Abstract
Ten functionals were used to assess their capability to compute a local reactivity descriptor coming from the Conceptual Density Functional Theory on a group of iron–based organometallic compounds that have been synthesized by Zohuri, G.H. et al. in 2010; these compounds bear the following substituent groups: H-, O2N- and CH3O- at the para position of the pyridine ring and their catalytic activities were experimentally measured by these authors. The present work involved a theoretical analysis applied on the aforementioned iron–based compounds thus leading to suggest a new 2,6-bis(imino)pyridine catalyst based on iron(II) bearing a fluorine atom whose possible catalytic activity is suggested to be near the catalytic activity of the complex bearing a hydrogen atom as a substituent group by means of the so called local hyper-softness (LHS) thus opening a chance to estimate a possible value of catalytic activity for a new catalyst that has not been synthesized yet without simulating the entire process of ethylene polymerization. Since Conceptual DFT is not a predictive theory, but rather interpretative, an analysis of the used reactivity descriptor and its dependence upon the level of theory was carried in the present work, thus revealing that care should be taken when DFT calculations are used for these purposes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today the Computational Chemistry has become an essential complementary tool to support scientific research in the field of Catalysis [1, 2]. Although the current computational capabilities allow to simulate several chemical reactions, the computational effort to mimic the complicated process of ethylene polymerization catalyzed by organometallic compounds is still a challenging task to be performed from the perspective of the Quantum Chemistry [3,4,5,6]. For that reason the analysis of chemical reactivity through the use of reactivity descriptors or reactivity indices should be able to provide us of a useful information concerning to certain key experimental parameters which are almost impossible to obtain through a typical quantum chemical calculation.
When reactivity of a family of molecules is understood, the computational design of a new molecule that belongs to that group should not be too complicated if at least one pure experimental parameter could be linked to a pure theoretical parameter. The latter means that the use of a reactivity descriptor (a pure theoretical parameter) would be a shortcut to obtain an approximated range of values of the target experimental parameter of interest associated to the new designed molecule (catalytic activity) without computing the entire chemical process and, of course, without performing the experimental procedure to measure the experimental parameter we are interested in. As a result, this alternate perspective of the Computational Chemistry lies not in the fact of reproducing an experimental parameter, but rather it points to obtain a value of a theoretical reactivity descriptor that explains certain interactions that are responsible of the value of the target experimental parameter aforementioned. By doing so this manner, the catalytic activity of an anticipatedly designed catalyst via computational quantum chemistry can be reasonably estimated through the use of a suitable pure theoretical parameter corresponding to a reactivity descriptor based on a robust physical support provided by the conceptual density functional theory [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27].
Ethylene polymerization catalyzed by some iron–based organometallic compounds
Within the context of ethylene polymerization, different catalysts were designed, but the productivity increased noticeably since the discovery of the metallocenes of group IVB by Kaminsky [28,29,30,31,32]. A metallocene is a catalytic precursor which after being assisted by a co-catalyst [33], is turned into an organometallic cation able to catalyze the polymerization process of ethylene. Since then, the ethylene polymerization has improved noticeably thanks to these organometallic catalysts. In order to use cheaper metals, during the ending of the past century until now there has been an interest in designing new catalysts of the type non-metallocene based on iron in order to be used just like metallocenes have been used to catalyze the polymerization of ethylene [34,35,36,37].
Zohuri et al., in 2010 [38] synthesized and characterized two late transition metal 2,6-bis(imino)pyridine precursor catalysts based on iron(II) of the type [Y−LFe(II)Cl2] where L is an organic ligand and Y−∈ {O2N−,CH3O−}, as depicted by Fig. 1, corresponds to the substituent group. These type of compounds bearing two chlorine atoms are also known as pre–catalysts because after activation with a cocatalyst (which is able to replace two chlorine atoms by one methyl group) [39,40,41], the pre–catalyst turns into a cationic species as follows:
where [Y−LFe(III)CH3]2+ is the real catalytic species which is able to catalyze the ethylene polymerization process and this kind of cationic species is our focus of attention at the present work.
