Abstract
Nitroazole derivatives are nitrogen-rich heterocyclic ring molecules with potential application as energetic materials. Thirty-three of them—nitroimidazoles, nitrotriazoles, and nitropyrazoles—were investigated. Computed density functional theory molecular charge densities were partitioned employing the accurate distributed multipole analysis (DMA) method. Based on the magnitude of the DMA atom-centered electric multipoles (monopole, dipole, and quadrupole values), mathematical models were developed to compute the impact sensitivity of the explosives composed of these molecules. Charge localization and delocalization of the ring nitrogen atoms as well as charges of the atoms of the nitro group affect the sensitivity of explosives composed of nitroazole derivatives. The sensitivity is strongly dependent on the ring position of the nitrogen atoms and the bonding site of the substituent groups. The N/C ratio and the repulsion of the non-bonding electron pairs of the vicinal nitrogen atoms of the ring also play an important role in the stability of nitroazoles. The influence of the withdrawing group (NO2) and the electron injector groups (NH2 and CH3) including their bonding position on the ring could be quantified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of military explosives undergoes extreme demands. In addition to an excellent energetic performance, they must have high resistance to external stimuli such as shock and heat [1] to reduce the risks of their handling and storage.
To develop low sensitivity/high performance explosives, a possible approach is to replace the traditional formulation of compounds based primarily on carbon atoms by one based on a high nitrogen content compound [2]. Five-membered heterocyclic rich nitrogen compounds such as nitroazoles have been widely used in this role [3]. Among the various types of nitrogen-rich explosives, imidazole, pyrazole, and triazole derivatives have been largely investigated due to their low initiation sensitivity and good performance [2,3,4,5,6,7].
Nitroazoles contains a five-atom ring with at least one nitrogen heteroatom. The number of nitrogen atoms can vary from one to three, which results in different chemical families. Figure 1 shows the structure of the possible nitroazole rings.
Pyrazole and imidazole with two nitrogen atoms differ only in the position of these atoms. In pyrazoles, the nitrogen atom is vicinal (position 1, 2) and in imidazoles, they are at position 1, 3: there is a carbon atom in-between. Triazole is a heterocyclic compound that has three nitrogen atoms in the ring. This class of compounds has aroused great interest because of applications ranging from drugs to explosives [8]. The nitro and nitramine derivatives of this heterocyclic family are of particular interest for investigating the electronic interaction between ring and ligands [9].
Explosives with only carbon atoms in the structure release energy from the oxidation of their chains and from the energy stored in cyclic and cage-type compounds with strained structures. However, for heterocyclic structures such as the nitrogen-rich nitroazoles, the energetic performance is largely due to high positive heats of formation and a higher volume of gas released per gram of explosive [4]. These properties also favor oxidation at lower temperatures, which makes nitrogen-rich nitroazole materials excellent candidates for use in ammunitions [10].
Additionally, besides their high energetic densities, nitroazoles are usually insensitive to impact. This property can be quantified by measuring h50 values, which is the height from which a given mass dropped upon a sample of the compound will initiate the reaction 50% of the time; naturally, h50 depend on the mass [11,12,13,14,15].
Although measurements of sensitivity by impact have different sources of uncertainty since they are affected by several experimental parameters, it was shown before that impact sensitivity can be correlated with chemical and electronic structure of molecules [16,17,18,19,20]. For this reason, use of accurate quantum chemical methods to establish correlations between molecular properties and sensitivity values often has been successful for the same chemical families [21,22,23,24,25].
Owens, Politzer, Murray, and other researchers correlated the electrostatic potential of C–NO2 bonds to sensitivity for several explosives [11, 26,27,28,29,30]. They also concluded that the correlation between bond strengths and impact sensitivity is not limited to certain classes of explosives [31]. Mathieu observed a relationship between the sensitivity of nitro compounds and their dissociation energy [32, 33] and proposed a unique mathematical model for predicting h50 for all classes of chemical substances according to specific bonds [15, 20]. Keshavarz and collaborators have succeeded in establishing correlations between purely structural molecular parameters related to the number and type of constituent atoms with sensitivities. They established relatively simple models with good results [34,35,36,37,38].
Concerning nitroazoles, there are some relevant theoretical studies. Cho and collaborators [39] analyzed the molecular structure of the nitroimidazoles 4ni, 5ni, and 45dni. From ab initio and density functional theory (DFT/B3LYP) calculations, they identified that the nitro group bonded to the carbon atom in position 4 (C4) is eclipsed and the one bonded to the C5 atom is planar. Sorescu et al. investigating the tautomerism of ANTA at the unrestricted Hartree-Fock level (UHF) level concluded that with more accurate theoretical methods, such as Moller-Plesset MP2, MP4 and DFT/BLYP and DFT/B3LYP, the geometric parameters of the molecule were closer to X-ray diffraction data. There was only a small discrepancy concerning the torsion angles of the nitro and amino groups [40].
Su and collaborators computed the heat of formation (HOF) of some nitroazoles as well as the dissociation energies of C–NO2 bonds at employing the DFT/B3LYP//6-311+G (3df, 2p) method [41]. The same group studied the relation between the ratio of the dissociation energy and total energy (BDE/E) and the impact sensitivity (h50) [42]. Their results showed that most of the studied compounds are insensitive towards impact stimuli when their h50 are larger than 60 cm. Furthermore, the computed BDEs and HOFs consistently indicate that C-nitro-substituted imidazoles are more stable than the corresponding N-substituted ones, even when a methyl is attached on those positions.
