Abstract
Undifferentiated sarcoma (US) is a frequent soft tissue sarcoma. Although the 10-year survival rate is around 60%, advanced US is highly resistant to chemo/radiotherapy. The tumor microenvironment (TME) is closely associated with tumor progression. However, few studies of infiltrated immune cells in US have been published. In this study, we evaluated tumor-associated macrophages (TAMs) and CD8-positive cytotoxic T lymphocytes (CTLs) in 28 cases of US. Iba1, CD163, and CD204 were used as markers for TAMs. The density of CTLs was positively correlated with the density of TAMs. However, a negative correlation was seen between the density of CTLs and the percentage of CD204-positive TAMs. We found no significant association between the density of Iba1-/CD204-/CD8-positive cells and clinicopathological factors. No significant correlation between immune cell infiltration and clinical outcome was observed. Although we found no significant association between immune cells and clinicopathological factors, these findings may provide new insight into the characterization of immune cells in the TME of US.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Undifferentiated pleomorphic sarcoma (UPS, changed to undifferentiated sarcoma, US, according to recent classification) is a frequent soft tissue sarcoma, and the 10-year survival rate is around 60% [1,2,3]. A larger tumor size and older age are well-known factors of worse clinical prognosis. Conversely, expression of PTEN and STAT3 is associated with a favorable clinical course [4, 5]. Advanced sarcomas including UPS show resistance to chemo/radiotherapy [6]. Notably, immunotherapy that blocks immune checkpoint molecules is a promising new therapy for advanced UPS, and significant tumor reduction was seen in 40% of UPS patients following treatment with anti-programmed death-1 (PD-1) antibodies [7].
The tumor microenvironment (TME) is closely associated with not only tumor progression but also resistance to chemo/radiotherapy and immunotherapy [8,9,10]. Macrophages that have infiltrated into the TME are associated with tumor progression via the secretion of soluble factors related to tumor cell growth, tumor invasion, metastatic niche formation, neovascularization, maintenance of stem-like cells, and immunosuppression [8,9,10]. Tumor-associated macrophages (TAMs) with heterogeneous phenotypes infiltrate into the TME. Over the last decade, the M1/M2 concept of macrophage phenotypes has been described, and heterogeneity of macrophage phenotypes is now considered to be involved in various diseases including malignant tumors and non-tumor diseases [11, 12]. Although a more complex activation status and phenotypical heterogeneity has been suggested by recent studies [13], CD163 and CD204, which are specific markers for macrophages, are highly expressed on TAMs in human malignant tumors with the protumor phenotype [14]. Animal studies have shown that CD163 and CD204 are involved in protumor activation of TAMs [15,16,17]. In addition, CD8-positive cytotoxic T lymphocytes (CTLs) infiltrating into tumor tissues are associated with anti-tumor immune responses and influence the clinical course in several types of tumors including colorectal cancer [18]. TAMs have immunosuppressive functions on CTLs in several malignant tumors [19, 20]. However, few studies have investigated the relationship between TAMs and CTLs in UPS. Therefore, we investigated TAMs and CTLs, as well as their correlations with clinicopathological factors using tumor samples diagnosed as UPS.
Materials and methods
Patients
We evaluated 28 tumors diagnosed as UPS that were registered in the Department of Anatomic Pathology, Kyushu University (Fukuoka, Japan). All samples were primary cases, and radiation-induced sarcomas and secondary sarcomas after chemotherapy were excluded. The reassessed diagnosis of UPS was made according to the World Health Organization 2013 classification [2]. Studies using these UPS cases were previously published. We evaluated the extent of necrosis and mitosis to define each tumor’s French Federation of Cancer Centers grade. Each tumor was staged according to the seventh edition of the American Joint Committee on Cancer staging system. The Institutional Review Board at Kyushu University approved this retrospective study (#27-78).
Immunohistochemistry (IHC)
ICH for Iba1, CD204, CD163, and CD8 was performed as described in previous studies. Reactions were visualized using a diaminobenzidine substrate system (Nichirei). Positively stained cells were counted in five randomly selected areas in a high-power field in a microscope by two pathologists who were blinded to information about the patients’ backgrounds or their prognosis. The Iba1 and CD163 data were the same as those published in a previous study [17].