Models of 2,6–bis(imino)pyridil iron–based catalysts, also called BIMP-Fe cation, where 2 + is the total net charge and H-S indicates that it corresponds to a high–spin configuration of sextet type (2S + 1 = 6) and \(\mathrm {Y-}\in \left \{\mathrm {O}_{2}\mathrm {N-},\mathrm {F-},\mathrm {H-},\text {CH}_{3}\mathrm {O-}\right \}\)
Along with the aforementioned two late transition metal organometallic compounds, the respective pre–catalyst with Y = H was used by them as a reference catalyst. The substituent Y−∈ {O2N−,H−,CH3O−} is located at the para position of the pyridine ring that belongs to the L organic ligand, and since it is the only different structural part amongst these compounds, under same experimental conditions, different values of catalytic activities are attributable to the long-range substituent effect exerted by Y on the metal atom where the polymerization process takes place by means of a cycle of coordination of monomers (ethyleno) leading to an increase of the carbon-chain length.
Zohuri et al. [38] obtained catalytic activities for these three organometallic compounds Y− ∈ {O2N−,H−,CH3O−} whose values are quoted in Table 1; these authors suggested that the more electron–withdrawing substituent (Y) leads to a higher catalytic activity. However, these authors did not mention the level of theory under which this conclusion was reached and neither they did not mention the type of population analysis from which they computed net charges.
Local hyper–softness, a new tool coming from the conceptual density functional theory
According to the Zohuri’s suggestions [38], net charge (also known as partial charge) on iron atom (the site of coordination for ethylene) might be used as a parameter to be linked to the catalytic activity (the target experimental parameter). However, since ethylene is a neutral molecule and iron being a transition metal, the interaction between the catalytic organometallic structure and ethylene molecule should be predominantly of covalent nature rather than electrostatic, so that the use of net charge should not be more encouraged to be used and another kind of local reactivity descriptor must be used instead of net charge. This suggestion is based on a recently published article involving the same iron–based organometallic systems [42] so that values of net charges computed on the iron atom, at three different population analysis, did not demonstrate to be better linked with catalytic activity in comparison with the so–called local hyper–softness (LHS) [15, 25,26,27, 43, 44]. Such an article suggested that the use of condensed values of LHS on atoms are preferred than the use of net charges on atoms.
Local hyper–softness (LHS) symbolized by s(2)(r) is defined as follows:
where S, f(2)(r), f(r) and γ are mathematically defined by the following expressions, respectively:
where E is the total energy, N is the number of electrons and υ(r) is the external potential, commonly due to the presence of nuclei. Definitions of S and f(r) are found in more detail in the reference about Conceptual DFT [13], while definition of f(2)(r) is found in its starting–point references [45, 46]. On the other hand, γ is called hyper–hardness whose physical meaning and relevance is under study [47]. Within the context of the Spin–Polarized Conceptual Density-Functional Theory [23,24,25], local hyper–softness (LHS) s(2)(r), global softness S, dual descriptor f(2)(r) and Fukui function f(r) are replaced by \(s_{_{\text {NNN}}}(\mathbf {r})\), \(S_{_{\text {NN}}}\), \(f_{_{\text {NNN}}}(\mathbf {r})\) and \(f_{_{\text {NN}}}(\mathbf {r})\), respectively [25].
LHS possesses an operational formula given by dual descriptor, \(f_{_{\text {NNN}}}(\mathbf {r})\), times the global softness [10, 12, 13] square, \(\mathrm {S}_{_{\text {NN}}}^{2}\). On the other hand, the term γ defined by Eq. 5 is neglected thus turning the Eq. 1 into the Eq. 6:
where \(\mathrm {S}_{_{\text {NN}}}\) can be calculated in terms of HOMO and LUMO energies provided that the Koopmans’ theorem [48,49,50] is satisfied as several articles have demonstrated [10, 12, 13, 49, 50], being the Janak’s theorem [51] the conceptual support in the framework of DFT calculations.
Notice that the work published by Nikitin et al. [52] supports the fact that catalytic activity is linked with \(\mathrm {S}_{_{\text {NN}}}\), the global softness during the ethylene polymerization catalyzed by phenoxy–imine titanium dichloride complexes activated by MAO, but they claim that “our estimates have qualitative nature, and further studies are needed for more accurate prediction of the catalytic activity”. According to the last statement, we suggest that an analysis of local reactivity as we have proposed might improve their analysis.
