Abstract
Revised International Prognostic (R-IPI) score is used widely for risk stratification of DLBCL cases, yet some patients belonging to same risk category tend to exhibit different outcomes. The role of T-cells and macrophages in prognostication of lymphomas has been a point of interest of late. We aimed to study the association of FOXP3 positive T-regulatory cells, cytotoxic T-cells and macrophages with the immunophenotypic subtypes, clinicopathological characteristics, treatment response and survival in nodal diffuse large B-cell lymphoma (DLBCL) patients. The clinicopathological and treatment data of 83 DLBCL patients diagnosed and treated at our institute from January 2015 to December 2018 were collected and followed up till June 2020. CD8, FOXP3 and CD68 immunostains were performed to highlight the cytotoxic T-cells, T-regulatory cells and macrophages respectively on the lymph node biopsies and the distribution of these cells and their association with clinico-pathological factors, treatment response and survival was analyzed. DLBCL cases with higher percentage of CD3 positive T-cells and CD8 positive cytotoxic T-cells had significant association with attainment of complete response to treatment. In addition, CD8 positive T-cells of more than 6.5% proved to be an independent predictor of treatment outcome (p = 0.022). Multivariate Cox regression survival analysis revealed that cases with ‘good’ R-IPI prognostic score and ‘high CD68 positive macrophages in tumor microenvironment’ had a significantly longer overall survival. Increased number of cytotoxic T-cells was significantly associated with complete response to treatment and higher number of macrophages correlated significantly with better overall survival signifying their antitumor effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype among the high-grade Non-Hodgkin Lymphomas (NHL) worldwide and represents 35% of all the NHL [1]. It occurs in lymph nodes as well as in extra nodal sites. Gene expression profiling of DLBCL has resulted in the identification of two clinically and prognostically distinct subtypes namely germinal center B-cell type (GCB) and the activated B-cell type (ABC) based on the cell of origin [1, 2]. R-CHOP (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisone) regimen has been the main stay of treatment for DLBCL [3]. However about 20% of patients relapse following completion of treatment. Revised International Prognostic Score (R-IPI) is the most widely used tool for risk stratification of DLBCL [4]. However it has been noted that patients belonging to the same risk category exhibit different outcomes.
In the last decade there has been emphasis on tumor microenvironment in lymphomas and its role in predicting the treatment outcome in these patients. The cells which constitute the tumor microenvironment are the innate and adaptive immune cells, microvasculature, fibroblasts and other stromal cells [5, 6]. In hematolymphoid malignancies, the interactions between the tumor microenvironment and the malignant cells are complex, and therefore it is difficult to predict the prognosis. The complex microenvironment may promote or constrain the development of lymphoma, depending on the relative distribution of various sub population of cells. In DLBCL, many researchers have studied the effect of Cytotoxic T-lymphocytes (CTL), T-regulatory cells (T-regs) and Tumor Associated Macrophages (TAM) on the treatment outcome and prognosis. Some of these studies have suggested that these cells provide anti-tumor immunity whereas other studies state that these cells promote tumor progression [6,7,8,9].
Even though CTL mount an anti-tumor immune response, in DLBCL an increase in the number of CTL creates a continuous immunogenic pressure on the tumor cells which results in downregulation of MHC class I expression on tumor cells, making it resistant to CTL mediated apoptosis [7]. The immunosuppressive function of T-regs have been well established in solid malignancies where these cells shields the tumor cells from immune detection, and thereby allow the tumor cells to survive and proliferate [10]. In NHL, the immunomodulatory role of T-regs is yet to be defined. T-regs have diverse interaction with tumor cells in lymphomas besides suppression of cytotoxic immune response. These cells can be directly cytotoxic to B-cells causing anti-tumor immunity in B-cell lymphomas like follicular lymphoma [11]. Even though much attention has been directed towards the role of T-regs in various NHLs, their role in the prognostication of DLBCL still remains controversial.
