Abstract
Spinel Li4Mn5O12 nanoparticles have been prepared by a very simple sol–gel method. Various initial conditions were studied in order to find the optimal conditions for the synthesis of pure Li4Mn5O12. X-ray diffraction results showed that spinel Li4Mn5O12 was obtained at a low temperature of 300 °C without any miscellaneous phase. Scanning electron microscope analyses indicated that the prepared Li4Mn5O12 powders had a uniform morphology with average particle size of about 50 and 100 nm. The prepared sample was firstly used as a cathode material in an asymmetric Li4Mn5O12/AC supercapacitor in aqueous electrolyte. The capacitive properties of the hybrid supercapacitor were tested by cyclic voltammetry, electrochemical impedance spectroscopy, and galvanostatic charge–discharge tests. The results showed that Li4Mn5O12 annealed at 450 °C for 4 h exhibited the best electrochemical capacitive performance within the potential range of 0–1.4 V in 1 M Li2SO4 solution. A maximum specific capacitance of 43 F g−1 based on the total active material weight of the two electrodes was obtained for the Li4Mn5O12/AC supercapacitor at a current density of 100 mA g−1. The capacitor showed excellent cycling performance and structure stability via 1,000 cycles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, many research works have been done in electrochemical capacitors aiming to increase power and energy density as well as reduce fabrication costs while using environmental friendly materials simultaneously. Electrochemical capacitors coupled with batteries are considered promising solutions providing the power peaks for acceleration and regenerative braking and recovering the breaking energy for electric vehicle (EV). Developing a hybrid system that consists of a double-layer capacitor (EDLC) electrode and a battery electrode was an effective way. For the hybrid supercapacitor, one electrode stores charge through a reversible non-faradaic reaction of ion adsorption/desorption on the surface of AC material and the other electrode utilizes a reversible redox faradaic reaction in a lithium-ion intercalated compound. In hybrid systems, the electrolyte works in the similar way to the EDLCs. The increasing of the working voltage and the high energy density of the battery electrode material in hybrid capacitors result in a significant increase in the energy density of the capacitors compared with that of EDLCs. Some lithium-ion intercalated materials such as Li2Ti3O7, Li4Ti5O12, and LiCoO2 have been used as the electrode materials of hybrid supercapacitor in organic electrolyte [1–4] and LiMn2O4, LiNi1/3Co1/3Mn1/3O2, and LiCoO2 in aqueous solution [5–7]. These hybrid pseudocapacitors show higher energy density, long cycle life, and fast charge–discharge rate.
Among all of the lithium-ion battery materials, spinel-type electrodes are becoming the promising electrode materials in terms of the intercalation potential, cyclability, and rate capability for the development of polymer lithium-ion batteries and hybrid supercapacitors [8–11]. The [M2]O4 framework of a Li[M2]O4 spinel is an attractive host structure for lithium insertion–extraction reactions because it provides a three-dimensional network of face-sharing tetrahedra and octahedra for lithium-ion diffusion.
The spinel-type lithium manganese oxides, which were seen as potentially low cost and environmentally benign electrode materials, have been examined extensively in recent years. Among them, the spinel LiMn2O4 has become of particular interest in the past decade [12–17]. However, large capacity fade on cycling is encountered in the 3 V region due to Jahn–Teller distortion as the average valence of manganese falls below +3.5 in LiMn2O4 [14, 18]. Li4Mn5O12 is a stoichiometric spinel with cubic symmetry and cationic arrangement Li [Li0.33Mn1.67] O4. With a manganese oxidation state of +4, the Jahn–Teller distortion, which causes the capacity fading of the spinel LiMn2O4 on cycling, can be suppressed in Li4Mn5O12 [19].
Furthermore, preparation of LiMn2O4 needs high calcinations temperature (about 800 °C) and long heated time (about 12~24 h) [20]. So people called it high-temperature spinel LiMn2O4 (HT-LiMn2O4). Compared with that of LiMn2O4, Li4Mn5O12 is conventionally synthesized at lower temperature (about 450 °C) and for shorter calcination times (about 4~6 h) [21]. So people called it low-temperature spinel Li4Mn5O12 (LT-Li4Mn5O12).
