Abstract
Nano-structured spinel Li2Mn4O9 powder was prepared via a combustion method with hydrated lithium acetate (LiAc·2H2O), manganese acetate (MnAc2·4H2O), and oxalic acid (C2H2O4·2H2O) as raw materials, followed by calcination of the precursor at 300 °C. The sample was characterized by X-ray diffraction, scanning electron microscope, and energy-dispersive X-ray spectroscopy techniques. Electrochemical performance of the nano-Li2Mn4O9 material was studied using cyclic voltammetry, ac impedance, and galvanostatic charge/discharge methods in 2 mol L−1 LiNO3 aqueous electrolyte. The results indicated that the nano-Li2Mn4O9 material exhibited excellent electrochemical performance in terms of specific capacity, cycle life, and charge/discharge stability, as evidenced by the charge/discharge results. For example, specific capacitance of the single Li2Mn4O9 electrode reached 407 F g−1 at the scan rates of 5 mV s−1. The capacitor, which is composed of activated carbon negative electrode and Li2Mn4O9 positive electrode, also exhibits an excellent cycling performance in potential range of 0–1.6 V and keeps over 98% of the maximum capacitance even after 4,000 cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Supercapacitor is a promising candidate for power sources of electric vehicles due to its high power density. Traditional double-layer supercapacitor is based on activated carbon (AC) or other similar materials. However, the energy density of double-layer AC/AC system is low (3–6 Wh kg−1). Today, much research in electrochemical capacitors aims to increase power and energy density as well as reduce fabrication cost while using environmental friendly materials [1–3]. One of the most useful approaches is to develop a hybrid system that typically consists of an electrochemical double-layer capacitor electrode and a battery electrode, such as AC/Ni(OH)2, AC/MnO2, AC/LiTi2(PO4)3, AC/Li4Mn5O12, and AC/LiMn2O4 systems [4–8]. Both the increase of the working voltage and the high energy density of the battery electrode material result in a significant increase in the overall energy density of the capacitors compared with that of AC/AC system.
Recently, we have demonstrated that the high cation-deficiency Li2Mn4O9 material exhibited excellent electrochemical performance in aqueous electrolytes [9]. We were interested in it because spinel Li2Mn4O9 is a very peculiar case in the Li–Mn–O system. This compound contains cation vacancies on both tetrahedral and octahedral sites [10–12]. Such a high cation deficiency could lead to high lithium intercalation capacity [11, 13–16]. It is well known that the performance of the powders used as electrode materials in supercapacitor or lithium-ion batteries is strongly affected by their preparation processes, so it is very important to select a suitable method to prepare high-performance Li2Mn4O9. Nano-structured materials, because of their unique characters, show remarkable properties in the energy storage applications. They have the advantages of large specific area, wider charge/discharge potential range, and higher specific capacitance, which attract much attention in the world as electrode material for supercapacitors in recent years [17–19].
The precursor materials reported in the literature for the solid state synthesis of Li2Mn4O9 [11, 16] are usually oxides, hydroxides, carbonates, or nitrates of manganese and lithium. We report here on the synthesis of nano-structured Li2Mn4O9 using an oxalic acid-assisted combustion method. The stoichiometry elemental analysis of Mn and O was determined by energy-dispersive X-ray spectroscopy (EDS). The electrochemical behavior of the material was demonstrated using simulative aqueous supercapacitor, in which AC was used as anode material, nano-structured Li2Mn4O9 as cathode material, and 2 mol L−1 LiNO3 aqueous solution as electrolyte.
Experimental
All chemicals were obtained from commercial sources and used without further purification. The Li2Mn4O9 was synthesized by the following procedure. Stoichiometric amount of Li(CH3COO)2·2H2O (AR), Mn(CH3COO)2·4H2O (AR), and a certain amount of oxalic acid (the molar ratio of oxalic acid to total metal ions was 1:1) were mixed together. The mixture was ground sufficiently in a ceramic mortar for about 1 h until the mixture changed to a light pink viscous complex intermediate. The mixture was dried at 75 °C under vacuum to obtain manganese oxide precursor. The precursor was then preheated at 300 °C in air for 2 h to decompose the organic components and further calcined at 300 °C for 8 h to obtain the final product. The AC was used as received (provided by Shanghai Aowei Technology Development Company Limited, China) and had a BET surface area of about 1,500 m2 g−1.
