWCNT was purified by sonication in a 6 mol L−1 HCl for 4 h. Later, 50 mg of purified MWCNT was dispersed in a 40 ml mixture of H2SO4/HNO3 (v:v = 3:1) prior to further sonication in a water bath for 7 h at 40 °C. After cooling at ambient temperature, the MWCNT was then centrifuged and washed to neutral. This pretreatment was subsequently repeated over again until all the acids were removed. The final solid was then dried to a constant weight and characterized by Fourier transform infrared (FT-IR) spectroscopy. A 0.5 mg mL−1 dry c-MWCNT was dispersed in either DMSO/THF/DMF/MeCN/water/acetone-ethanol (v:v = 1:2) prior to 20 min sonication. The dispersed solutions were then ready for EPD treatment.
Electrode preparation
The pencil lead was obtained by completely removing the outer wooden sheath of the pencil using a sharp knife. It was then cut at 6.0-cm length before use as CPGE. The CPGE was cleaned in an ultrasonic bath first in acetone and then in water for a period of 10 min each. A tip of disposable polypropylene pipette was cut to a suitable size to fit the CPGE and then sealed with epoxy resin. The surface of CPGE disc was polished on a polishing paper until a mirrorlike surface appeared. The CPGE disc was later polished in 0.05 μm alumina slurry before rinsing thoroughly with water and, finally, sonicated in ethanol and water for 5 min each. For conventional method, the disc CPGE was first dried in a desiccator prior to modification with c-MWCNT. The experimental design of EPD is shown (Scheme 1). For each series of EPD experiments, c-MWCNT was deposited onto the CPGE disc by applying a constant voltage in the range of 20–25 V, with deposition times ranging from 1 to 3 min, and 2-cm distance between electrodes. After EPD, electrodes were dried upright in a desiccator for 24 h prior to be used.
Characterization and application
Cyclic voltammetry of 5 mmol L−1 K4[Fe(CN)6] in 1.0 mol L−1 KCl on the modified electrodes was carried out with potential range from −0.2 to 0.8 V. The EIS measurement was immediately performed after cyclic voltammetry. Amplitude of 5 mV root-mean-square sinusoidal signal was applied. The modulus and phase were plotted over a frequency range of 100 kHz to 100 mHz. The FESEM with an acceleration voltage of 15 kV was used to characterize the surface morphologies of the discs.
Results and discussion
Influence of deposition time
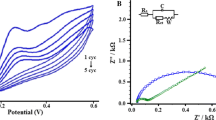
EIS is a highly effective means of probing the features of surface-modified electrodes. Thus, it has been used to characterize the fabricated electrode by utilizing 5 mmol L−1 K4[Fe(CN)6] in 1.0 mol L−1 KCl as a redox probe. Figure 1 shows that the proposed Randles equivalent circuit fits the impedance of the electrodes satisfactorily. The coating of c-MWCNT is strongly affected by the deposition times. Periods of time ranging from 10 s to 10 min have been found suitable for the deposition. Figure 2 shows that the bare 6B has a higher charge transfer resistance (R ct; 1,011 Ω cm2), while the value is much reduced when the electrode is coated with c-MWCNTs.
It is obvious that the unmodified CPGE is a pure resistor at lower frequency range before becoming a good capacitor at higher frequency range. But by depositing c-MWCNT, the CGPE has become a pure capacitor and more so as the layer thickened. With increasing thickness of the layer, the modulus decreases which correlates with decreasing of R ct and increasing in charge transfer rate. As the R ct reaches to a minimal value 5.64 Ω cm2 at 1 min, it remains stable until the duration time reaches 3 min. However, a longer period of coating causes increasing of R ct. This could be due to blocking effect of the mass transport of the thickening c-MWCNT film. Bode’s plot also shows that bare 6B CPGE has a symmetrical peak with a maximum value of 56° at 127 Hz which corresponds to the relaxation process of the 6B lead–solution interface. Modification of the electrode led to the disappearance of this relaxation with new ones observed at different maxima (69.18° at 0.67 Hz for 10 s, and 80.02° at 0.25 Hz).