In this work, LT-Li4Mn5O12 was prepared by a simple sol–gel method with citric acid as chelating agent at a very low temperature of 300 °C. The pseudocapacitive properties of Li4Mn5O12 were firstly examined in electrochemical capacitor Li4Mn5O12/AC in which Li4Mn5O12 was used as the cathode material and activated carbon (AC) was used as the anode material. The combination of Li4Mn5O12 and AC within aqueous electrolyte provides good cycle life and the use of aqueous electrolyte can overcome the drawbacks of safety hazards from the use of highly toxic and flammable organic solvents.
Experimental
Preparation of Li4Mn5O12
Li4Mn5O12 was prepared by a sol–gel process with LiAc·2H2O (AR, 99%) and MnAc2·4H2O (AR, 99%) used as starting materials. All the chemicals were provided by Chengdu KeLong Industries Co., China. Required amounts of the raw materials with the molar ratio of Li:Mn = 4:5 were dissolved in appropriate distilled water and stirred. The aqueous solution of citric acid was added to the mixed solution under stirring to obtain a crystal clear solution; the ratio of citric acid to total metal ions was 1:1. And then, ammonia solution was dropped until pH value of the admixture solution was about 8–9 to obtain a sol. The clear sol was continued to be heated and stirred until water was removed from the sol and then a viscous gel was obtained. The resulting gel was dried at 100 °C over 6 h to extract excess water and yield organic precursors. The gel precursors were preheated at 300 °C for 2 h in air and then calcined at 250, 300, 450, 600, and 700 °C for 4 h in air, respectively.
Activated carbon (AC) was provided by the Research Institute of Chemical Defense—Beijing, China.
Characterization of Li4Mn5O12
The samples were characterized by X-ray diffraction (XRD), using a D/max-rA diffractometer with Cu Kα radiation operated at 40 kV and 100 mA. Data were collected in the range 10–70° (λ = 0.15418 nm). The scanning electron microscope (SEM, Hitachi-S-450, Japan) was used to observe the morphology and size of prepared particles.
Measurement of electrochemical properties
The cathode electrode was prepared by mixing 75 wt.% of the Li4Mn5O12 with 20 wt.% acetylene black and 5 wt.% polyvinylidene fluoride (PVDF) using N-methy1–2-pyrrolidine as the solvent. After the mixture was sufficiently blended, the obtained slurry was subsequently brush-coated on to a stainless steel grid and then dried to obtain the Li4Mn5O12 electrode. Preparation of AC anode electrode was similar to that of Li4Mn5O12 electrode. The AC anode electrode contained 65 wt.% AC, 5 wt.% PVDF, and 30 wt.% acetylene black. The fabricated hybrid supercapacitors were tested in different aqueous electrolytes (1 mol l−1 Li2SO4, 1 mol l−1 (NH4)2SO4, 1 mol l−1 Na2SO4, 1 mol l−1 K2SO4) at different current densities (100–400 mA g−1) in the range of 0–1.4 V at room temperature by Neware Battery Program Control Test System.
The cyclic voltammetry was characterized by using a three-electrode cell, in which a stainless steel grid coated with Li4Mn5O12 was used as the working electrode and platinum and saturated calomel electrode (SCE, 0.242 V) as the counter and reference electrode, respectively. The measurements were performed on an LK2005 electrochemical workstation system.
The electrochemical impedance spectroscopy experiment was also carried out by using a three-electrode cell, and it was performed by using Potentiostat/Galvanostat IM6ex (ZHANER Elektrik; Germany) instrument. The frequency limits were typically set between 1000 kHz and 0.01 Hz.