Powder X-ray diffraction (XRD) data were collected on a Rigaku D/MAX-rA diffractometer with CuK α radiation (λ = 1.5418 nm), operating at 40 kV and 100 mA. The scanning electron microscope (SEM, JSM-5900LV, Japan) was used to observe the morphology and particle size. The chemical composition of the compound was confirmed by a field emission scanning electron microscope (JSM6700F) equipped with an EDS (Oxford INCA).
The Li2Mn4O9 electrode was prepared by mixing 75 wt% active material, 20 wt% acetylene black and 5 wt% polyvinylidene fluoride (PVDF) binder together using N-methyl-2-pyrrolidine as the solvent. After drying the obtained mixture slurry overnight under vacuum at 80 °C, the mixture was pressed onto a stainless steel grid to obtain the Li2Mn4O9 electrode. The preparation of AC electrode was similar to that of Li2Mn4O9 electrode. The ratio of AC, acetylene black, and PVDF was 65:30:5 (wt.%).
Cyclic voltammetry (CV) were carried out in a three-electrode glass cell in 2 mol L−1 LiNO3 electrolyte using a LK2005 electrochemical workstation system. Platinum foil was used as a counter electrode, and saturated calomel electrode (SCE, 0.242 V) as the reference electrode. Charge/discharge test of hybrid capacitor was performed using a two-electrode glass cell consisting of Li2Mn4O9 positive electrode and activated carbon negative electrode with Neware Battery Program-control Testing System. Electrochemical impedance measurements were performed on an Autolab PGSTAT 12 potentiostat (Amsterdam, The Netherlands) using a three-electrode system in which platinum foil and SCE were used as the counter and reference electrodes.
Results and discussion
Li2Mn4O9 is a high cation-deficiency spinel material [10]. The crystal structure of spinel Li2Mn4O9 is depicted in Fig. 1. The MnO6 octahedra share edges with six octahedral-site neighbors, two of which are located in the same plane and the remaining four are above and below the plane. The LiO4 tetrahedral sites share corners with octahedral sites. In the Li2Mn4O9 spinel matrix, lithium ions are located at the tetrahedral 8a sites, manganese ions are distributed at the octahedral 16d sites, and the oxygen ions occupy the 32e sites. Both the tetrahedral and octahedral sites contain cation vacancies. Cock [10] deduced that the spinel Li2Mn4O9 possesses the structural formula of \( \left( {{\hbox{L}}{{\hbox{i}}_{{8}/{9}}}{\square_{{1}/{9}}}} \right)\left[ {{\hbox{M}}{{\hbox{n}}_{{16}/{9}}}{\square_{{2}/{9}}}} \right]{{\hbox{O}}_4} \), in which □ (white square) indicates vacancy in the tetrahedral and octahedral sites.
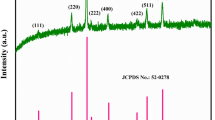
Figure 2 shows the X-ray diffraction patterns of Li2Mn4O9 powders. The calculated unit parameter of a = 8.1592 Å is very close to the previous results obtained by Kostecki et al. [20]. The crystallite size of Li2Mn4O9 calculated by the Scherrer's formula from (1 1 1) diffraction peak is about 29 nm. In addition, all the diffraction peaks can be indexed in the space group Fd3m (JCPDS No. 88–1608) of a cubic spinel, indicating that Li2Mn4O9 pure phase formed [21].
However, it is difficult to prepare fully oxidized spinel Li2Mn4O9 as is well known [12]. Strict control of experimental conditions such as temperature, time, and particle size of the precursor materials is essential for producing fully oxidized, single-phase material. By studies to date, the fully oxidized Li2Mn4O9 phase has never been prepared successfully. The value of z in Li2Mn4O8+z has generally been reported to be in the range of 0.2 to 0.5 [22, 23]. In order to determine the elemental ratio of Mn and O, EDS analysis was performed (Fig. 3). Results of EDS microprobe elemental analyses on Li2Mn4O9 gave a molar ratio of about 4:8.39 for Mn:O. That means the value of z in Li2Mn4O8+z is about 0.39, which is close to the highest value reported in the literature [22, 23].