Tumor Associated Macrophages (TAM) also has a prognostic impact on DLBCL, as shown in several recent studies. High CD68 mRNA levels as well as increased number of CD68 positive macrophages are associated with better progression free survival (PFS) in the patients who received chemo-immunotherapy, whereas the same was associated with poor PFS in the patients treated with chemotherapy alone [12]. Rituximab (anti CD20 antibody) exerts its action by antibody dependent cellular phagocytosis and antibody dependent cytotoxicity which is mediated efficiently by the macrophages. This role of the macrophages can be attributed to the favorable outcome in patients who received rituximab. Nevertheless, according to Hasselblom et al. and Meyer et al., there was no significant correlation between TAM and survival in DLBCL patients and thus suggested that TAM does not play a major role in DLBCL prognostication [13, 14].
With this background, the distribution of CTLs, T-regs and TAM in nodal DLBCL using CD8, FOXP3 and CD68 immunohistochemical stains respectively were studied to identify the association of these cells with the histological subtypes, immunophenotypes and clinicopathological characteristics of nodal DLBCL. The prognostic utility of CTLs, T-regs and TAM in predicting the end of treatment response/outcome and survival in DLBCL patients was also studied to identify new prognostic biomarkers.
Materials and Methods
Clinical Data
A total of 103 cases of nodal DLBCL were diagnosed in Department of Pathology from the period of January 2015 to December 2018 and the formalin fixed paraffin embedded blocks of these cases were available in our archives. Out of the 103 cases, 83 cases had complete clinical and treatment details and were included in the study. Baseline demographics, patient and disease related characteristics, laboratory parameters, treatment course, response and outcomes were collected from medical records in the Department of Medical Oncology and the hospital information system. Staging was done according to the modified Ann-Arbor and cases were divided into early stage (Stage I and II) and advanced stage (Stage III and IV) [15]. Risk assessment was done using R-IPI score and the cases were divided into very good, good and poor prognostic groups [4].
Histopathological and Immunohistochemical Data
The diagnostic Hematoxylin and Eosin (H and E) stained lymph node biopsy slides were reviewed along with their corresponding IHC slides to confirm the morphological variant and the immunophenotype. Immunophenotypic sub-typing of lymph node biopsies was done according to Han’s algorithm into germinal center B-cell type (GCB) and the activated B-cell type (ABC). CD8, CD68 and FOXP3 immunostains were performed in all the 83 cases on standard sections of 2 to 3 micron thickness.
Interpretation of Immunohistochemical Stains
CD3, CD8, FOXP3 and CD68 highlighted panT-cells, cytotoxic T-cells, T-regulatory cells and macrophages in the tumour microenvironment respectively. CD3 (Polyclonal, PathnSitu) immunostain highlighted the T-cells in membranous staining pattern. CD8 (clone: C8/144B. DAKO) showed membranous staining pattern in the cytotoxic T-cells. CD68 (clone: PG-M1, DAKO) showed membranous and cytoplasmic granular staining pattern in the macrophages. Forkhead box P3/ scrufin (FOXP3) is a transcription factor which is crucial for the immunosuppressive function of T-regulatory cells. FOXP3 (clone 236A/E7, ABCAM) immunostain showed nuclear staining pattern in the T-regulatory cells. The IHC-stained slides were independently viewed by two pathologists, who manually quantified the cells using the following method. Ten high-power fields (40 × magnification) where the maximum density of IHC positive cells was observed were selected as hot spots. The number of CD3, CD8, FOXP3 and CD68 positive cells were counted in these 10 hot spots of the respective slides. The total number of stained cells was divided by the total number of cells viewed in each hot spot to arrive at percentage of cells positive for a particular immunostain. The mean value was calculated from the 10 hot spots. The average of the values obtained by the two pathologists was taken as the final value.
Treatment and Follow Up Data
Patients were treated with six cycles of first line chemotherapy (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisone – CHOP/ R-CHOP regimen). Concurrent involved site radiotherapy (ISRT) was given to patients with bulky disease (> 10 cm). Assessment for treatment response was done at the end of four cycles (interim response) and at the end of six cycles (end of treatment response) by PET-CT/CECT scan as recommended by Cheson et al. in 2014 [16]. The cases were classified under four response categories namely ‘Complete Response’ (CR), Partial Response (PR), Stable Disease (SD) and Progressive Disease (PD). Further treatment for patients with PR, SD and PD was based on treating physician’s discretion. Follow up was done till June 2020 and any relapse or progressive disease was recorded from the patient’s records. Overall survival (OS) and progression free survival (PFS) were calculated. Overall survival (OS) was defined as the time interval between the date of diagnosis to the date of death or the date of the last follow-up visit. Progression free survival (PFS) was defined as the time from initial diagnosis to relapse, death, or last follow-up, whichever came first. The median follow-up time was 23 months and ranged from 16.9–29 months.