Results and discussions
XRD analysis
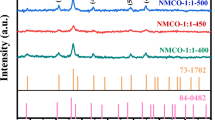
Figure 1 showed the powder X-ray diffraction patterns of Li4Mn5O12 prepared at 250, 300, 450, 600, and 700 °C for 4 h in air. The results indicated that the sample obtained at 250 °C contained MnO2 and Mn2O3 miscellaneous phase. When heating temperature was increased to above 300 °C, all the XRD patterns are in agreement with JCPDS card no. 46-0810. The cubic lattice parameter of Li4Mn5O12 at 300, 450, and 600 °C was calculated to be a = 8.1705 Å, a = 8.1677 Å, and a = 8.1799 Å. These results were very close to JCPDS card no. 46-0810 (a = 8.161 Å) and in good agreement with those obtained by C. M. Julien et al. [9] and Toshimi Takada et al. [22]. The results indicated that all samples are spinel Li4Mn5O12 single phase.
As can be seen in Fig. 1, the diffraction peaks of Li4Mn5O12 gradually sharpened with the increasing of temperature, which indicates an increase of crystallinity as may occur from the growth of grain size and ordering of local structure. All the diffraction peaks can be indexed based on a face-centered cubic spinel structure with Fd3m space group, where lithium ions are located at the tetrahedral 8a sites, and tetravalent manganese ions and lithium ions are randomly distributed at octahedral 16d sites by the ratio of Li/Mn = 1:5, while oxygen ions are located at the 32e sites. Thus, Li4Mn5O12 can be described as [Li]8a[Li1/3Mn5/3]16d[O4]32e [23].
SEM analysis
The SEM images of Li4Mn5O12 powders prepared at 300, 450, and 600 °C for 4 h are shown in Fig. 2. As can be seen in the figure, powders heated at 300 and 450 °C for 4 h were composed of some irregular analogue globular nanoparticles. The average particle sizes were about 50 nm and 100 nm, respectively. When temperature increased to 600 °C, the average particles size of Li4Mn5O12 sample increased obviously. This indicated that the soft-chemistry method and the lower annealing temperature are favorable for forming a product with smaller particle size, which are favorable for improving pseudocapacitance properties of Li4Mn5O12..
Electrochemical performance
Electrochemical performance of Li4Mn5O12 prepared at different temperatures
Figure 3 shows the 20th cycle discharge curve of Li4Mn5O12 1 M Li2SO4/AC hybrid aqueous pseudocapacitor at a current rate of 100 mA g−1 between 0 and 1.4 V. Li4Mn5O12 sample was prepared at different annealing temperatures of 300, 450, and 600 °C for 4 h in air, respectively. As shown, a good linear variation of potential versus time was observed for both curves, which is a typical characteristic of an ideal supercapacitor. Based on the discharge curves, the discharge specific capacity (C s) of the hybrid pseudocapacitors were calculated as the following formula:
Where i is the current of charge–discharge, Δt is the time of discharge, ΔV is the discharge voltage difference, and m is the mass of active materials based on the total weight of positive electrode and negative electrode within the capacitor.
According to the above equation, specific capacitances of hybrid capacitor based on Li4Mn5O12 material calcined at 300, 450, and 600 °C were calculated to be 40, 43, and 42 F g−1, respectively. Cycling performance of the hybrid supercapacitor with Li4Mn5O12 materials calcined at 300, 450, and 600 °C are shown in Fig. 4. As can be seen in Fig. 4a and c, the hybrid supercapacitors exhibited poor cycling life. After 200 cycles, the specific capacitance of the two capacitors decreased to 30 and 35 F g−1, respectively. While in Fig. 4b, the hybrid pseudocapacitor showed a good cycle performance with the specific capacitance remained 39 F g−1 after 200 cycles. The above results indicated that the hybrid supercapacitor fabricated with spinel Li4Mn5O12 heated at 450 °C showed the best electrochemical properties in 1 M Li2SO4 aqueous electrolyte.