Figure 4a and b show SEM images of Li2Mn4O9 sample prepared by combustion process with low and high magnifications, respectively. The images clearly show that Li2Mn4O9 sample is composed of many small nano grains with the average particle size of about 50 nm without agglomeration. In our previous work [24], we synthesized Li4Ti5O12 with oxalic acid as a chelating agent and a fuel and found that oxalic acid played an important role in the formation of nano Li–Ti–O material. According to our previously study, oxalic acid forms a mixed precursor, which acts as a substrate for the homogeneous distribution of the metal oxide phase. Upon calcination in air, the carbonaceous substrate is oxidized to carbon dioxide, leaving behind a finely divided oxide phase. In this case, oxalic acid had the similar effect.
Figure 5 show the cyclic voltammograms curves of Li2Mn4O9 electrodes in various potential ranges at a scan rate of 5 mV s−1 in 2 mol L−1 LiNO3 aqueous solution. It is clearly shown that no peaks of the H2 and O2 evolution are found in the widest potential range of 0 to 1.4 V, indicating that capacitive performance of the Li2Mn4O9 electrode is stable in the potential limits. When potential voltage is in 0–1.4 V (vs. SCE), the electrode exhibits the best capacitive performance. The oxidation/reduction peak-potential centered at 0.5 and 1.2 V (potential vs. SCE) suggests that the reaction of Li2Mn4O9 electrode in aqueous Li salt electrolyte is a quasi-reversible Li+ insertion/extraction process, which is in good agreement with the extraction/insertion reaction of Li2Mn4O9 in the organic electrolyte [12]. Faradic redox reaction occurred both on the surface and in the inner matrix of the Li2Mn4O9 material and this could provide high pseudocapacitance [9].
According to the cyclic voltammetry, the specific capacitance of the single Li2Mn4O9 electrode was calculated by the following equation [8]:
where Q refers to the charge integrated from the cathodic sweep, ΔV and m refer to the difference in the voltage window and weight of the single Li2Mn4O9 electrode, respectively. According to the equation, specific capacitances of the single Li2Mn4O9 electrode were calculated to be 407 F g−1 during 0–1.4 V at the scan rates of 5 mV s−1. This is a relatively high value for supercapacitor electrode materials. It is lower than that of mesoporous MnO2 (a maximum capacitance of 449 F g−1) [25] and PSC–Co3O4 electrode (a maximum capacitance of 453 F g−1) [26], but higher than most of other electrode materials. For example, a maximum specific capacitance of 344 F g−1 was obtained for the layered structure MnO2 [27]. A hydrothermally prepared nano-structured MnO2 showed a high specific capacitance of 168 F g−1 [28]. Hydrous ruthenium dioxide (RuO2·xH2O) prepared in a modified sol–gel process by Chang et al. [29] delivered a specific capacitance of 390 F g−1. A chemically deposited nanocrystalline NiO thin films showed maximum specific capacitance of 167 F g−1 [30].
According to the result obtained from the SEM, the sample synthesized by an oxalic acid combustion method had nano-structured particles. It is well known that the particle size, shape, and homogeneity of electrode materials have a definite effect on the electrochemical properties. Among them, particle size is the main factor. According to Singhal et al. [31], smaller particles have shorter diffusion distances for intercalated Li-ions, resulting in a higher charge rate for intercalated electrode materials. Since electrochemical lithium intercalation and deintercalation are in general limited by the rate of diffusion. The aforementioned features are important because smaller grain size can favor the lithium-ion mobility in the particles by reducing the ion-diffusion pathway and the active material could be utilized sufficiently.
Figure 6a–c shows the galvanostatic charge/discharge curves of the AC/Li2Mn4O9 hybrid aqueous supercapacitor in 2 mol L−1 LiNO3 between 0 and 1.6 V at various current rates from 100 to 1,000 mA g−1. The curves are almost linear and there is no obvious sudden drop of potential at the beginning of charge and discharge observed due to the ohmic resistance of the cell. Figure 7 gives the changes of specific capacitance of the AC/Li2Mn4O9 hybrid supercapacitor dependence on charge/discharge current rates. The discharge specific capacitance of the supercapacitor was calculated according to the following equation:
where I is the discharge current, ΔV is the discharge voltage, Δt is the discharge time, m is the total mass of active materials including positive and negative electrodes in the supercapacitor.