The geometry and some structural properties of certain molecules, among them some nitropyrazoles, were studied using DFT/B3LYP//aug-cc-pVDZ [43]. They showed that the bonding position of the NH2 group on polynitropyrazoles is directly related to the sensitivity. They also concluded that the position of the nitro groups determines the stability and the sensitivity of nitroazoles [43, 44].
Similarly to Su et al., Ghule and collaborators [45] predicted the stability of nitroazole compounds by assessing the dissociation energy of the weaker C–NO2 bond. They also explored the aromaticity of the heterocycles to predict the stability of these compounds and used the charge of the nitro group to predict the sensitivity of the molecules.
The effect of substituent groups on the nitroazole ring has been studied by several groups in the last decade. Cho and collaborators showed the influence on the energetic performance of the electron withdrawal nitro group and the electron injector amino groups for the nitroimidazoles [39, 46, 47]. Moxnes et al. showed that NTO and ANTA are low-sensitivity explosives with properties comparable with TNT and RDX. Calculations indicated that the majority of the nitrated derivatives of these nitroazoles have sensitivity values similar to RDX [48].
We have been investigating families of molecules looking for correlations between molecular properties and impact sensitivities . For this purpose, the accurate distributed multipole analysis (DMA) partition method of the molecular charge density was employed to build models that lead to good results for predicting h50 for nitrobenzenic [18] and non-aromatic nitramine molecules [49]. The DMA method was also employed in the analysis of molecular charge properties of promising explosives such as 1,1-diamino-2,2-dinitroethylene - FOX-7 [19] and diazo-cyclopropane derivatives [17]. DMA was employed before to rationalize very distinct chemical phenomena such as molecular adsorption in catalysis including microwave effects [50,51,52,53]. Excited states of energetic molecules have been also investigated [54,55,56,57,58].
The extensively developed aforementioned ideas from the group of Politzer and Murray of employing the electrostatic potentials of energetic molecules bearing nitro groups to analyze sensitivity to impact are directly related to the DMA method of partition of the molecular charge density and nicely agree with the major conclusions of the present work. The reason is that the molecular electrostatic potential equals the sum of the integrated nuclear and electron (i.e., charge) densities over the volume; hence, the accuracy of both approaches is directly related to the quality of the employed quantum-chemical method.
In the present work, we apply the DMA method to partition the molecular charge density to investigate the impact sensitivity of a set of 33 nitroimidazoles, nitropyrazoles, and nitrotriazoles.
Methods
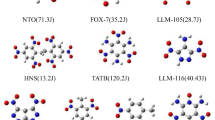
The set of 33 nitroazoles is composed of 18 nitroimidazoles, 8 nitrotriazoles, and 7 nitropyrazoles (Fig. 2) with great potential for use as energetic materials. The IUPAC names for all the molecules are collected in Table 2S of the Supplementary Material.
The conformational analysis of the molecules was initially performed by applying the Monte-Carlo and simulated annealing methods at the DFT//SVWN/DN* level employing the Spartan Pro software [59]. In this way, different molecule conformations were generated by random rotation of bonds and ring bends. The set of some of the most diverse conformations produced was used as a starting point to determine the lowest energy geometry of each molecule as described in the next paragraph.
The lowest energy geometry of each of the 33 molecules was found from optimization employing DFT [59, 60] with the B3LYP hybrid functional [61] combined with the 6-311+G(d) basis set. These calculations were performed using the Gaussian 03 program [62]. Vibrational frequency calculations were carried out for these molecules in order to ensure that imaginary frequencies were not obtained.
The molecular charge densities of the converged geometries were decomposed via the distributed multipoles analysis (DMA) method using the GDMA2 program [63].
In the DMA method, the charge distribution of a molecule is described as an expansion of electric multipoles located at the atomic sites of the molecule [64,65,66,67]. The starting point of the method is the description of the electronic density calculated from ab initio or DFT methods using Gaussian basis sets. The molecular charge density is written as a sum of products of atomic Gaussian basis functions. If the orbitals are on different atoms, then each pair of Gaussian functions produces a finite series of multipoles at a point between the two atoms determined by the Gaussian exponents involved. Combining the charge densities of electrons with the positive charge values of the nuclei, one can obtain the molecular charge density [63].
Thus, the product of two “s” functions is spherically symmetrical and corresponds to a point charge (monopole located at the atomic site). The product of an “s” function with a “p” function represents a charge and a dipole, the overlapping of two “p” functions represents charge, dipole, and quadrupole and so on.
The multipole expansion components are interpreted in a chemically intuitive way. The monopole represents charges located at the atomic sites. Dipole moments, which express charge separation in an atom (i.e., polarization), are represented by a vector that points from a negative charge to a positive one of the same magnitude. The polarization at the atomic site results from interactions between distinct atoms with different electronegativities in the environment of the molecule, since an isolated, perfectly spherical atom has no polarization. The quadrupole moments, the first to include contributions of electron density outside the plane of the molecule, are associated with delocalized π electrons, such as non-bonded electron pairs or those of unsaturated bonds, for example [50].
The monopole, as a point charge, is represented by a negative or positive numerical value depending on the charge it represents. For dipole moments, a vector property, their magnitudes are reported and vectors are drawn to represent them graphically, whereas for the quadrupole moments, a tensor, a number corresponding to the square root of the sum of all components of the squared elements is given in units of 4.486 × 10–40 C.m2 [64,65,66,67].