According to the results obtained from the SEM and XRD, the sample synthesized at 450 °C had the smaller particles and relatively high crystallinity; it also had the better electrochemical properties. For Li4Mn5O12/1 M Li2SO4/AC hybrid supercapacitor, the AC electrode stores charge through a reversible non-faradaic reaction of ion adsorption/desorption on the surface of the material, and the lithium-ion intercalated Li4Mn5O12 electrode material utilizes a reversible redox faradaic reaction. So in hybrid systems, the particle size and morphology of lithium-ion intercalated Li4Mn5O12 materials have a definite effect on the electrochemical properties of supercapacitors. According to A. Singhal et al. [24], smaller particles have shorter diffusion distances for intercalated Li-ion materials. C. M. Julien et al. [9] reported that the decrease in the particle size of Li4/3Ti5/3O4 spinels is unequivocally correlated with kinetics of grain formation using the proposed HEBM synthesis, which favors the tendency of small grains, since electrochemical lithium intercalation and deintercalation are in general limited by the rate of diffusion. The aforementioned features are important since smaller grain size can favor the lithium-ion mobility in the particles by reducing the ion-diffusion pathway. In our case, the Li4Mn5O12 material had the similar characteristics. So samples that had smaller particle size had the better performance. For this reason, in all latter studies, Li4Mn5O12 samples annealed at 450 °C for 4 h was chosen for the cathode of Li4Mn5O12/AC hybrid pseudocapacitor.
Cycling stability of the Li4Mn5O12/AC hybrid aqueous pseudocapacitors was examined by means of charge–discharge tests in a potential range of 0–1.4 V at a current rate of 100 mA g−1. As indicated in Fig. 5, Li4Mn5O12/AC pseudocapacitor exhibits good cycling performance with the capacity fading rate of only 0.0078 F g−1 per cycle after 1,000 cycles.
The structure of Li4Mn5O12 prepared at 450 °C before and after 50th cycle is analyzed by XRD. The typical XRD patterns of Li4Mn5O12 before charge–discharge cycles and after 50th cycle are given in Fig. 6. It can clearly be seen that the sample after 50th cycle still remained as single-phase spinel Li4Mn5O12, indicating that the lattice structure of Li4Mn5O12 prepared at 450 °C was stable when Li-ions are rapidly inserted and extracted.
Effect of different electrolytes
The influence of various aqueous solutions on the capacitive behavior of supercapacitor was investigated in order to find the most appropriate electrolyte. Figure 7 shows the typical discharge curves of Li4Mn5O12/AC measured in 1 M Li2SO4, 1 M (NH4)2SO4, 1 M Na2SO4, and 1 M K2SO4 solutions between 0 and 1.4 V at a scan rate of 100 mA g−1.
The results indicated that the Li4Mn5O12/AC capacitor exhibited the best electrochemical performance in 1 M Li2SO4 solution than in other aqueous electrolytes, which indicates that the species and size of the cation such as Li+, Na+, NH4 +, and K+ in the systems had definite influence on the electrochemical performance of supercapacitor. The data also showed that the charge storage mechanism of the Li4Mn5O12 cathode material in the potential range of 0–1.4 V in aqueous electrolyte is associated with the insertion/deinsertion of Li-ion, which is similar to the charge storage mechanism of Li4Mn5O12 material in organic electrolyte.
The structure of the spinel Li4Mn5O12 could easily accommodate to insert Li+ ions. During discharge process, Li-ions first intercalate into 16c sites in Li4Mn5O12 structure, and then into the tetrahedral 8a sites, which also migrate to 16c sites. Eventually, the 16c sites are occupied by Li-ions. As demonstrated above, there is the mechanism proposed for the charge–discharge storage in Li4Mn5O12 electrode, which is based on the concept of intercalation of Li+ ions [25]. It can be described as follows:
During the charge process, Li-ions in spinel Li4 + x Mn5O12 material were easily deinserted to form Li4Mn5O12, leading to many Li-ion vacant sites produced in the electrode material. During the discharge process, Li-ions in Li2SO4 solution could be easily reinserted into the vacant sites, thus obtaining higher discharge specific capacitance.