Statistics
Statistical analyses were carried out using JMP10 (SAS Institute, Chicago, IL, USA) and StatMate III (ATOMS, Tokyo, Japan). The Kaplan–Meier method and the Cox hazard test were used to analyze associations with the clinical course. Differences between two groups were examined for statistical significance using the Mann–Whitney U test. A value of P < 0.05 was considered statistically significant.
Results
The densities of TAMs and CTLs in UPS
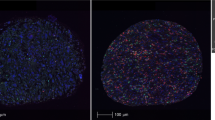
First, we evaluated the densities of CD204-positive TAMs and CD8-positive CTLs in tumor tissues using IHC (Fig. 1); these densities were 479 ± 390 and 381 ± 433/mm2, respectively (Fig. 2a). The densities of Iba1-positive TAMs and CD163-positive TAMs in the same tissue samples, which were reported in our previous study, were 814 ± 515 and 670 ± 368/mm2, respectively. The densities of Iba1-, CD163-, and CD204-positive TAMs were significantly and positively correlated with each other (Fig. 2b).
The correlations between TAMs and CTLs
Next, we tested the association between the densities of TAMs and CTLs. The densities of CTLs were positively correlated with the density of Iba1-, CD163-, and CD204-positive TAMs (Fig. 3a). Although a slightly negative correlation was seen between the density of CTLs and the percentage of CD204-positive TAMs, it was not statistically significant (P = 0.10) (Fig. 3b). We also found no significant association between the density of CTLs and the percentage of CD163-positive TAMs (Fig. 3b).
The density of macrophages and CTLs and their correlations with clinicopathological factors
As shown in Table 1, the density of CD163-positive macrophages was high in older patients, and the percentage of CD163-positive cells among Iba1-positive total macrophages was higher in patients with a smaller tumor size. However, we found no significant association between the density of Iba1-/CD204-/CD8-positive cells and clinicopathological factors (Table 1). We also found no association between expression of these immune cell markers and clinical outcome, although a high density of CD163- or CD204-postive cells tended to be associated with a lower disease-free survival rate and lower overall survival rate (Fig. 4; Table 2).
Discussion
In this study, we found no correlation between clinical outcome and infiltrating immune cells, especially CTLs. We believe this study is the first report to investigate the correlation between infiltrating CTLs and clinical outcome in UPS. Although we found no significant involvement of CTL density in the clinical course, we found that the density of CTLs was well correlated with the density of TAMs.
The density of CTLs was positively correlated with the density of Iba1-, CD163-, and CD204-positive TAMs, and interestingly, we found negative correlations between the density of CTLs and the ratio of CD163- or CD204-positive cells among Iba1-positive TAMs. Iba1 is a well-known marker of macrophages, whereas CD163 and CD204 are promising markers for protumor TAMs as described below. High infiltration of CTLs into the TME is well correlated with a better clinical outcome in several malignant tumors such as colorectal cancer and liver cancer [18, 19], whereas no association with outcome is present with other types of tumors such as breast cancer and ovarian cancer [20, 21]. CTL infiltration into the tumor cell nest has been focused on as a preferential marker for better prognosis and is considered to reflect anti-tumor immune responses in some types of tumors including lung squamous cell carcinoma [22]. Recent studies indicate that TAMs suppress the cytotoxic functions and chemotaxis of CTLs in the TME [23, 24]. Whether TAMs affect CTL functions in UPS will be an interesting topic for a future study.
Macrophages have multiple functions and have been broadly classified into classically activated macrophages (M1/kill/inflammatory macrophages) and alternatively activated macrophages (M2/repair/anti-inflammatory macrophages) according to their biological functions and expression markers [23]. In this study, we investigated CD163 and CD204 as markers for M2-like or protumor TAM subpopulations [25]. An in vitro study revealed that CD163 is specifically up-regulated by stimulation with interleukin-10 and can be considered a marker for M2c-like macrophages [25]. CD204 is highly expressed in both M1-like and M2-like macrophages in vitro, and the CD204+ subpopulation is thought to differ somewhat from the CD163+ subpopulation [25]. In this study, the number of CD204-positive cells was slightly lower than that of CD163-positive cells. Similar results were seen in kidney cancer [26], and these indicated the existence of CD163-positive and CD204-negative TAMs in TME. The significance of this population in TME has never been clarified; however, macrophages positive for both CD163 and CD204 preferentially express high levels of PD-L1 and have strong immunosuppressive function [27]. Studies using knock-out mice have shown that CD163 and CD204 are involved in protumor activation of TAMs [15,16,17]. TAMs engulf tumor cell-derived soluble factors via CD204, and CD204 also inhibits Toll-like receptor-mediated macrophage activation by scavenging Toll-like receptor ligands [28]. CD163 binds the hemoglobin/haptoglobin complex and is associated with the production of protumor cytokines such as interleukin-6 and CXCL2 by TAMs [17].