The only way that LHS can be used quantitatively is performing an condensation (integration) on the kth–atom [18, 19, 23]. Through an appropriate integration within the kth–atomic domain Ωk, LHS turns into a local reactivity index associated to the kth–atom: Ωk [18,19,20, 53]:
This implies that a condensation-on-atoms of dual descriptor which can be performed through an appropriate integration within the kth–atomic domain Ωk:
Then, the use of LHS allows us make sure that the measurement of local reactivity on a molecular system can be comparable with local reactivity of other systems in spite of their size differences. This descriptor should be able to measure electronic effects that are exerted by substituent groups in agreement with the molecular size [43].
Since iron atom corresponds to the catalytic site where the ethylene coordination occurs to initiate the polymerization reaction, the interest of measuring the local reactivity will be focused on iron atom so that the kth–atom is iron: \(\mathrm {s}_{_{\text {NNN}}}\left \{\mathrm {k}\right \}\equiv \mathrm {s}_{_{\text {NNN}}}\left \{\text {Fe}\right \}\) and our hypothesis establishes that a high value of \(\mathrm {s}_{_{\text {NNN}}}\left \{\text {Fe}\right \}\) leads to a high value of catalytic activity, thus leading to estimate catalytic activities. As a consequence, and in order to measure local reactivity, LHS must be condensed onto the iron atom. It is expected that the main local reactivity is concentrated on the iron atom because during ethylene polymerization, the monomer (ethylene) takes part of the coordination cycle onto the iron atom in the complex.
Computational methods
Catalytic systems bearing substituent groups Y−∈ {O2N−,F−,H−,CH3O−} were taken into account at a high–spin configuration (sextet) along with a total net charge of 2+, thus meaning Fe(III) as depicted by Fig. 1, the latter is based on previous calculations concerning to its oxidation state [36, 37]. Geometrical optimizations were performed in gas phase with the LANL2DZ [54,55,56] with effective core potentials for all atoms by means of the following functionals: HCTH (GGA, 0% of Hartree–Fock exchange) [57,58,59], M06 (global hybrid meta–GGA, 27% of Hartree–Fock exchange) [60], M06L (meta–GGA, 0% of Hartree–Fock exchange) [61], B3LYP (global hybrid GGA, 20% of Hartree–Fock exchange) [62,63,64,65], B97D (GGA+D, 0% of Hartree–Fock exchange) [66], BP86 (GGA, 0% of Hartree–Fock exchange) [62, 67], VSXC (meta–GGA, 0% of Hartree–Fock exchange) [68], M062X (global hybrid meta–GGA, 54% of Hartree–Fock exchange) [60], B3PW91 (global hybrid GGA, 20% of Hartree–Fock exchange) [62, 67, 69,70,71] and CAM-B3LYP (range–separated hybrid GGA, 19–65% of Hartree–Fock exchange) [72]. Same set of functionals were used to perform identical calculations, but applying the LANL2TZ+ pseudopotential [54,55,56, 73] on the metal atom solely, while non metal atoms were represented by the 6-311+G(d,p) [74,75,76,77,78,79,80,81,82,83,84] basis set.
Frequency calculations were then performed to identify the stationary points as minima [85]. However, notice that all of those results corresponding to the substituents Y− ∈ {O2N−,H−,CH3O−} performed through the use of the B3LYP [62,63,64,65], BP86 [62, 67], B97D [66] and VSXC [68] functionals were extracted directly from a previous publication [42] so that they were not computed again for the present work, but they are quoted in Tables included in Supplementary Materials.
All calculations were carried out using the Gaussian 09 [86] software package. To obtain condensed values of LHS on iron atom (\(\mathrm {s}_{_{\text {NNN}}}\left \{\text {Fe}\right \}\)), the AOMix software was used [87, 88]. Those functionals that gave \(\mathrm {s}_{_{\text {NNN}}}\left \{\text {Fe}\right \}\) values for substituents −Y = {−NO2,−F,−H,−OCH3} following the same trend as indicated by catalytic activity values were called “well-behaved” functionals; on the contrary; the “ill–behaved” functionals are those ones did not agree with the observed trend given by the catalytic activities values.