When using the K2SO4-, Na2SO4-, and (NH4)2SO4-based electrolytes, the charge process was similar to that in Li2SO4 solution. Li-ions in spinel Li4 + x Mn5O12 material were easily deinserted to form Li4Mn5O12, leading to Li-ion vacant sites produced in the electrode material. But the discharge process was different. During the discharge process, M+ (K+, Na+, NH4 +) in electrolyte solution was difficult to reinsert into the vacant sites because of their larger sizes. Even if a small amount of cations such as K+, Na+, and NH4 + were inserted into the Li4Mn5O12, the stability of the lattice structure would have seriously decreased. Therefore, the capacitive behavior of Li4Mn5O12/AC capacitor was worse in Na2SO4, K2SO4, and (NH4)2SO4 solution. In the next experiments, Li2SO4 solution was chosen as the electrolyte of the Li4Mn5O12/AC supercapacitor.
Effect of working voltage
It is well known that working voltage is one of the predominant factors influencing the energy density of the hybrid pseudocapacitors. To guarantee the electrolyte not to decompose during charge/discharge process in aqueous system, it is important to control a safe potential window without O2 and H2 evolution on the surface of the electrodes. The CV curves of Li4Mn5O12 and AC in different voltage range in 1 M Li2SO4 electrolyte solution at scan rate of 5 mV s−1 are shown in Figs. 8 and 9, respectively. A sharp peak in Fig. 8 showed that O2 evolution occurred at above 1.4 V (vs. SCE), which is higher than the potential of O2 evolution (1.23 V vs. NHE). So the safe potential window for Li4Mn5O12 material is controlled between 0 and 1.4 V in 1 mol l−1 Li2SO4.
From the CV curves of Li4Mn5O12 in the potential range of 0–1.4 V, a pair of broad redox peaks located at about 0.5 and 1.2 V (vs. SCE) are observed. When changing the potential scanning direction, the current direction does not turn around instantly, indicating that the insertion/extraction of Li-ions occurs not only on the surface of the Li4Mn5O12 powders but also into the inner lattice of the material. The diffusion rate of Li-ions is much lower in the inner lattice of the material than on the surface of the material, so it leads to slower current response.
Figure 9 shows the CV curves of the AC electrode in various potential ranges in 1 mol l−1 Li2SO4 aqueous electrolyte. As shown in the figure, the AC electrode exhibits a rectangular-shaped curve in the potential range of −0.4–1.0 V (vs. SCE) in 1 mol l−1 Li2SO4 aqueous electrolyte, which is the typical characteristic of double-layer capacitance. As clearly shown in Fig. 9, H2 evolution occurred at below −0.8 V vs. SCE. From all the above results, the conclusion can be drawn that no oxygen evolution occurs at Li4Mn5O12 cathode and no hydrogen evolution occurs at AC anode in the voltage range of 0–1.4 V. So the charge/discharge voltage of the Li4Mn5O12/AC hybrid pseudocapacitors was controlled between 0 and 1.4 V in 1 M Li2SO4 electrolyte.
Effect of scan rate
In order to get more information on the electrochemical behavior of the Li4Mn5O12 cathode material, CV characterizations were done at different scan rates of 1, 3, 5, 10, and 20 mV s−1 and the results are shown in Fig. 10. From the results of CV, two pairs of redox peaks located at 0.64 and 1.2 V vs. SCE were observed for Li4Mn5O12 at a scan rate of 1 mV s−1, which agrees with the insertion/extraction reaction of Li4Mn5O12 in the organic electrolyte [22], suggesting that Li-ions could diffuse into the inner lattice of the material.
From the CV recorded at various scan rates, SC values of the single Li4Mn5O12 electrode are calculated by using the following equation [26]:
where Q refers to the charge integrated from the cathodic sweep and ΔV and m refers to the difference in the voltage window (here 1.4 V) and weight of the single Li4Mn5O12 electrode, respectively. The SC values calculated from the CV were 291, 247, and 97 F g−1 at scan rates of 1, 5, and 10 mV s−1, respectively. The specific capacitance decreased with the increase of the scan rate. The fact can be explained that, when the scan rate increases, the participation of the Li4Mn5O12 electrode is limited almost to the outer surface and difficult to the inner lattice of Li4Mn5O12 matrix, leading to a lower specific capacitance with increasing of the scan rate. The active material is utilized insufficiently and faradaic redox reactions occurred almost on the surface of the electrode.