The DMA method converges rapidly and provides an accurate description of the charge density of a molecule because it is capable of dealing with the different electrostatic anisotropic contributions of the interactions between different atoms. It is, in a sense, a more general and much more precise population analysis than that of Mulliken; however, without presenting its deficiencies [64]. The method goes beyond the simple quantitative analysis of atom-centered charges by computing the dipole vectors, quadrupole tensors, and even higher atom-centered terms.
Finally, the DMA method can be considered a complete method to accurately describe the molecular density of charge and presents a rapid convergence and independence of the size of the basis set [18, 63].
Results and discussion
Molecular charge density of the selected nitroazoles
Figure 3 collects DMA monopole and quadrupole values of representative molecules of the set. The electric multipoles of the remaining molecules are shown in Figure 1S of the Supplementary Material.
The behavior of the nitro group is similar for all the 33 molecules. The monopole (charge) value of the nitrogen atom is always positive due to the inductive effect of the more electronegative oxygen atoms that attract the electronic density of the N atoms. The overall result is the electron-attraction character of the nitro group.
Figure 3 shows that the oxygen monopole values are always negative (the most negative of all atoms, with values in the range from −0.2 to −0.3e) due to the aforementioned attraction effect. The corresponding quadrupole values are large, around 0.9 ea02, which are comparable with the nitrobenzenic values [18]. These large values are due to the isolated electron pairs of the oxygen atoms and the characteristic electron delocalization of this group [68].
Those properties of the nitro group are also confirmed by the dipole moment vectors (Fig. 4) of the 4ni, llm-116, and NTO molecules. This large polarization of the oxygen atoms in the nitro group is also due to their isolated pairs. The comparable magnitude of the dipole vector of one of the ring nitrogen atoms of the molecules in Fig. 4 is due to the inductive effect of the closest oxygen atom of the nitro group; the same happens in the other molecules of the set not shown.
Concerning the influence of the nitro group on the ring atoms, for the nitroimidazoles increasing the number of nitro groups attached to the ring, which leads to more sensitive explosives similarly to the nitrobenzenics [18], results in a less negative nitrogen atom at the position 3 of the ring. This is a general effect: for the 2ni, 24dni, and 245tni series of molecules (Fig. 5), the nitrogen at position 3 has charge values of − 0.224 e, − 0.167 e, and − 0.160 e, respectively.
For nitropyrazoles and nitroimidazoles, the aforementioned effect can be observed in the nitrogen atoms at position 2; this is the case, for example, of the 34dnpy and 345tnpy nitropyrazoles that have charge values of − 0.151e and − 0.105e, respectively (Fig. 5). Similarly for the 3n124triaz and 35dn124triaz nitrotriazoles, their charge values are − 0.150 e and − 0.115 e, respectively.
On the other hand, the presence of an electron injector group such as NH2 increases electron delocalization of the ring atoms (i.e., increases their Q2 values). This is the case of the 45dni, 34dnpy, 35dnpy, 24dni, and 25dni molecules, which upon inclusion of a NH2 group produces the 2a45dni, 5a34dnpy, 4a35dnpy, 5a24dni, and 4a25dni molecules, respectively: the sum of the Q2 values of the ring atoms increases in the latter, as shown in Table 1S of the supplementary material.
Concerning the methyl group (CH3), its electronic induction is less effective for increasing the electronic delocalization in the ring, since this induction effect is propagated only through the bond with the nearest atom and thus is not of great magnitude in farther atoms.
In spite of the aforementioned difference of the role of the NH2 and CH3 groups, in both cases electron donation increases the total charge of the nitro group. Since the electron density increases over the nitro group, its electron attracting effect is not so effective, causing an increasing of the quadrupole value of the ring atoms (Table 1S).
The quadrupole values of the ring atoms of the nitroazoles, which lie in the range from 0.8 to 1.3 ea02, are comparable with those of the ring carbon atoms of nitrobenzenics [18]. This can be explained by the fact that nitroazoles have also aromatic rings, which possess high electronic delocalization (i.e., large Q2 values) over their atoms.
The nitrogen atom at position 1 of the ring, when bonded to a hydrogen atom or a to a methyl group, displays the largest quadrupole values among all the atoms, in the range from 1.1 to 1.4 ea02. The exception is the nitrogen atom of the amino group, which has large Q2 values of the order of 1.6 ea02, since, in this case, it is not bonded to more electronegative atoms. On the other hand, the nitrogen atoms displaying π-bonds (i.e., having sp2 hybridization) have quadrupole values in the 0.8–0.9 ea02 range. This difference is because the nitrogen atom at position 1 has sp3 hybridization, with orbital angles around 106°, whereas the π-bonded ring nitrogen atoms have a sp2 hybridization. For ring resonance to occur, a perfect alignment of the p orbitals of the nitrogen atoms with the p orbitals or the remaining ring atoms must exist. Given that the sp3 nitrogen atom at position 1 is bonded to planar sp2-hybridizated atoms, it is “forced” to change its geometry, hence becoming almost planar. Therefore, the p orbital becomes coplanar with the p orbitals of the ring atoms thus allowing the existence of resonance.