Results and discussion
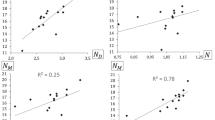
Table 2 provides the following trend for global softness \(S_{_{\text {NN}}}\):
thus supporting Nikitin et al. observations concerning to the relation between catalytic activity and the HOMO-LUMO gap [52].
From Table 3, readers can notice that the column corresponding to \(s_{_{\text {NNN}}}\{\text {Fe}\}\) values provides the following order relation for each functional: O2N− > F− > H− >CH3O−.
Then the [F−LFe(II)Cl2] pre–catalyst after being activated by a cocatalyst would present a catalytic activity slightly higher than that one exhibited by the [H−LFe(II)Cl2] pre–catalyst.
Table 4 implies a subtle different trend for global softness \(S_{_{\text {NN}}}\) in comparison to the trend obtained from Table 2 as follows:
Similarly, from Table 5, we observe that LHS presents the following order relation:
excepting when the B97D functional is employed which gives that:
Given that the LHS condensed value of the complex bearing a fluorine atom is too near to the condensed LHS value of the complex bearing an hydrogen atom, it indicates that they both are catalytic species that will present similar values of catalytic activities. As will be discussed in next paragraphs, we can explain the “ill-behavior” of M062X, B3PW91 and CAM-B3LYP functionals in spite of using different basis sets and pseudotentials [LANL2DZ on all atoms versus LANL2TZ+ on iron atom only and 6-311+G(d,p)]. But on the other hand, the fact that B3LYP and VSXC acquired a “ill-behavior” when passing from LANL2DZ to LANL2TZ+ and 6-311+G(d,p) could be attributable to the use of an incomplete mathematical expression like that one given by the Eq. 6 where the abscence of the term S3γ f(r) could be increasing the number of “ill–behaved” functionals, we suspect that this occurs because this term could become more and more important as more electrons are included in quantum chemical calculations.This is the type of discrepancy that should encourage us to use the Eq. 1 instead of Eq. 6 in order to keep the accuracy in our results and provide more confidence in the use of descriptors of reactivity coming from the Conceptual DFT. Even so, the use of the B97D, BP86, HCTH, M06 and M06L functionals is recommended in these types of compounds because a trend of reactivity given by the global and local reactivity descriptors \(S_{_{\text {NN}}}\) and \(s_{_{\text {NNN}}}\{\text {Fe}\}\), respectively is conserved in agreement with experimental results expressed in values of catalytic activities. In reference to the fact that M062X, B3PW91 and CAM-B3LYP functionals always behave badly, we can claim that within the foundations of DFT, it has been established that an universal density functional indeed exists. This density functional is universal in the sense that it can be applied to the calculation of all the chemical and physical properties of any given system. The electronic density of that system is supposed to contain all the information related to it. As it is well known, although there is a theorem that assures it existence, we do not know the functional form of that universal density functional. Thus, it has been necessary to resort to approximations, and there is a long and rich story of people doing research to build better and improved density functionals. Indeed, there is a plethora of information in the literature about these efforts [89, 90]. However, most of the density functionals that have been developed during the years are far from being universal. Thus, there are density functionals that have been created for the reproduction of certain chemical or physical properties, and we call them property-dependent density functionals. There are instead another group of density functionals that work with a particular chemical or physical system, but behave poorly for others. These are system-dependent density functionals. In order to verify how well a particular density functional can be used for the description of a particular chemical or physical property, or for a given molecular system, some researchers have compiled large databases with huge information about an extended number of molecular systems that can be compared with the predictions that outcome from the use of the different density functionals [89]. An inspection of that comparisons can give us a clue to understand the results of our particular research. In our case, we have studied the possible catalytic activity of a new iron-based organometallic compound for ethylene polymerization through the use of the condensed local hypersoftness descriptor (LHS) that arise from Conceptual DFT. To this end, ten density functionals have been considered: B3LYP, B97D, BP86, VSXC, HCTH, M06, M06L, M062X, B3PW91 and CAM-B3LYP to the study of that given molecular systems with four different substituents. We have found that, although some of them (B3LYP, B97D, BP86, VSXC, HCTH, M06 and M06L) are “well–behaved”, in the sense that the calculated LHS over the Fe atom of the catalysts is linked to the catalytic activity in relation to the substituent group when pseudopotentials are applied on all atoms. Meanwhile, B97D, BP86, HCTH, M06 and M06L functionals demonstrated a good behavior when pseudopotentials are applied on iron atoms only, thus revealing that the LHS is a robust tool when using B97D, BP86, HCTH, M06 and M06L functionals. The other density functionals considered here (M062X, B3PW91 and CAM-B3LYP) are “ill–behaved”, that is, a different ordering has been found.