High expression of CD163 on TAMs was significantly associated with a poor clinical outcome in our previous study of 62 patients [17], but we found no association between TAMs and clinical outcome in this study. This discrepancy may be due to the limitation of the small number of enrolled cases in this study.
Taken together, we found positive correlations between the densities of TAMs and CTLs in UPS. The density of CTLs was negatively correlated with the percent of CD204-positive TAMs. Although we found no significant correlation between immune cells and clinical outcome in the present cases, these findings may provide new insight into the characterization of immune cells in the TME of UPS.
References
Oda Y, Yamamoto H, Kohashi K, Yamada Y, Iura K, Ishii T, Maekawa A, Bekki H (2017) Soft tissue sarcomas: from a morphological to a molecular biological approach. Pathol Int 67:435–446
Fletcher CDM, Chibon F, Mertens F (2013) Undifferentiated/unclassified sarcomas. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (eds) WHO classification of tumours of soft tissue and bone. International Agency for Research on Cancer, Kyon, pp 236–238
Delisca GO, Mesko NW, Alamanda VK, Archer KR, Song Y, Halpern JL, Schwartz HS, Holt GE (2015) MFH and high-grade undifferentiated pleomorphic sarcoma-what’s in a name? J Surg Oncol 111:173–177
Roland CL, May CD, Watson KL, Al Sannaa GA, Dineen SP, Feig R, Landers S, Ingram DR, Wang WL, Guadagnolo BA, Feig B, Hunt KK, Cormier JN, Lazar AJ, Torres KE (2016) Analysis of clinical and molecular factors impacting oncologic outcomes in undifferentiated pleomorphic sarcoma. Ann Surg Oncol 23:2220–2228
Bekki H, Kohashi K, Yamada Y, Iura K, Ishii T, Maekawa A, Otsuka H, Yamamoto H, Hakozaki M, Nabeshima K, Iwamoto Y, Oda Y (2017) Phosphorylation of STAT3 in undifferentiated pleomorphic sarcoma is correlated with a favorable prognosis. Pathobiology 84:161–169
Mahmood ST, Agresta S, Vigil CE, Zhao X, Han G, D’Amato G, Calitri CE, Dean M, Garrett C, Schell MJ, Antonia S, Chiappori A (2011) Phase II study of sunitinib malate, a multitargeted tyrosine kinase inhibitor in patients with relapsed or refractory soft tissue sarcomas. Focus on three prevalent histologies: leiomyosarcoma, liposarcoma and malignant fibrous histiocytoma. Int J Cancer 129:1963–1969
Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, D’Angelo S, Attia S, Riedel RF, Priebat DA, Movva S, Davis LE, Okuno SH, Reed DR, Crowley J, Butterfield LH, Salazar R, Rodriguez-Canales J, Lazar AJ, Wistuba II, Baker LH, Maki RG, Reinke D, Patel S (2017) Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 18:1493–1501
Kitamura T, Qian BZ, Pollard JW (2015) Immune cell promotion of metastasis. Nat Rev Immunol 15:73–86
Engblom C, Pfirschke C, Pittet MJ (2016) The role of myeloid cells in cancer therapies. Nat Rev Cancer 16:447–462
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14:399–416
Komohara Y, Jinushi M, Takeya M (2014) Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 105:1–8
Komohara Y, Takeya M (2017) CAFs and TAMs: maestros of the tumour microenvironment. J Pathol 241:313–315
Lewis CE, Harney AS, Pollard JW (2016) The multifaceted role of perivascular macrophages in tumors. Cancer Cell 30:18–25
Takeya M, Komohara Y (2016) Role of tumor-associated macrophages in human malignancies: friend or foe? Pathol Int 66:491–505
Neyen C, Plüddemann A, Mukhopadhyay S, Maniati E, Bossard M, Gordon S, Hagemann T (2013) Macrophage scavenger receptor a promotes tumor progression in murine models of ovarian and pancreatic cancer. J Immunol 190:3798–3805