The effect of charge/discharge current densities
The rate capability of Li4Mn5O12/AC hybrid pseudocapacitor was also examined at various current rates from 100 to 400 mA g−1. Figures 11 and 12 show the charge/discharge curves and cycling stability of Li4Mn5O12/1 M Li2SO4/AC hybrid aqueous pseudocapacitors at different current densities. Apparently, the Li4Mn5O12/1 M Li2SO4/AC hybrid pseudocapacitors had the maximum specific capacitance of 43 F g−1 and good cycling performance at the current density of 100 mA g−1. While at the current density of 400 mA g−1 the maximum specific capacitance and cycle ability were very poor. Hence, it is clear that, at a smaller current density, Li+ ions could diffuse into the inner lattice of the material and the redox reactions occurred both on the surface and in the inner lattice of material, and at high current density, the diffusion of Li+ ions was limited almost to the outer surface of electrode and the redox reactions occurred almost on the surface of the electrode, which agrees with the result obtained in cyclic voltammetry test in Fig. 10.
The first four cycles of the charge–discharge charge/discharge curves of Li4Mn5O12/1 M Li2SO4/AC hybrid pseudocapacitor at a current rate of 100 mA g−1 between 0 and 1.4 V are shown in Fig. 13. The E-t behavior as mirror-like during the charge–discharge process meant that a reversible reaction occurred in the electrode materials during the charge and discharge steps, which indicates a typical pseudocapacitance behavior of an ideal supercapacitor and in agreement with the analysis of CV measurement.
Electrochemical impedance analysis
Electrochemical impedance spectroscopy of Li4Mn5O12 before charge/discharge and after 50 cycles between 0 and 1.4 V is shown in Fig. 14. The EIS curves include two parts of a semicircle and a straight line; in low frequency of 100 kHz–0.01 Hz, the semicircle is related to the lithium-intercalation process and in the high frequency of 100–1,000 kHz, a straight line, which keeps at an angle of 70° with the x-axis, has a typical capacitive characteristic. As shown in Fig. 14, there is only a little increase in resistance after 50 cycles in comparison with that before charge/discharge. The fact supports the result of XRD analysis before and after cycling and the capacity fading less upon cycling, indicating that Li4Mn5O12 electrode has a good capacitive behavior and superior cycling performance.
Conclusion
In this study, we firstly presented hybrid aqueous pseudocapacitors in which the activated carbon (AC) was used as an anode material and a lithium-ion intercalating compound Li4Mn5O12 as a cathode material in aqueous electrolyte. The research results indicated that calcinations temperature, working voltage, current density; electrolyte species and scan rate had great effect on the electrochemical performance of Li4Mn5O12 material. The sample annealed at 450 °C for 4 h possesses remarkable electrochemical capacitive behavior and superior cycling performance. Li4Mn5O12/AC pseudocapacitor exhibited the best electrochemical performance in 1 M Li2SO4 solution than in other aqueous electrolytes such as 1 mol l−1 K2SO4, (NH4)2SO4, and Na2SO4, which indicate that the charge/discharge mechanism of Li4Mn5O12/1 M Li2SO4/AC is associated with the transfer of Li-ions between two electrodes. This hybrid cell exhibited an estimated specific capacity of 43 F g−1 based on the total weight of the active electrode materials. Li4Mn5O12/AC pseudocapacitor also exhibited good cycling performance with the capacity fading rate of 0.0078 F g−1 per cycling over 1,000 cycles at the current density of 100 mA g−1.