The aforementioned properties of the molecules, and the major conclusions concerning h50 prediction (discussed below) and previously found for nitrobenzenics [18], nicely agree (as expected) with the aforementioned works of Politzer and Murray on other classes of energetic molecules possessing nitro and other groups including three of the studied molecules (24dn1, 245tni, and 2-nitroimidazole). This agreement concerns especially the identification from molecular electrostatic potentials that the imbalance between charge delocalization and NO2 electron-withdrawing effects is a determinant property of the impact sensitivity of an energetic material compound [24].
Mathematical models for h 50 prediction
Given that the investigated nitroazoles are aromatic, first, we applied models initially developed for the nitrobenzenic molecules [18].
For this purpose, the molecules of this work were divided in two groups: the training group was composed of 7 molecules with known experimental h50 values (1met245tni, 24dni, 245tni, 1met35dn124triaz, llm-116, 34dnpy, and 1met25dni) and the test group was composed of the remaining 26 molecules with 4 of them (3n124triaz, ANTA, 4n123triaz, and llm-116) also having known experimental h50 values.
The first model, called model 1A, includes the contributions of the magnitude of the total charge and the number of atoms in the nitro group according to Eqs. 1 and 2:
where Σ|Q0(NO2)| and [Σn(NO2)]2 are, respectively, the sum of the DMA charge magnitudes and the square of the number of nitro groups; both terms are summed over the number of nitro groups in each molecule. The fitted constants here and for all the other models were obtained using the molecules of the training set of the present work. The unit of Г(Q, M) is e and for the fitted constants in Eq. 2 are respectively e–1 cm and cm.
The second model (model 2A) refines model 1A by including the quadrupole values (Q2) of the carbon atoms of the aromatic ring according to Eqs. 3 and 4,
where Σ|Q0(NO2)|, Σn(NO2), and Σ[Q2(x)] are, respectively, the sum of the DMA charge magnitudes, the number of nitro groups, and the sum of the DMA quadrupole moment values of the ring atoms of each molecule (x = C and N). The unit for Г(Q, M) is e2a02 and for the fitted constants in Eq. 4 are e−2a0−2 cm and cm, respectively.
The third model (model 3A), in addition to the parameters of model 2A, includes the magnitude of the dipole moment vector of the atoms of the nitro group and the average C–NO2 bond length, as shown by Eqs. 5 and 6.
where Σ|Q0(NO2)|, Σn(NO2), Σ[Q2(x)], Σ|Q1(NO2)|, and [R(C-NO2)]3 are, respectively, the sum of the DMA charge magnitudes, the number of nitro groups, the sum of the DMA quadrupole moment values of the ring atoms of each molecule (x = C or N), the sum of the magnitude of the vector dipole moments of the nitro group atoms, and the average C–NO2 bond length. The unit for Г(Q, M) is ea0Å−3 and for the fitted constants in Eq. 6 are e−1a0−1Å3 cm and cm, respectively.
Table 1 summarizes the results from the application of the models to the eleven molecules with known h50 values.
There are notable differences between nitrobenzenes and nitroazoles; the deviations are relatively large because we applied models to the latter originally designed for the former. Whereas nitrobenzenes have homocyclic six-membered rings and thus bear perfect aromaticity, nitrazoles are less aromatic given they are five-membered heterocyclic rings. Moreover, nitroazoles have a number of π electrons larger than the number of ring atoms because one of the heteroatoms (N) contributes to the resonance with a pair of electrons rather than with just one electron [69].
To improve the prediction of the impact sensitivity of nitroazoles, we developed other types of models taking into account the particularities of the nitroazole molecules, especially the presence of nitrogen heteroatoms in the ring.
In a preliminary screening, we investigated a total of 70 models divided into three categories: model 1-type is based on the total charge of the nitro group(s); model 2-type included the sum of the quadrupole values (Q2) of the ring atoms in two different ways: sum of the Q2 values of all atoms indistinctively, or the sum of the Q2 values of the carbon and nitrogen values separately. Model 3-type also includes the magnitude of the dipole moment vectors of the atoms in the nitro group.
The molecules, similarly to the nitrobenzenic molecules, were divided in two groups: a training group, composed of 7 molecules with known experimental h50 values (1met245tni, 24dni, 245tn, 1met35dn124triaz, nto, 34dnpy, and 1met25dni) and the test group, composed of the remaining 26 molecules, 4 (3n124triazol, nto, anta, 4n123triazol) of them having experimental h50 values.
From all those initial 70 models, the one that presented the best agreement with the experimental h50 values and more consistent sensitivity values for the molecules with unknown experimental values was a model 2-type according to the equations
where Σ|Q0(NO2)|, [Σn(NO2)]2, ΣQ2(Nring) Σ|Q0(Nring)| are, respectively, the sum of the charges of the nitro group, the square of the number of nitro groups, the sum of the quadrupole values of the ring nitrogen atoms, and the sum of the charges of the ring nitrogen atoms. The unit for Г(Q, M) is e3a02 and for the fitted constants in Eq. 8 are e−3a0−2 cm and cm, respectively.
According to Eq. (7), it seems that charge localization and delocalization over the ring N atoms are the key molecular properties for predicting the sensitivity of nitroazoles. As will be discussed below, the number and distribution of the nitrogen atoms in the ring structure of these compounds are also important features for the molecular stability and consequently for the sensitivity of the nitroazoles. It is known, for example, that for triazoles, the electronic repulsion between the isolated pairs of electrons from the vicinal nitrogen atoms destabilizes the ring [70].
The results obtained with the application of model 2 to the molecules having experimental h50 values are collected in Table 2.