Statistical Tests
All statistical analysis was done using SPSS software IBM PASW statistics (SPSS) – version 20.0. Normality of the quantitative variables was tested by Kolmogrov Smirnoff test. The association of CD3 positive T-cells, CD8 positive T-cells, FOXP3 positive T-cells and CD68 positive macrophages with clinical characteristics and treatment outcome was done using ‘independent student’s t-test’. The cutoff values of the quantitative variables that had significant association with treatment outcome were calculated from ROC curve using Youden’s index. The independent factors associated with the response to treatment were explored using binary logistic regression analysis. Kaplan Meir curves with log-rank test and Cox proportional hazard regression analysis were used to estimate the survival and factors affecting it. The p-values of all these tests were based on two-tailed statistical analysis, considering p-values below 0.05 as statistically significant.
Results
Clinicopathological Characteristics and Treatment Data
The median age of our study group was 55 years (range—14 to 80 years) with a male: female ratio of 2.1:1. Advanced clinical stage was seen in 49 (59%) cases and B-symptoms were more commonly observed in cases who presented with advanced stage disease (67%). Based on the R-IPI score the cases were divided into three prognostic groups (very good, good and poor). None of the cases had an R-IPI score of zero. There were 56 patients (67.5%) in the ‘good’ prognostic group and 27 patients (32.5%) in the ‘poor’ prognostic group. ABC was the most common immunophenotype comprising of 52 cases (62.7%) followed by GCB subtype (37.3%). Bone marrow involvement was assessed by either PET-CT or bone marrow biopsy and this data was available for 81 patients (97%). Marrow involvement by DLBCL in bone marrow biopsy was confirmed by performing CD20 immunohistochemistry. Marrow involvement by DLBCL was observed in 11 (13.6%) cases. The baseline clinico-pathological details are summarized in Table 1.
Chemotherapy with or without additional ISRT was given to 76 (91.5%) patients in the form of CHOP regimen (7 patients; 9.2%) R-CHOP regimen (66 patients; 86.9%) or dose adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) regimen (3 patients; 3.9%). Two patients (2.4%) expired and five patients (6.1%) lost to follow up before starting chemotherapy. Out of these 76 patients who received chemotherapy, 53 (69.7%) patients completed six cycles of chemotherapy and were subjected to response assessment at the end of treatment. Eleven patients (14.5%) expired and 12 patients (15.8%) lost to follow up before completion of treatment. Nine patients (10.8%) had relapsed after treatment completion and were given salvage chemotherapy, and three out of the nine relapsed patients had undergone autologous stem cell transplant.
Tumor Microenvironment
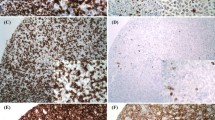
The mean values of CD3 positive T-cells, CD8 positive T-cells, FOXP3 positive T-cells and CD68 positive macrophages in the tumor microenvironment were 36.6% ± 18.4%, 12.6% ± 11.5%, 8.8% ± 11.1% and 14.3% ± 10.4% respectively. Figures 1 and 2 show the expression of CD3 positive T-cells, CD8 positive CTL, FOXP3 positive T-regs and CD68 positive macrophages in the tumor microenvironment of GCB – DLBCL and ABC – DLBCL respectively.