Komohara Y, Takemura K, Lei XF, Sakashita N, Harada M, Suzuki H, Kodama T, Takeya M (2009) Delayed growth of EL4 lymphoma in SR-A-deficient mice is due to upregulation of nitric oxide and interferon-gamma production by tumor-associated macrophages. Cancer Sci 100:2160–2166
Shiraishi D, Fujiwara Y, Horlad H, Saito Y, Iriki T, Tsuboki J, Cheng P, Nakagata N, Mizuta H, Bekki H, Nakashima Y, Oda Y, Takeya M, Komohara Y (2018) CD163 is required for protumoral activation of macrophages in human and murine sarcoma. Cancer Res 78:3255–3266
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW (2011) The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105:93–103
Ohnishi K, Komohara Y, Saito Y, Miyamoto Y, Watanabe M, Baba H, Takeya M (2013) CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci 104:1237–1244
Shiota T, Miyasato Y, Ohnishi K, Yamamoto-Ibusuki M, Yamamoto Y, Iwase H, Takeya M, Komohara Y (2016) The clinical significance of CD169-positive lymph node macrophage in patients with breast cancer. PLoS One 11:e0166680
Clarke B, Tinker AV, Lee CH, Subramanian S, van de Rijn M, Turbin D, Kalloger S, Han G, Ceballos K, Cadungog MG, Huntsman DG, Coukos G, Gilks CB (2009) Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol 22:393–402
Kurozumi S, Fujii T, Matsumoto H, Inoue K, Kurosumi M, Horiguchi J, Kuwano H (2017) Significance of evaluating tumor-infiltrating lymphocytes (TILs) and programmed cell death-ligand 1 (PD-L1) expression in breast cancer. Med Mol Morphol 50:185–194
Mills CD, Lenz LL, Harris RA (2016) A breakthrough: macrophage-directed cancer immunotherapy. Cancer Res 76:513–516
Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J, Ouakrim H, Régnier F, Lupo A, Alifano M, Damotte D, Donnadieu E (2018) Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci USA 115:E4041–E4050
Fujiwara Y, Hizukuri Y, Yamashiro K, Makita N, Ohnishi K, Takeya M, Komohara Y, Hayashi Y (2016) Guanylate-binding protein 5 is a marker of interferon-γ-induced classically activated macrophages. Clin Transl Immunol 5:e111
Motoshima T, Miura Y, Wakigami N, Kusada N, Takano T, Inoshita N, Okaneya T, Sugiyama Y, Kamba T, Takeya M, Komohara Y (2018) Phenotypical change of tumor-associated macrophages in metastatic lesions of clear cell renal cell carcinoma. Med Mol Morphol 51:57–63
Kubota K, Moriyama M, Furukawa S, Rafiul HASM, Maruse Y, Jinno T, Tanaka A, Ohta M, Ishiguro N, Yamauchi M, Sakamoto M, Maehara T, Hayashida JN, Kawano S, Kiyoshima T, Nakamura S (2017) CD163+ CD204+ tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci Rep 7:1755
Ohnishi K, Komohara Y, Fujiwara Y, Takemura K, Lei X, Nakagawa T, Sakashita N, Takeya M (2011) Suppression of TLR4-mediated inflammatory response by macrophage class A scavenger receptor (CD204). Biochem Biophys Res Commun 411:516–522
Acknowledgements
We thank Ms. Ikuko Miyakawa and Mr. Takenobu Nakagawa for their technical assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (nos. 16H05162, 17H04060).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no financial competing interests to declare.
Rights and permissions
About this article
Cite this article
Komohara, Y., Takeya, H., Wakigami, N. et al. Positive correlation between the density of macrophages and T-cells in undifferentiated sarcoma. Med Mol Morphol 52, 44–51 (2019). https://doi.org/10.1007/s00795-018-0201-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-018-0201-3