The deviations of the h50 values of the training group used to fit the models are less than 5% with two exceptions: 1met245tni (− 22.24 cm, 45.39%) and 245tni (12.6 cm, − 18.52%). Concerning the four molecules of the test group, the largest deviations are for llm-116 (99.39 cm, − 59.54%) and especially for 4n123triaz (− 213.84, 855.38%). The latter does not display any peculiarity in the molecular structure as compared with the others and similar deviations were found in all the 70 screened models for predicting h50. Another group that develop models based on basic molecular properties to predict h50 values reported also a very large deviation (612%) for 4n123triaz [75].
The second largest deviation value was for llm-116 (99.39 cm, − 59.54%). This may be due to the fact that it is the only molecule in the test set to have an amino group adjacent to a nitro group resulting in a intramolecular hydrogen bond. This molecule thus has higher thermal stability and less impact sensitivity [76]. This effect, not found in any of the molecules composing the training group, may have produced such a deviation. Although the ANTA molecule has an amino group, the intramolecular hydrogen bond does not involve a nitro group.
In Table 3 that collects the predicted h50 values of molecules without experimental data, the nitroimidazoles have the highest predicted h50 values. In contrast, the nitrotriazoles, which have a greater number of nitrogen atoms in the ring, are the more sensitive. The nitropyrazoles also present low sensitivity because of their vicinal polarized nitrogen atoms.
Although the nitropyrazoles have two nitrogen atoms in the ring similarly to the nitroimidazoles, the former are the most sensitive molecules in Table 3. This may be related to the ease of ring opening and consequent release of N2 by the nitropyrazoles. Moyano [77] and Silva [78] showed that substituted pyrazole rings undergo the thermal decomposition presented in Fig. 6.
In the nitrazoles, the nitrogen atoms of the heterocyclic rings affect the behavior of these molecules [79]. Regarding sensitivity, three properties are the most relevant [69]. First, is the number of heteroatoms in the ring because the higher the N/C ratio, the smaller is the electron delocalization, hence the larger the sensitivity, as expected from previous studies of another type of ring explosives, the nitroaromatics and other types [11, 18, 24, 80]. Due to this small delocalization, there is a low tendency of these molecules to undergo electrophilic substitution reactions as verified experimentally [69]. For the present molecules, this fact is confirmed by the Q2ring values collected in Table 1S which shows that triazoles have the lowest total quadrupole values. The predicted h50 values of the present molecules with no experimental data (Table 3) are in agreement with these features, as can be seen in the case of 45dni (111.14 cm) and 45dn123triaz (69.14 cm), for instance, which have distinct Q2 values (45dni—Q2ring = 5.558 ea02; 45dn123triaz—Q2ring = 5.066 ea02).
The second relevant property affecting sensitivity is the position of the nitrogen atoms in the five-membered heterocyclic rings of nitroazoles. As mentioned, the repulsion of the non-bonded electron pairs is one of the factors affecting the sensitivity in nitrotriazoles. Therefore, 1,2,3 nitrotriazoles are more prone to this effect, as confirmed by the low predicted h50 value for 45dn123triaz (69.14 cm) as compared with the 1,2,4 nitrotriazoles which has a much larger calculated h50 value, e.g., 35dn124triaz has 107.43 cm. Considering that nitroimidazole molecules do not have nitrogen atoms in adjacent positions, they have accordingly large predicted h50 values—see Table 3. Moreover, in the case of triazoles, two of the three ring nitrogen atoms are the most polarized in the cycle. For the 123 triazoles, these atoms are first-neighbors, which causes a great repulsion between them, a phenomenon similar to what happens with non-binding electron pairs. In the case of the 124 triazoles, this repulsion is not observed since these well-polarized atoms are not first-neighbors. Figure 7 illustrates this fact showing the prominent vectors of the dipole moment of the 35dn124triaz and 45dn123triaz molecules.
The effect of substituent groups on the ring is the third relevant property affecting the sensitivity of nitroazoles as expected from chemical intuition, e.g., it is known that the larger the number of nitro groups in the molecule, the more sensitive the explosive will be ([18, 49] and references therein). The reason for that is the electron withdrawing character of NO2 which reduces the electronic delocalization of the ring atoms as seen before in nitrobenzenics [11, 18, 21, 81]. Similarly, for the nitroimidazoles, the largest predicted h50 values correspond to those molecules that have only one nitro group bonded to the ring; the addition of a further nitro group increases the sensitivity of the molecule accordingly. The predicted h50 values of the nitroimidazole molecules bearing two nitro groups is about three times smaller as compared with molecules bearing one: for example, 5ni = 358.33 cm and 45dni = 111.14 cm. The same occurs for nitropyrazoles: for 35dnpy having two nitro groups, the predicted h50 value is more than 1.5 times larger than for the 345tnpy having three nitro groups.
Similarly also to the nitrobenzenic molecules, the CH3 and NH2 electron injector groups in the nitrazoles exert an opposite influence as compared with the nitro group. For instance, the presence of an amino group increases the predicted h50 value from 107.32 cm in 25dni to 154.46 cm in 3a25dni as expected from the lower ring electron delocalization. The same occurs for the methyl group, for example, in 4ni, which h50 value changes from 312.56 to 385.85 cm in 1met4ni.
Conclusions
In this work, we investigated possible correlations between electronic properties and the impact sensitivity of a group of 33 nitroazole derivatives. For this purpose, DFT calculations and the partition of the molecular charge density (DMA) were employed.