Expression of tumor microenvironment in Germinal Centre B cell subtype of DLBCL (A) photomicrograph showing medium to large tumor cells showing round to ovoid nuclei, vesicular chromatin and multiple tiny nucleoli, H&EX 400; B) CD10 positive; C) BCL6 positive; D) MUM1 negative; E) CD3 positive T-cells; F) CD8 positive T-cells; G) CD68 positive macrophages; H) FOXP3 positive T-cells X DAB 400
Expression of tumor microenvironment in Activated B Cell subtype of DLBCL (A) photomicrograph showing medium to large tumor cells showing round to ovoid nuclei, vesicular chromatin and prominent nucleoli, H&EX 400; B) CD10 negative; C) BCL6 positive; D) MUM1 positive; E) CD3 positive T-cells; F) CD8 positive T-cells; G) CD68 positive macrophages; H) FOXP3 positive T-cells X DAB 400
Association of Tumor Microenvironment with Clinicopathological Characteristics
The expression of CD3 positive T-cells (p = 0.002) and CD8 positive CTLs (p = 0.003) were significantly lower is cases with age more than 60 years. Similarly, there was a higher expression of TAM in cases presenting with early-stage disease (p = 0.020). There were no significant differences in the expression of these cells among the immunophenotypic subgroups and other clinical characteristics. The association of CD3 positive T-cells, CD8 positive CTL, FOXP3 positive T-regs and CD68 positive macrophages with the clinico-pathological characteristics are summarized in Table 2.
Association of Tumor Microenvironment with End of Treatment Response
The association of CD3 positive T-cells, CD8 positive CTL, FOXP3 positive T-regs and CD68 positive macrophages with the treatment outcome at the end of 6 cycles (end of treatment response) are summarized in Table 3. For the ease of statistical analysis, the patients were divided into two groups based on treatment outcome as “complete response (CR)” and “less than CR” which included PR, SD and PD. There was a significant difference in the expression of CD3 positive T-cells (p = 0.044) and CD8 positive CTLs (p = 0.041) between the ‘CR’ group and ‘less than CR’ group.
Cutoff values for CD3 and CD8 were derived using ROC and AUC. The optimal cutoff value for CD3 positive T-cells was 34.5% with AUC of 0.681 (95% CI: 0.530–0.830; p = 0.042). The optimal cutoff value for CD8 positive CTLs was 6.5% with AUC of 0.697 (95% CI: 0.540–0.855; p = 0.026). These 53 cases were divided into two groups based on the cutoff values and the association of these groups with the end of treatment response is shown in Table 4.
The odds of attaining complete response to treatment was significantly higher in cases having high CD3 expression (Odd’s Ratio: 3.84; 95% CI: 1.08–13.63; p = 0.032) and high CD8 expression (Odd’s Ratio: 6.64; 95% CI: 1.77–24.82; p = 0.003). Also, when a binary logistic regression analysis was performed by incorporating the significant variables like R-IPI score, CD3 and CD8 cutoff groups, ‘CTLs more than 6.5%’ was identified to be the independent predictor of treatment outcome (Adjusted Odd’s Ratio: 5.984; 95% CI: 1.29–27.69; p = 0.022).
Survival Analysis
The median OS time period was 50.2 months (95% CI: 42.9–57.3 months) and the median PFS time period was 37.6 months (95% CI: 31.4–43.8 months). Among the clinical characteristics, R-IPI score had a significant effect on better OS (p = 0.002) and PFS (p = 0.010). The Kaplan Meier curves for OS and PFS according to R-IPI prognostic groups are shown in Fig. 3.
The effect of CD3 positive T-cells, CD8 positive T-cells, FOXP3 positive T-cells and CD68 positive macrophages on OS and PFS is shown in Table 5. CD68 positive macrophages had a significant effect on the OS (p = 0.032). There was no significant effect of CD3 positive T-cells, CD8 positive T-cells and FOXP3 positive T-cells on either OS or PFS. The variables that were statistically significant in the univariate Cox regression analysis were incorporated in the multivariate model to identify the combined impact of these variables on the OS. Cases with ‘good’ R-IPI prognostic score have a 72% reduction in risk of death when compared to cases with ‘poor’ R-IPI prognostic score (HR: 0.282 with 95% CI: 0.118–0.671; p = 0.004). There is a 5.5% reduction in the risk of death for every 1% rise in background CD68 positive macrophages (HR: 0.945 with 95% CI: 0.898–0.995; p = 0.036).