The first point to be observed is that all “well–behaved” density functionals (with the exception of B3LYP and M06) are pure functionals, that is, without the addition of a certain percentage of HF exchange. In turn, the group of “ill–behaved” density functional are different types of hybrid GGA DFT. A recently published study about the quest for a universal density functional, the accuracy of a large number of density functionals have been tested across a broad spectrum of databases in chemistry and physics. There are four databases in that work that are of interest for our purposes because they are related with the molecular catalysts that we have studied. The first one is SRMBE13 (single-reference metal bond energies) and the second is MRBE10 (multi-reference bond energies). The others include metal bond energies (MBE18) which comprehends all metal bond energies in the chemistry set and is composed of all data in SRMBE13 and five data from MRBE10, and transition metal bond energies (TMBE15) that collects the transition metals bond energies from the SRMBE13 and MRBE10.
Systems containing transition metals, and/or exhibiting high multi-reference character [89] lead to mean unsigned errors (MUE) for the pure density functionals that are lower than those for the hybrid functionals. This explains while in our work the “ill–behaved” functionals are those of the hybrid kind (M062X, B3PW91 and CAM-B3LYP). While the inclusion of HF exchange delivers density functionals which perform well for the prediction of the bandgap of solids, this does not seem to be the case for metallorganic systems. However, the hybrid GGA B3LYP and meta-GGA M06 belong to “well–behaved” density functionals. Perhaps, the explanation to this could be found in the fact that amount of HF exchange that have been included in every functional. For example, M06 is a global hybrid meta-GGA with 27% of HF exchange, that leads to a overall good performance for chemistry, while M06-2X is a global hybrid meta-GGA with 54% HF exchange, with top-level performances in all areas of chemistry including thermochemistry and reaction kinetics, but excluding multi-reference systems, such as many systems containing transition metals. Finally, a comparison between B3LYP (in the “well–behaved” group) and B3PW91 (in the “ill–behaved” group), both with an equal amount of HF exchange (in any case, lower than M06), indicates that the difference must be in the correlation part. As a matter of fact, an inspection of the tables in the mentioned work, reveals that the MUEs against the MRBE10 are lower for B3LYP than for B3PW91 (although worse than for the pure density functionals). Thus, the difference on how correlation is accounted in both functionals could explain the different behaviors that we found.
Conclusions
The main purpose of the present work was not only to assess the performance of a set of functionals to study the catalytic activity, but also to obtain insights about local reactivity by means of the most rough operational formula which is usually employed to obtain 3D plots and condensed values of dual descriptor in a fast way.
-
Bar graphics given by Figs. 2 and 3 illustrates better our selection of functionals in this context. The recommended functionals to study reactivity on these type of compounds are: HCTH, M06, M06L, B97D and BP86. However we must to say that other functionals gave anomalous results (M062X, B3PW91 and CAM-B3LYP). These undesirable values are due to under–estimations or over–estimations of values of the energies of HOMO and LUMO and therefore these were called “ill–behaved” functionals, meanwhile all those functionals that provided us results that were capable to follow the same order relation in values of catalytic activities were called “well–behaved” functionals. For more details concerning to this comparison, please refer to the Supplementary Materials. The increase of the number of “ill–behaved” functionals (B3LYP and VSXC) when pseudopotentials are applied on metal atoms only in the same type of calculations can be attributable to the use of the Eq. 6 instead of Eq. 1 where the S3γ f(r) term could be playing a more important role when more electrons are included in our calculations, because to the best of our knowledge, the way of calculation of the γ index has not been tested in terms of energy of frontier molecular orbitals in this type of organometallic compounds. Besides, dealing with multi–reference organometallic compounds should encourage to use suitable functionals for these systems, i.e. MN12-SX [91] or MN15 [92]. The latter is a pending task that will be broached in next articles.