In potential range of 0–1.6 V, the specific capacitance of AC/Li2Mn4O9 asymmetric capacitor decreases from 64 F g−1 at a current rate of 100 mA g−1 to 47 F g−1 at 1,000 mA g−1. In potential range of 0–1.4 V, the capacitance decreases from 57 F g−1 at 100 mA g−1 to 45 F g−1 at 1,000 mA g−1. When current rate increases, specific capacitance of the hybrid supercapacitor decreases slowly. The results suggest that the AC/Li2Mn4O9 hybrid capacitor presents excellent rate capability in the above working windows. The AC/Li2Mn4O9 hybrid capacitor also has a good specific energy density and power density. For example, in potential range of 0–1.6 V, the specific energy is 22.8 Wh kg−1 at a power density of about 100 W kg−1 and still keeps 16.7 Wh kg−1 at a power density of 1,000 W kg−1. For comparison, the specific capacitance change of AC/AC symmetric supercapacitor in potential range of 0–1.6 V is also listed. As seen in the figure, the capacitance decreases from 26 F g−1 at 100 mA g−1 to 17 F g−1 at 1,000 mA g−1, which is much lower than the value of AC/Li2Mn4O9 hybrid capacitor.
The cycling stability of the AC/Li2Mn4O9 hybrid supercapacitor upon charge/discharge cycling was tested in 2 mol L−1 LiNO3 at a current rate of 300 mA g−1 as is illustrated in Fig. 8. The hybrid supercapacitor exhibited an excellent cycle profile and maintained over 98% of its maximum specific capacitance after 4,000 cycles between 0 and 1.6 V. The capacitor also exhibits an excellent cycling performance and keeps over 96% of the maximum capacitance after 4,000 cycles in potential range of 0–1.4 V. Cycling behavior of AC/AC symmetric supercapacitor in 2 mol L−1 LiNO3 is also shown in inset of Fig. 8. The maximum specific capacitance at 300 mA g−1 is 20.4 F g−1 and it keeps 15.9 F g−1 after 10,000 cycles. The AC/AC symmetric supercapacitor also has excellent cycling performance in LiNO3 electrolyte, but the specific capacitance is much lower than that of AC/Li2Mn4O9 hybrid capacitor. The results show that nano-Li2Mn4O9 electrode plays a key role in the hybrid supercapacitor. It provides a high specific capacitance for the capacitor and maintains excellent cycling performance at the same time.
Figure 9a shows the impedance plot of the Li2Mn4O9 electrode in 2 mol L−1 LiNO3 solution. The plot is composed of a semicircle and a straight line. The semicircle at high frequency region should be attributed to the charge transfer process at electrode/electrolyte interface, and the straight line at lower frequency region should be ascribed to the diffusion process in solid [32]. The semicircle is small, indicating that the electrochemical reaction resistance for Li2Mn4O9 electrode in 2 mol L−1 LiNO3 electrolyte is small. The Bode plots for the Li2Mn4O9 electrode is shown in Fig. 9b. As can be seen in the figure, the total impedance increased with decreasing in the frequency. In the lower frequency (0.01 Hz), the negative values of the phase angle reach −65° for the Li2Mn4O9 electrode. Both of the results indicate that the nano-Li2Mn4O9 electrode has good capacitive performance.
Conclusions
A nano-structured Li2Mn4O9 material for supercapacitor has been synthesized by an oxalic acid-assisted combustion method, and characterized by XRD, EDS analysis, and SEM observation. CV results indicate that the nano-structured Li2Mn4O9 material exhibits a high specific capacitance of 407 F g−1 at a scan rate of 5 mV s−1. Charge/discharge tests show that hybrid AC/Li2Mn4O9 supercapacitor possessed excellent rate capability and long cycle life in 2 mol L−1 LiNO3 aqueous electrolyte. The hybrid capacitor delivered a maximum discharge capacitance of 64 F g−1 at the current density of 100 mA g−1 and remained 47 F g−1 at 1,000 mA g−1, which is much higher than the value of AC/AC symmetric supercapacitor. After 4,000 cycles, it still remained above 98% of its initial capacitance. EIS impedance result demonstrated that the charge transfer resistance for the nano-Li2Mn4O9 electrode is small. The nano-Li2Mn4O9 material is a promising candidate for the power sources of electric vehicles and other large power devices owning to its low price, non-toxicity, long cycle life, excellent rate behavior, and easy preparation.