It was found that increasing the number of electron withdrawing nitro groups in the nitroazole rings decreases the negative charge on the nitrogen atom on position 3 of the nitroimidazole ring. This also occurs on the atom on position 2 of nitropyrazoles and nitrotriazoles rings. The overall effect when the C carbon atoms are also considered is a decrease in the charge delocalization of the ring atoms that leads to more sensitive explosives. A similar behavior was found in the previous investigations of nitrobenzenic molecules [18] and for other classes of molecules. Employed approaches in this regard include distinct theoretical models such as electrostatic potentials [24], Mulliken charges [23], and more recently Wiberg bond indices [82, 83].
The presence of NH2 and CH3 electron injector groups bonded to the carbon atoms of nitroazole molecules increases the charge of the nitro groups. In this case, the nitro group is less able to attract electrons from the ring; hence, electron delocalization increases. This effect contributes to the lower sensitivity of the molecules bearing these groups.
The computed DMA multipole values of the 33 molecules were used to propose different mathematical models for predicting the impact sensitivity. The one that presented the smallest deviations as compared with experimental data includes the charge of the nitro group, and the charge and the quadrupole values of the nitrogen atoms of the azolic ring. Consistent h50 sensitivity values of molecules with no experimental data were also computed.
References
Sikder AK, Sikder N (2004) A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J Hazard Mater 112(1–2):1–15
Turker L (2016) Azo-bridged triazoles: green energetic materials. Def Technol 12(1):1–15
Singh RP, Ga H, Meshri DT, Shreeve JM (2007) Nitrogen-rich heterocycles. In: Klapotke TM (ed) High energy density materials. Structure and bonding, vol 125. Springer-Verlag, Berlin, pp 35–83. https://doi.org/10.1007/430_2006_055
Badgujar DM, Talawar MB, Asthana SN, Mahulikar PP (2008) Advances in science and technology of modern energetic materials: an overview. J Hazard Mater 151(2–3):289–305
Zhao GZ, Jia JF, Wu HS (2016) Design and selection of triazole-based compounds with high energetic properties and stabilities. J Chem Sci 128(8):1223–1236
Turker L, Atalar T (2006) Quantum chemical study on 5-nitro-2,4-dihydro-3H-1,2,4-triazol-3-one (NTO) and some of its constitutional isomers. J Hazard Mater 137(3):1333–1344
Turker L (2009) Structure-impact sensitivity relation of certain explosive compounds. J Energ Mater 27(2):94–109
Xia Y, Li W, Qu FQ, Fan ZJ, Liu XF, Berro C, Rauzy E, Peng L (2007) Synthesis of bitriazolyl nucleosides and unexpectedly different reactivity of azidotriazole nucleoside isomers in the Huisgen reaction. Org Biomol Chem 5(11):1695–1701
Agrawal JP (2010) High energy materials - propellants, explosives and pyrotechnics. Wiley, Essex
Sivabalan R, Anniyappan M, Pawar SJ, Talawar MB, Gore GM, Venugopalan S, Gandhe BR (2006) Synthesis, characterization and thermolysis studies on triazole and tetrazole based high nitrogen content high energy materials. J Hazard Mater 137(2):672–680
Rice BM, Hare JJ (2002) A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J Phys Chem A 106(9):1770–1783
Zeman S (2007) Sensitivities of high energy compounds. High energy density materials, vol 125. Structure and Bonding. Springer-Verlag, Berlin, pp 195–271. https://doi.org/10.1007/430_2006_052
Mathieu D (2013) Toward a physically based quantitative modeling of impact sensitivities. J Phys Chem A 117(10):2253–2259
Mathieu D, Alaime T (2014) Predicting impact sensitivities of nitro compounds on the basis of a semi-empirical rate constant. J Phys Chem A 118(41):9720–9726
Mathieu D, Alaime T (2015) Impact sensitivities of energetic materials: exploring the limitations of a model based only on structural formulas. J Mol Graph 62:81–86
Kamlet MJ, Adolph HG (1979) Relationship of impact sensitivity with structure of organic high explosives: . polynitroaromatic explosives. Propellants and Explosives 4(2):30–34
Borges I (2008) Conformations and charge distributions of diazocyclopropanes. Int J Quantum Chem. 108(13):2615–2622
Anders G, Borges I (2011) Topological analysis of the molecular charge density and impact sensitivy models of energetic molecules. J Phys Chem A 115(32):9055–9068
Giannerini T, Borges I (2015) Molecular electronic topology and fragmentation onset via charge partition methods and nuclear Fukui functions: 1,1-diamino-2,2-dinitroethylene. J Braz Chem Soc 26(5):851–859
Mathieu D (2016) Physics-based modeling of chemical hazards in a regulatory framework: comparison with quantitative structure-property relationship (QSPR) methods for impact sensitivities. Ind Eng Chem Res 55(27):7569–7577
Zhang CY, Shu YJ, Huang YG, Zhao XD, Dong HS (2005) Investigation of correlation between impact sensitivities and nitro group charges in nitro compounds. J Phys Chem B 109(18):8978–8982