-
Not only from the global point of view revealed through global softness (\(S_{_{\text {NN}}}\)), but also from the local one through the values of LHS condensed on iron atom (\(s_{_{\text {NNN}}}\{\text {Fe}\}\)), we claim that a catalytic activity value is a consequence of local and global reactivity of the catalyst provided that experimental conditions are kept constant including the nature of monomer and in consequence, the catalytic activity is an experimental parameter that is a consequence of the global reactivity of the catalyst along with its local reactivity focused on the iron atom where the polymerization process takes place. It is possible to notice that during the entire analysis the monomer (ethylene) is irrelevant and we focused on the intrinsic reactivity of the iron–based complexes. The use of condensed values of LHS was possible to perform because the steric effect is another constant parameter which does not play an essential role for the catalytic activity in this context, so that all observed differences in catalytic activity values are exclusively attributable to the electronic effect given by the substituent groups.
-
The use of a bigger basis set to represent all electrons of non metal atoms and the use of a pseudopotential focused only on the metal revealed that the fine trend has been altered due to modifications on values of energy of frontier molecular orbitals, thus indicating that the frontier molecular orbital approximation must be abandoned and the Eq. 1 should be used in its more complete form without excluding any term in order to check if the deleted term allows to keep the same trend between LANL2DZ for all atoms and LANL2TZ+ for iron and 6-311+G(d,p) for non metal atoms. Clearly, the next step will lead us to refine our calculations by the use of a balanced basis–set on every atom. In addition, our calculations did not include a solvent model for toluene, so our results have revealed the intrinsic reactivity of these catalysts. This work concerning to the use of a more accurate operational formula and the inclusion of a solvent model is in progress. In consequence the present work is a previous approach to get an systematic insight about the global and local reactivity of these organometallic systems within the framework of the catalysis of the ethylene polymerization.
-
To end, the new suggested 2,6-bis(imino)pyridine catalyst based on iron(II) bearing a fluorine atom as a substituent group (not been synthesized yet to the best of our knowledge), should present a catalytic activity value less than that the nitro Fe–based and greater than the methoxy Fe–complexes, but pretty near to the catalytic activity value of the Fe–based complex bearing an hydrogen atom simply.
References
Thiel W (2014) Angew Chem Int Ed 53:8605–8613
Jesús Jover J, Fey N (2014) Chem Asian J 9:1714–1723
Das P, Dockter D, Fahey D, Lauffer D, Hawkins G, Li J, Zhu T, Cramer C, Truhlar D, Dapprich S, Froese R, Holthausen M, Liu Z, Mogi K, Vyboishchikov S, Musaev D, Morokuma K (1998) Ethylene polymerization by zirconocene catalysts. Research Report UMSI 98/112, Supercomputing Institute for Digital Simulation and Advanced Computation, 599 Walter Library, 117 Pleasant St. SE, Minneapolis, MN 55455
Cruz VL, Muñoz Escalona A, Martínez-Salazar J (1998) J Polym Sci A Polym Chem 36:1157–1167