References
Conway BE, Birss V, Wojtowicz J (1997) J Power Sources 66:1–14
Conway BE (1991) J Electrochem Soc 138:1539–1548
Sarangapani S, Tilak BV, Chen CP (1996) J Electrochem Soc 143:3791–3799
Lika SM, Reisner DE, Dai J, Cepulis R (2001) Proceedings of the 11th International Seminar on Double Layer Capacitors, Florida Educational Seminars Inc.
Brousse T, Toupin M, Belanger D (2004) J Electrochem Soc 151:A614–A622
Wang YG, Luo JY, Wang CX, Xia YY (2006) J Electrochem Soc 153:A1425–A1431
Luo JY, Liu JL, He P, Xia YY (2008) Electrochim Acta 53:8128–8133
Hao YJ, Wang YY, Lai QY, Zhao Y, Chen LM, Ji XY (2009) J Solid State Electrochem 13:905–912
Hao YJ, Lai QY, Wang L, Xu XY, Chu HY (2010) Synth Met 160:669–674
de Kock A, Rossouw MH, de Picciotto LA, Thackeray MM (1990) Mater Res Bull 25:657–664
Strobel P, Ibarra Palos A, Anne M (2001) J Power Sources 97–98:381–384
Masquelier C, Tabuchi M, Ado K, Kanno R, Kobayashi Y, Maki Y, Nakamura O, Goodenough JB (1996) J Solid State Chem 123:255–266
Kilroy WP, Ferrando WA, Dallek S (2001) J Power Sources 97–98:336–343
Xia YY, Yoshio M (1996) J Power Sources 63:97–102
Wang GX, Zhong S, Bradhurst DH, Dou SX, Liu HK (1998) J Power Sources 74:198–201
Ibarra Palos A, Anne M, Strobel P (2001) J Solid State Chem 160:108–117
Arumugam D, Paruthimal Kalaignan G, Manisankar P (2009) J Solid State Electrochem 13:301–307
Wang J, Wang G, Yang L, Ng SH, Liu H (2006) J Solid State Electrochem 10:250–254
Farsi H, Gobal F (2007) J Solid State Electrochem 11:1085–1092
Kostecki R, Kong F, Matsuo Y, McLarnon F (1999) Electrochim Acta 45:225–233
Masquelier C, Tabuchi M, Ado K (1996) J Solid State Chem 123:255–266
Xia Y, Yoshio M (1997) J Electrochem Soc 144:4186–4194
Dallek S (1998) Characterization of Li2Mn4O9 Cathode Material by Thermogravimetry. In: Proceeding of the 38th Power Sources Conference, 8–11 June 1998, pp 378–381
Hao YJ, Lai QY, Lu JZ, Wang HL, Chen YD, Ji XY (2006) J Power Sources 158:1358–1364
Xue T, Xu CL, Zhao DD, Li XH, Li HL (2007) J Power Sources 164:953
Li Y, Huang K, Liu S, Yao Z, Zhuang S. J Solid State Electrochem. doi:10.1007/s10008-010-1128-3
Zolfaghari A, Ataherian F, Ghaemi M, Gholami A (2007) Electrochim Acta 52:2806–2814
Subramanian V, Zhu H, Wei B (2006) J Power Sources 159:361–364
Chang K-H, Hu C-C, Chou C-Y (2009) Electrochim Acta 54:978–983
Patil UM, Salunkhe RR, Gurav KV, Lokhande CD (2008) Appl Surf Sci 255:2603–2607
Singhal A, Skandan G, Amatucci G (2004) J Power Sources 129:38–44
Liu L, Wang W, Zou W, He B, Sun M, Wang M, Xu X (2007) J Solid State Electrochem. doi:10.1007/s10008-010-1051-7
Acknowledgement
The authors acknowledge the financial support from the National Natural Science Foundation of China (no. 20701029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hao, YJ., Wang, L. & Lai, QY. Preparation and electrochemical performance of nano-structured Li2Mn4O9 for supercapacitor. J Solid State Electrochem 15, 1901–1907 (2011). https://doi.org/10.1007/s10008-010-1206-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-010-1206-6