Zhang CY (2008) Investigation of the correlations between nitro group charges and some properties of nitro organic compounds. Propellants Explos Pyrotech 33(2):139–145
Zhang CY (2009) Review of the establishment of nitro group charge method and its applications. J Hazard Mater 161(1):21–28
Murray JS, Concha MC, Politzer P (2009) Links between surface electrostatic potentials of energetic molecules, impact sensitivities and C-NO2/N-NO2 bond dissociation energies. Mol Phys 107(1):89–97
Yan QL, Zeman S (2013) Theoretical evaluation of sensitivity and thermal stability for high explosives based on quantum chemistry methods: a brief review. Int J Quantum Chem. 113(8):1049–1061
Owens FJ, Jayasuriya K, Abrahmsen L, Politzer P (1985) Computational analysis of some properties associated with the nitro groups in polynitroaromatic molecules. Chem Phys Lett 116(5):434–438
Politzer P, Murray JS (1996) Relationships between dissociation energies and electrostatic potentials of C-NO2 bonds: applications to impact sensitivities. J Mol Struct 376:419–424
Murray JS, Lane P, Politzer P (1998) Effects of strongly electron-attracting components on molecular surface electrostatic potentials: application to predicting impact sensitivities of energetic molecules. Mol Phys 93(2):187–194
Politzer P, Murray JS (2016) High performance, low sensitivity: conflicting or compatible? Propellants Explos Pyrotech 41(3):414–425
Ren FD, Cao DL, Shi WJ, Gao HF (2016) A theoretical prediction of the relationships between the impact sensitivity and electrostatic potential in strained cyclic explosive and application to H-bonded complex of nitrocyclohydrocarbon. J Mol Model 22(4):8
Fried LE, Manaa MR, Pagoria PF, Simpson RL (2001) Design and synthesis of energetic materials. Ann Rev Mater Res 31:291–321
Mathieu D (2012) Theoretical shock sensitivity index for explosives. J. Phys. Chem. A 116(7):1794–1800
Mathieu D (2017) Sensitivity of energetic materials: theoretical relationships to detonation performance and molecular structure. Ind Eng Chem Res 56(29):8191–8201
Keshavarz MH (2010) Simple relationship for predicting impact sensitivity of nitroaromatics, nitramines, and nitroaliphatics. Propellants Explos Pyrotech 35(2):175–181
Keshavarz MH (2013) A new general correlation for predicting impact sensitivity of energetic compounds. Propellants Explos Pyrotech 38(6):754–760
Keshavarz MH, Ghaffarzadeh M, Omidkhah MR, Farhadi K (2017) New correlation between electric spark and impact sensitivities of nitramine energetic compounds for assessment of their safety. Z Anorg Allg Chem 643(19):1227–1231
Keshavarz MH, Ghaffarzadeh M, Omidkhah MR, Farhadi K (2017) Correlation between shock sensitivity of nitramine energetic compounds based on small-scale gap test and their electric spark sensitivity. Z Anorg Allg Chem 643(24):2158–2162
Keshavarz MH, Abadi YH (2018) Novel organic compounds containing nitramine groups suitable as high-energy cyclic nitramine compounds. ChemistrySelect 3(28):8238–8244
Cho SG (2011) A predictive study on molecular and explosive properties of 1-aminoimidazole derivatives. Bull Kor Chem Soc 32(7):2319–2324
Sorescu DC, Bennett CM, Thompson DL (1998) Theoretical studies of the structure, tautomerism, and vibrational spectra of 3-amino-5-nitro-1,2,4-triazole. J Phys Chem A 102(50):10348–10357
Su XF, Cheng XL, Ge SH (2009) Theoretical investigation on structure and properties of 2,4,5-trinitroimidazole and its three derivatives. Theochem-J Mol Struct 895(1–3):44–51
Su XF, Cheng XL, Meng CM, Yuan XL (2009) Quantum chemical study on nitroimidazole, polynitroimidazole and their methyl derivatives. J Hazard Mater 161(1):551–558
Ravi P, Gore GM, Tewari SP, Sikder AK (2012) A DFT study of aminonitroimidazoles. J Mol Model 18(2):597–605
Ravi P, Gore GM, Tewari SP, Sikder AK (2010) Quantum chemical studies on the fused nitroazoles. J Mol Struct THEOCHEM 955(1–3):171–177
Ghule VD, Sarangapani R, Jadhav PM, Tewari SP (2011) Theoretical studies on nitrogen rich energetic azoles. J Mol Model 17(6):1507–1515
Yu Z, Bernstein ER (2013) Sensitivity and performance of azole-based energetic materials. J Phys Chem A 117(42):10889–10902
Ravi P, Tewari SP, Ramaswamy R (2013) A DFT study on the structures and energies of isomers of 4-amino-1,3-dinitro-1,2,4-triazol-5-one-2-oxide: new high energy density compounds. Propellants, Explosives, Pyrotechnics 38(3):425–432
Moxnes JF, Frøyland Ø, Risdal T (2017) A computational study of ANTA and NTO derivatives. J Mol Model 23(8):8
Oliveira MAS, Borges I (2019) On the molecular origin of the sensitivity to impact of cyclic nitramines. Int J Quantum Chem 119(8):14
Borges I, Silva AM, Aguiar AP, Borges LEP, Santos JCA, Dias MHC (2007) Density functional theory molecular simulation of thiophene adsorption on MoS2 including microwave effects. Theochem-J Mol Struct 822(1–3):80–88
Silva AM, Borges I (2011) How to find an optimum cluster size through topological site properties: MoSx model clusters. J Comput Chem 32(10):2186–2194
Borges I, Silva AM (2012) Probing topological electronic effects in catalysis: thiophene adsorption on NiMoS and CoMoS clusters. J Braz Chem Soc 23(10):1789–1799