Petitjean L, Pattou D, Ruiz-López MF (2001) J Mol Struct THEOCHEM 541:227–235
Woo T, Deng L, Margl P, Ziegler T (2000) In: Scheirs J, Kaminsky W (eds) Metallocene-based polyolefins. Wiley Series in Polymer Science, Chichester, pp 69–88
Hohenberg P, Kohn W (1964) Phys Rev 136:B864–B871
Kohn W, Sham LJ (1965) Phys Rev 140:A1133–A1138
Gázquez J, Galván M, Vela A (1990) J Mol Struct THEOCHEM 210:29–38
Parr R, Yang W (1989) Density–functional theory of atoms and molecules. Oxford University Press, New York
Senet P (1996) J Chem Phys 105:6471–6489
Chermette H (1999) J Comput Chem 20:129–154
Geerlings P, Proft FD, Langenaeker W (2003) Chem Rev 103:1793–1874
Johnson P, Bartolotti L, Ayers P, Fievez T, Geerlings P (2012) In: Gatti C, Macchi P (eds) Modern charge-density analysis. Springer, New York, pp 715–764
Ayers P, Morell C, De Proft F, Geerlings P (2007) Chem Eur J 13:1521–3765
Geerlings P, De Proft F (2008) Phys Chem Chem Phys 10:3028–3042
Geerlings P, Ayers PW, Toro-Labbé A, Chattaraj PK, Proft FD (2012) Acc Chem Res 45:683–695
Contreras RR, Fuentealba P, Galván M, Pérez P (1999) Chem Phys Lett 304:405–413
Fuentealba P, Pérez P, Contreras R (2000) J Chem Phys 113:2544–2551
Galván M, Vela A, Gázquez JL (1988) J Phys Chem 92:6470–6474
Galván M, Vargas R (1992) J Phys Chem 96:1625–1630
Ghanty T, Ghosh S (1994) J Am Chem Soc 116:3943–3948
Chamorro E, Pérez P (2005) J Chem Phys 123:114107
Pérez P, Chamorro E, Ayers P (2008) J Chem Phys 128:204108
Chamorro E, Pérez P, Duque M, De Proft F, Geerlings P (2008) J Chem Phys 129:064117
Labet V, Morell C, Grand A, Cadet J, Cimino P, Barone V (2008) Org Biomol Chem 6:3300–3305
Labet V, Morell C, Cadet J, Eriksson LA, Grand A (2009) J Phys Chem A 113(11):2524–2533
Heiland K, Kaminsky W (1992) Die Makromolekulare Chemie 193:601–610
Kaminsky W (1996) Macromol Chem Phys 197:3907–3945
D’Agnillo L, Soares J, Penlidis A (1998) Macromol Chem Phys 199:955–962
Kaminsky W, Laban A (2001) Appl Catal Gen 222:47–61
Pédetour JN, Radhakrishnan K, Cramail H, Deffieus A (2001) Macromol Rapid Commun 22:1095–1123
Chen EYX, Marks T (2000) Chem Rev 100:1391–1434
Small BL, Brookhart M (1998) J Am Chem Soc 120:7143–7144
Britovsek GJP, Gibson VC, McTavish SJ, Solan GA, White AJP, Williams DJ, Britovsek GJP, Kimberley BS, Maddox PJ (1998) Chem Commun, pp 849–850
Martínez J, Cruz V, Ramos J, Gutiérrez-Oliva S, Martínez-Salazar J, Toro-Labbé A (2008) J Phys Chem C 112:5023–5028
Raucoules R, de Bruin T, Raybaud P, Adamo C (2008) Organometallics 27:3368–3377
Zohuri G, Seyedi S, Sandaroos R, Damavandi S, Mohammadi A (2010) Catal Lett 140:160–166
Zurek E, Woo T, Firman T, Ziegler T (2001) Inorg Chem 40:361–370
Zurek E, Ziegler T (2001) Inorg Chem 40:3279–3292
Zurek E, Ziegler T (2002) Organometallics 21:83–92
Martínez-Araya JI, Grand A, Glossman-Mitnik D (2015) Phys Chem Chem Phys 17:29764–29775
Martínez-Araya JI (2013) J Phys Chem C 117:24773–24786
Morell C, Hocquet A, Grand A, Jamart-Grégoire B (2008) J Mol Struct THEOCHEM 849:46–51
Morell C, Grand A, Toro-Labbé A (2005) J Phys Chem A 109:205–212
Morell C, Grand A, Toro-Labbé A (2006) Chem Phys Lett 425:342–346
Morell C, Grand A, Toro-Labbé A, Chermette H (2013) J Mol Model 19:2893–2900
Koopmans T (1933) Physica 18:104–113
Stowasser R, Hoffmann R (1999) J Am Chem Soc 121:3414–3420
Zevallos J, Toro-Labbé A (2003) J Chil Chem Soc 48:39–47
Janak JF (1978) Phys Rev B 18:7165–7168