Figure 3 indicates that the coating of c-MWCNT has catalytic effects on the peak currents (I p) as well as kinetic properties of the electrode. Even though anodic peak current (I pa) is gradually increased as the film becomes thicker, peak separation (ΔE p) deviates from the reasonable value and double-layer capacitance (Cdl) starts to increase at 3 min and beyond. Hence, a short duration of deposition time between 1 and 3 min is chosen in this study.
Influence of deposition voltage
The Bode’s plot (see Fig. S1) of c-MWCNT/6B electrode at various constant voltage ranging from 2.5 to 25 V shows that the electrode starts to increase relaxation from 2.5 V (65.5° at 1.08 Hz) and exhibits larger relaxation (80.77° at 0.1 Hz) at 20 V with more refined and narrower distribution peak before the value becomes stable and changed slightly at 25 V (80.79° at 0.1 Hz). It appears that, at higher voltages, a more homogeneous coating of c-MWNCT is being produced. Hence, deposition voltage of 20 to 25 V is selected for future study (see Fig. S2).
Influence of dispersing solvent
Figure 4a and b shows DMF is the most suitable solvent for dispersing of MWCNT. However, Fig. 4e and f indicates that there are cavities in the c-MWCNT film formed during the EPD process. As a result, the surface coverage of c-MWCNT prepared in DMF is less compact than that in MeCN (Fig. 4g and h). Although the solvent mixture of acetone/ethanol (v:v = 1:2) is reported [29] to form a uniform CNT film for different purposes, this study, however, fails to show its obvious advantage over the others. The influence of dispersing solvent on the R ct of c-MWCNT/6B is in the order of: acetone/ethanol (21.02 Ω cm2) > DMF (12.92 Ω cm2) > THF (10.16 Ω cm2) > H2O (7.8 Ω cm2) > DMSO (6.58 Ω cm2) > MeCN (5.64 Ω cm2).
Influence of hardness of pencil lead
The Nyquist and Bode plots of bare CPGE (from 6H to 8B) are presented in Fig. 5a and b. By comparison, the bare CPGE have exhibited a nonideal capacitive behavior over the whole frequency range and the imaginary impedances reach higher values than the c-MWCNT/CPGE (Fig. 5c and d). This can be attributed to a decrease in resistivity of the c-MWCNT/CPGE. By and large, a significant decrease in the R ct values is observed for all the c-MWCNT/CPGE compared to the bare CPGE. Also, the rather high R ct for CPGE is due to nonconformity of its surface compared to c-MWCNT/CPGE. It appears that the electron transfer process of the c-MWCNT/CPGE is much superior than the bare CPGE, CPE, and glassy carbon electrode (GCE; Fig. 6). The faster electron transfer rate of the c-MWCNT/CPGE is shown through the increase in peak current (I p). The k app values of the bare CPGE fit well with the results of EIS, indicating convenient electron transfer at the interfacial layer. The k app values of the 2H (rigid CPGE) and 6B (soft CPGE) are 5.42 × 10−7 and 7.43 × 10−7 cm s−1, respectively. The remarkable increase in k app values is observed at the c-MWCNT-modified 2H (1.8 × 10−4 cm s−1) and 6B (1.3 × 10−4 cm s−1) electrodes.
Deposition of the c-MWCNT has occurred at the anode as the zeta potential of the acid-treated CNTs is negative [38, 39]. The FT-IR result (Fig. 7) indicates that carboxylic group has been introduced to bulk MWCNT. The respective sharp peaks at 3,446, 1,712, and 1,215 cm−1 are due to OH stretching, C = O stretching and COOH stretching. The peak at 1,587 cm−1 is nonetheless attributed to C-C stretching of the bulk MWCNT. Thus, the pretreated process of CNTs not only causes the surface oxides to electrostatically stabilize the suspensions in most solvents but also reduces the length of the CNTs that breaks up the aggregates present in the commercial CNTs. The 0.5 mg mL−1 suspension was chosen in this study to produce the homogeneous film. In conclusion, the c-MWCNT/CPGE with the c-MWCNT film obtained at 20–25 V and deposition time of 1–3 min possess high-quality coating and good electrochemical activities. The FESEM images indicate the formation of the entangled CNT web like on 2 H, but a better array and orientation coating of CNTs on 6B. This is illustrated, in general, in Scheme 1a, while c-MWCNT coatings of 2H and 6B are displayed in Scheme 1b and c, respectively.