Borges I, Silva AM, Modesto-Costa L (2018) Microwave effects on NiMoS and CoMoS single-sheet catalysts. J Mol Model 24(6):8
Borges I (2008) Excited electronic and ionized states of N,N-dimethylnitramine. Chem Phys 349:256–262
Borges I (2008) Excited electronic and ionized states of the nitramide molecule, H2NNO2, studied by the symmetry adapted-cluster configuration method. Theor Chem Accounts 121:239–246
Borges I, Barbatti M, Aquino AJA, Lischka H (2012) Electronic spectra of nitroethylene. International Journal of Quantum Chemistry 112: 1225-1232
Borges I (2014) Electronic and ionization spectra of 1,1-diamino-2,2-dinitroethylene, FOX-7. J Mol Model 20(3):1–7
Modesto-Costa L, Uhl E, Borges I (2015) Water solvent effects using continuum and discrete models: the nitromethane molecule, CH3NO2. J Comput Chem 36(30):2260–2269
Spartan Pro (1999). Wavefunction, Irvine
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140(4A):1133–1138
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98(2):1372–1377
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi B, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski J, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith TJ, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003). Gaussian 03, Revision C.02 edn. Gaussian, Inc., Pittsburgh
Stone AJ (2005) Distributed multipole analysis: stability for large basis sets. J Chem Theory Comput 1(6):1128–1132
Stone AJ (1981) Distributed multipole analysis, or how to describe a molecular charge-distribution. Chem Phys Lett 83(2):233–239
Price SL, Stone AJ (1983) A distributed multipole analysis of charge-densities of the azabenzene molecules. Chem Phys Lett 98(5):419–423
Stone AJ, Alderton M (1985) Distributed multipole analysis - methods and applications. Mol Phys 56(5):1047–1064
Stone AJ (2000) The theory of intermolecular forces. International Series of Monographs on Chemistry. Oxford University Press, Oxford
March J, Smith MB (2007) Advanced organic chemistry 6th edn. Wiley, New York
Pozharskii AF (1977) Concept of π-surplus character in the chemistry of heteroaromatic compounds (review). Chem Heterocycl Compd 13(6):583–598
Balaban AT, Oniciu DC, Katritzky AR (2004) Aromaticity as a cornerstone of heterocyclic chemistry. Chem Rev 104(5):2777–2812
Schmidt NB, Lee GS, Mitchell AR, Gilardi R (2001) Synthesis and properties, of a new explosive, 4-amino-3,5-dinitro-1 H-Pyrazole(LLM-116), Lawrence Livermore National Laboratory, Lawrence Livermore National Laboratory
Mathieu D (2015) Prediction of gurney parameters based on an analytic description of the expanding products. J Energ Mater 33(2):102–115
Bulusu NB (1990) Chemistry and physics of energetic materials, vol 309. 1st edn. Kluwer Academic Publishers, Dordrecht
Depaz JLG, Ciller J (1994) Structure and tautomerismo of Anta (aminonitrotriazole). Propellants Explos Pyrotech 19(1):32–41
Keshavarz MH, Jaafari M (2006) Investigation of the various structure parameters for predicting impact sensitivity of energetic molecules via artificial neural network. Propellants Explos Pyrotech 31(3):216–225
Agrawal JP, Hodgson RD (2007) Organic chemistry of explosives. 1 edn. Wiley, Essex
Moyano EL, Yranzo GI, Elguero J (1998) Flash vacuum pyrolysis of pyrazoles as an alternative way to study vinylcarbenes. J Org Chem 63(23):8188–8191
da Silva G (2009) Thermal decomposition of pyrazole to vinylcarbene + N-2: a first principles/RRKM study. Chem Phys Lett 474(1–3):13–17
Larina L, Lopyrev V (2009) Nitrazole: synthesis, structure and applications. Springer Science, New York
Politzer P, Murray JS (2015) Some molecular/crystalline factors that affect the sensitivities of energetic materials: molecular surface electrostatic potentials, lattice free space and maximum heat of detonation per unit volume. J Mol Model 21(2):11
Politzer P, Murray JS, Lane P, Sjoberg P, Adolph HG (1991) Shock-sensitivity relationships for nitramines and nitroaliphatics. Chem Phys Lett 181(1):78–82
Harper LK, Shoaf AL, Bayse CA (2015) Predicting trigger bonds in explosive materials through Wiberg bond index analysis. ChemPhysChem 16(18):3886–3892
Shoaf AL, Bayse CA (2018) Trigger bond analysis of nitroaromatic energetic materials using wiberg bond indices. J Comput Chem 39(19):1236–1248
Itamar Borges, Adélia J. A. Aquino, Hans Lischka, (2014) A Multireference Configuration Interaction Study of the Photodynamics of Nitroethylene. The Journal of Physical Chemistry A 118 (51):12011–12020
Funding
We thank the Brazilian National Research Council (CNPq) through research grants 304148/2018-0 and 409447/2018-8 and the Brazilian Army for the support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1965 kb)
Rights and permissions
About this article
Cite this article
de Oliveira, R.S.S., Borges, I. Correlation between molecular charge densities and sensitivity of nitrogen-rich heterocyclic nitroazole derivative explosives. J Mol Model 25, 314 (2019). https://doi.org/10.1007/s00894-019-4195-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4195-0