Nikitin SV, Nikitin VV, Oleynik II, Oleynik IV, Bagryanskaya EG (2016) J Mol Catal A Chem 423:285–292
Zielinski F, Tognetti V, Joubert L (2012) Chem Phys Lett 527:67–72
Hay PJ, Wadt WR (1985) J Chem Phys 82:270–283
Wadt WR, Hay PJ (1985) J Chem Phys 82:284–298
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Hamprecht FA, Cohen AJ, Tozer DJ, Handy NC (1998) J Chem Phys 109:6264–6271
Boese AD, Doltsinis NL, Handy NC, Sprik M (2000) J Chem Phys 112:1670–1678
Boese AD, Handy NC (2001) J Chem Phys 114:5497–5503
Zhao Y, Truhlar DG (2008) Theor Chem Accounts 120:215– 241
Zhao Y, Truhlar DG (2006) J Chem Phys 125:194101
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Miehlich B, Savin A, Stoll H, Preuss H (1989) Chem Phys Lett 157:200–206
Vosko SH, Wilk L, Nusair M (1980) Can J Phys 58:1200–1211
Grimme S (2006) J Comput Chem 27:1787–1799
Perdew JP (1986) Phys Rev B 33:8822–8824
Van Voorhis T, Scuseria GE (1998) J Chem Phys 109:400– 410
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Phys Rev B 46:6671–6687
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1993) Phys Rev B 48:4978–4978
Perdew JP, Burke K, Wang Y (1996) Phys Rev B 54:16533–16539
Yanai T, Tew DP, Handy NC (2004) Chem Phys Lett 393:51–57
Roy L, Hay P, Martin R (2008) J Chem Theory Comput 4:1029–1031
Wachters A (1970) Physics 52:1033–1036
Hay P (1977) J Chem Phys 66:4377–4384
Krishnan R, Binkley J, Seeger R, Pople J (1980) J Chem Phys 72:650–654
McLean A, Chandler G (1980) J Chem Phys 72:5639–5648
Raghavachari K, Trucks G (1989) J Chem Phys 91:1062–1065
Binning R, Curtiss LA (1990) J Comput Chem 11:1206–1216
McGrath M, Radom L (1991) J Chem Phys 94:511–516
Curtiss L, McGrath M, Blaudeau JP, Davis N, Binning R Jr, Radom L (1995) J Chem Phys 103:6104–6113
Blaudeau JP, McGrath M, Curtiss L, Radom L (1997) J Chem Phys 107:5016–5021
Clark T, Chandrasekhar J, Spitznagel G, Schleyer PVR (1983) J Comput Chem 4:294–301
Frisch M, Pople J, Binkley J (1984) J Chem Phys 80:3265–3269
Schlegel HB (1982) J Comput Chem 3:214–218
Frisch M et al (2009) Gaussian 09 revision e.01. Gaussian Inc. Wallingford CT
Gorelsky SI (2013) Aomix: Program for molecular orbital analysis; version 6.88 University of Ottawa. http://www.sg-chem.net/
Gorelsky SI, Lever ABP (2001) J Organomet Chem 635:187–196
Peverati R, Truhlar DG (2014) Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences 372(2011):20120476
Jensen F (2017) Introduction to computational chemistry, 3rd edn. Wiley, Chichester
Peverati R, Truhlar DG (2012) Phys Chem Chem Phys 14:16187–16191
Yu HS, He X, Li SL, Truhlar DG (2016) Chem Sci 7:5032–5051
Acknowledgments
J.I. Martínez-Araya wishes to thank the financial support coming from FONDECYT grant N∘ 1140289 and ICM, Millennium Nucleus Chemical Processes and Catalysis (CPC) grant N∘ 120082. D. Glossman-Mitnik is a researcher of CIMAV and CONACYT (Mexico) and acknowledges both institutions for partial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection P. Politzer 80th Birthday Festschrift
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martínez-Araya, J.I., Glossman-Mitnik, D. Assessment of ten density functionals through the use of local hyper–softness to get insights about the catalytic activity. J Mol Model 24, 42 (2018). https://doi.org/10.1007/s00894-017-3576-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3576-5