Electrochemical studies of hyperin by c-MWCNT/CGPE
The practical uses of the developed EPD-modified electrode on hyperin are studied by chronoamperometry (Fig. 8a) and cyclic voltammetry (Fig. 8b), while calibration curve and reproducibility are tested by differential pulse voltammetry (DPV; Fig. 8c). A linear regression equations of I pa = 2.1560v 1/2–7.4247 (μA, mV s−1, R = 0.9971) and I pc = −1.5898v 1/2 + 8.5759 (μA, mV s−1, R = 0.9845) are obtained between peak currents and scan rates indicating this is a diffusion controlled process and with a fast charge-transfer kinetics. Figure 8a shows a straight line plot of net current vs. t −1/2 is obtained by subtracting the background current point-by-point. This demonstrates that the transient current has been controlled by a diffusion process. Under the selected conditions of DPV parameters, a linear regression equation Fig. 8c (i) of I pa = 1.6496c + 10.063 (μA, mg L−1, R = 0.9958) is obtained with limit of detection (LOD) of 0.051 mg L−1, which implies the good sensitivity of the c-MWCNT/CGPE.
A Influence of scan rate on cyclic voltammograms of c-MWCNT/6B electrode in 10 mg l−1 hyperin with phosphate buffer (pH 6.9) solution. Inset showed the comparable linear relationship of anodic and cathodic peak current versus square root of scan rate. B Chronoamperograms of c-MWCNT/6B with the presence of hyperin over a concentration range of 0.5 to 4.0 mg l−1. The applied potential steps were 0.0 and 0.5 vs. (Ag/AgCl)/V, respectively. Inset showed the dependency of transient current on t −1/2. C Differential pulse voltammograms of hyperin in phosphate buffer (pH 6.9) with concentration (mg l−1): 0.1, 0.5, 1, 2, 4, 6, 8, 10, 12, and 14. Inset a The calibration curve, Inset b the calibration plots for the determination of hyperin in dry fruit of A. sessiliflorus
The c-MWCNT/CGPE can be regenerated by repetitive cycling in a blank solution for several times. A 10 mg L−1 hyperin solution is determined with the same electrode regenerated after every determination. The good operational stability of the fabricated modified electrode is shown through the relative standard deviation of I pa at 3.6 %. After repetitive use (over 30 times), the response of the electrode has decreased only slightly, which indicates a good storage stability of the c-MWCNT/CPGE produced through EPD method.
Electrochemical studies of hyperin in dry fruits of A. sessiliflorus
The c-MWCNT/CPGE is investigated for the determination of hyperin in dry fruits of A. sessiliflorus. A linear regression equation Fig. 8c (ii) of I pa = 1.66c + 10.357 (μA, mg L−1, R = 0.9997) is obtained when certain amount of standard quercetin solution is constantly added to the real sample. Table 1 shows that the amount of hyperin found in the A. sessiliflorus powder is 0.093 mg g−1, with recoveries ranging between 98.2 % and 103 %. This indicates a successful application of the proposed method for determination of hyperin.
Conclusion
A simple EPD procedure at a constant voltage to produce a uniform and homogeneous c-MWCNT film on CPGE has been demonstrated. This study also shows that the commercial pencil lead regardless of the hardness once modified can become relatively superior to the nonmodified pencil lead, CPE and GCE. Overall, the c-MWCNT/CPGE has effectively improved the stability, electron transfer rate, redox reversibility, and reproducibility of the bare CPGE. The c-MWCNT/CPGE is also viable to be used in analysis of hyperin and with satisfactory results.