References
Amatucci GG, Badway F, Pasquier AD, Zheng T (2001) J Electrochem Soc 148:A930, doi:10.1149/1.1383553
Huang BH, Yang P, Zhang BH, Shi QM (2006) J Power Sources 30:560, Chinese
Chen F, Li RG, Hou M, Liu L, Wang R, Deng ZH (2005) Electrochim Acta 51:61, doi:10.1016/j.electacta.2005.03.047
Pasquier AD, Laforgue A, Simon P (2004) J Power Sources 125:95, doi:10.1016/j.jpowsour.2003.07.015
Wang YG, Xia YY (2006) J Electrochem Soc 153:A450, doi:10.1149/1.2140678
Wang YG, Lou JY, Wu W, Wang CX, Xia YY (2007) J Electrochem Soc 154:A228, doi:10.1149/1.2432056
Wang YG, Lou JY, Wang CX, Xia YY (2006) J Electrochem Soc 153:A1425, doi:10.1149/1.2203772
Scrosati B, Panero S, Reale P, Satolli D, Aihara Y (2002) J Power Sources 105:161, doi:10.1016/S0378-7753(01)00935-1
Julien CM, Massot M, Zaghib K (2004) J Power Sources 136:72, doi:10.1016/j.jpowsour.2004.05.001
Zaghib K, Armand M, Gauthier M (1998) J Electrochem Soc 145:3135, doi:10.1149/1.1838776
Zaghib K, Simoneau M, Armand M, Gauthier M (1999) J Power Sources 300:81–82, doi:10.1016/S0378-7753(99)00209-8
Tarascon JM, Wang E, Shokoohi FK (1991) J Electrochem Soc 138:2859, doi:10.1149/1.2085330
Wen SJ, Richardson TJ, Ma L (1996) J Electrochem Soc 143:L136, doi:10.1149/1.1836902
Levi E, Levi MD, Salitra G (1999) Solid State Ionics 126:109, doi:10.1016/S0167-2738(99)00219-2
Park YJ, Kim JG, Kim MK (2000) Solid State Ionics 130:203, doi:10.1016/S0167-2738(00)00551-8
Liu DQ, He ZZ, Liu XQ (2007) Mater Lett 61:4703, doi:10.1016/j.matlet.2007.03.012
Zeng RH, Li WS, Lu DS, Huang QM (2007) J Power Sources 174:592, doi:10.1016/j.jpowsour.2007.06.120
Ohzuku T, Kitagawa M, Hirayi T (1990) J Electrochem Soc 137:769, doi:10.1149/1.2086552
Thackeray MM, De Kock A, Rossouw MH (1992) J Electrochem Soc 139:363, doi:10.1149/1.2069222
Liu D-Q, He Z-Z, Liu X-Q (2007) J Alloy Comp 440:69, doi:10.1016/j.jallcom.2006.09.013
Thackeray MM, Mansuetto MF, Johnson CS (1996) J Solid State Chem 125:274, doi:10.1006/jssc.1996.0297
Takada T, Hayakawa H, Akiba E, Chakoumakos BC (1997) J Power Sources 68:613, doi:10.1016/S0378-7753(96)02570-0
Julien CM, Zaghib K (2004) Electrochim Acta 50:411, doi:10.1016/j.electacta.2004.03.052
Singhal A, Skandan G, Amatucci G (2004) J Power Sources 129:38, doi:10.1016/j.jpowsour.2003.11.010
Brett A, Deborah J, Roziere J, Burns J, Gary R (1995) Chem Mater 7:2151, doi:10.1021/cm00059a024
Hu CC, Wang CC (2002) J. Electrochem Commun 4:554, doi:10.1016/S1388-2481(02)00371-5
Acknowledgement
The authors acknowledge the financial support from the National Natural Science Foundation of China (no. 20701029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hao, YJ., Wang, YY., Lai, QY. et al. Study of capacitive properties for LT-Li4Mn5O12 in hybrid supercapacitor. J Solid State Electrochem 13, 905–912 (2009). https://doi.org/10.1007/s10008-008-0627-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-008-0627-y