References
Ding RG, Lu GQ, Yan ZF, Wilson MA (2001) J Nanosci Nanotechnol 1:7–29
Balasubramanian K, Burghard M (2005) Small 1:180–192
Wan J, Bi J, Du P, Zhang S (2009) Anal Biochem 386:256–261
Liu C, Hu J (2008) Electroanalysis 20:1067–1072
Baskaran D, Mays JW, Bratcher MS (2005) Polymer 46:5050–5057
Zhang M, Gorski W (2005) Anal Chem 77:3960–3965
Wang J, Musameh M (2003) Anal Chem 75:2075–2079
Xiong J, Zheng Z, Qin X, Li M, Li H, Wang X (2006) Carbon 44:701–2707
Minnikanti S, Skeath P, Peixoto N (2009) Carbon 47:884–893
Xu GR, Kim S (2006) Electroanalysis 18:1786–1792
Wang HS, Li TH, Jia WL, Xu HY (2006) Biosens Bioelectron 22:664–669
Zhang M, Liu K, Xiang L, Lin Y, Su L, Mao L (2007) Anal Chem 79:6559–6565
Profumo A, Fagnoni M, Merli D, Quartarone E, Protti S, Dondi D, Albini A (2006) Anal Chem 78:4194–4199
Tsai YC, Chen JM, Marken F (2005) Microchim Acta 150:269–276
Sohaefer DW, Brown JM, Anderson DP, Zhao J, Chokalingam K, Tomlin D, Ilavski J (2003) J Appl Crystallogr 36:553–557
Chen PY, Vittal R, Nien PC, Ho KC (2009) Biosens Bioelectron 24:3504–3509
Song Z, Huang JD, Wu BY, Shi HB, Anzai JI, Chen Q (2006) Sensors Actuators B 115:626–633
Wang YZ, Li Q, Hu SS (2005) Bioelectrochemistry 65:135–142
Salimi A, Banks CE, Compton RG (2004) Analyst 129:225–228
Erdem A, Papakonstantinou P, Murphy H (2006) Anal Chem 78:6656–6659
Deng PH, Fei JJ, Feng YL (2010) Sensors Actuators B 148:214–220
Cheng YX, Liu YJ, Huang JJ, Xian YZ, Zhang W, Zhang ZH, Jin LT (2008) Talanta 75:167–171
Zhao GY, Zhan XJ, Dou WC (2011) Anal Biochem 408:53–58
Liu JS, Zhou DZ, Liu XP, Wu KB, Wan CD (2009) Colloids Surf B 70:20–24
Li Y, Shi XW, Hao JC (2006) Carbon 44:2664–2670
Wang LL, Chen YW, Chen T, Que WX, Sun Z (2007) Mater Lett 61:1265–1269
Samant KM, Chaudhari VR, Kapoor S, Haram SK (2007) Carbon 45:2126–2139
Thomas BJC, Bocaccini AR (2005) J Am Ceram Soc 88:980–982
Du CS, Heldbrant D, Pan N (2002) Mater Lett 57:434–438
Qin YX, Hu M (2009) Appl Surf Sci 255:7618–7622
Kaya C (2008) Ceram Int 34:1843–1847
Cho J, Konopka K, Rozniatowski K, García-Lecina E, Shaffer MSP, Boccaccini AR (2009) Carbon 47:58–67
Zhang CW, Zhou YQ, Chen L (2007) Anhui Med Pharm J 11:961–963, Ch
Li MF, Li H, Wang XM (2008) Chin J Infect Tradit Chin Med 4:102–104, Ch
Wan DG, Pei J, Yan ZY (2004) J Chin Med Mater 6:397–398, Ch
Liu ZY, Tao XY, Zhang CW, Lu YH, Wei DZ (2005) Biomed Pharmacother 59:481–490
Lin L, Liu ZH, Qian F (2009) Chin Pharm Affairs 3:279–280, Ch
Sun J, Gao L, Li W (2002) Chem Mater 14:5169–5172
Esumi K, Ishigami M, Nakajima A, Sawada K, Honda H (1996) Carbon 34:279–281
Acknowledgments
This work was supported by Universiti Sains Malaysia Postgraduate Research Grant Scheme (USM-RU-PRGS) 1001/PKIMIA/843050 and Incentive Postgraduate Research Grant Scheme (PRGS) 1001/PKIMIA/821155.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Bode plots of impedance phase (a) and modulus (b) of 5.0 mmol l−1 K4 [Fe(CN)6] in 1.0 mol l−1 KCl at c-MWCNT/6B with different deposition potentials (DOC 59 kb)
Fig. S2
Influence of ΔE p and I pa values as a function of deposition potential on c-MWCNT/6B (red) and c-MWCNT/2 H (black) electrodes (DOC 175 kb)
Rights and permissions
About this article
Cite this article
Zhu, QG., Sujari, A.N.A. & Ab Ghani, S. Electrophoretic deposited MWCNT composite graphite pencils and its uses to determine hyperin. J Solid State Electrochem 16, 3179–3187 (2012). https://doi.org/10.1007/s10008-012-1749-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1749-9