Discussion
DLBCL is one of the most common aggressive B-cell malignancy with heterogeneity in its morphological and genetic characteristics. Evaluation of tumor microenvironment has been one of the areas of recent interest in understanding the pathophysiology and outcomes of this disease. We present one of the first study from India, which explored the association of tumor microenvironment on the clinicopathological characteristics, treatment outcome and survival of DLBCL. Our results suggest that higher number of T-cells, especially cytotoxic T-cells in the tumor microenvironment of DLBCL were associated with better complete response at the end of treatment, and additionally, higher number of CD68 positive macrophages in the tumor microenvironment correlated significantly with better overall survival.
In our study, ‘good’ R-IPI score was significantly associated with complete response to treatment, longer OS and PFS. This result is in concordance with the study by El-Galaly et al., in which R-IPI score was able to identify significant difference in survival between the R-IPI prognostic subgroups with 100% ‘3 years’ PFS in the ‘very good’ prognostic group [17]. However in our study, none of the cases were eligible to be classified under ‘very good prognostic’ group. One more limitation encountered in our study was that approximately 30% of our patients were not available for PFS or end of treatment response assessment due to death or lost to follow up.
Our study found that a high expression of T-lymphocytes (CD3 > 34.5%) and CTLs (CD8 > 6.5%) in the tumor microenvironment were significantly associated with attainment of complete response to treatment. In addition, high CTL expression was found to be the independent predictor of treatment outcome. Our data was similar to studies by Chang et al. and Linderoth et al., where they had reported that increased expression of CTL in the tumor bed was associated with favorable prognosis in DLBCL [18, 19]. Similarly, Lippman et al. studied the immunohistochemical expression of CTL in DLBCL using Leu2 + as the immunostain and found that less than 6% CTL correlated with reduced relapse free survival [20]. However, there was no significant association between the CTLs and clinical characteristics and survival in our study. On the other hand, Muris et al. observed in his study that a significant number of DLBCL cases which had the presence of activated CTL more than 15% had failed to attain complete remission [7]. Many recently conducted studies have proved that increased expression of CTL were associated with poor outcome and survival of DLBCL patients [7, 21]. A plausible explanation for the poor outcome could be down regulation of surface expression of MHC class I by the tumor cells leading to immune evasion.
A higher expression of CD68 positive macrophages in tumor microenvironment had a significant association with better OS in both univariate and multivariate Cox regression analysis. There was a 5.5% reduction in the risk of death for every 1% rise in background TAM. Our result was in concordance with Nam et al. and Riihijarvi et al. where an increase in CD68 positive cells were related to improved OS in patients who received R-CHOP [12, 22]. A higher number of TAM were also found in cases which attained complete response to treatment, but the association was statistically insignificant. Many recent studies have hypothesized that higher expression of CD163 positive M2 macrophages in DLBCL predicted poor clinical outcome [23, 24]. This discrepancy between our results and existing literature could be explained by the polarization of macrophages towards M1 subtype in our study. CD68 being a pan-macrophage marker, highlights both the M1 macrophages involved in anti-tumor immunity and M2 macrophages which participates in tumor progression. This hypothesis of polarization of M1 and M2 macrophages could have been studied better if CD163 was used in our study along with CD68.
Many studies have found that the presence of increased percentage of FOXP3 positive T-regs in DLBCL correlated with longer overall survival (OS) [25, 26]. In a study on DLBCL by Tzankov et al., increased T-regs were associated with better survival in GCB subtype and poor survival in ABC subtype [9]. But in our study, FOXP3 did not have any effect on the treatment outcome, OS and PFS. This result was concordant with study by Hasselblom et al. where he stated that T-regs could have a minimal role in DLBCL when compared to CTL [21]. The disparities in the results between various studies can be explained by the fact that T-regs have diverse interaction in lymphomas apart from suppression of cytotoxic immune response, T-regs can also be directly cytotoxic to B-cells causing anti-tumor immunity in certain B-cell lymphomas.
The dissimilarity in the results published by various studies including our study can be explained by the heterogeneity in IHC staining protocols, varying clones of the antibody used, different methods used for assessment of these cells, selection of tumor areas for counting the cells and variation in sample size. Use of automated scoring platforms can improve the objectivity of the study and aid in providing high throughput results. In addition, evaluation of other markers in the tumor microenvironment can be performed to understand the biology of the tumor microenvironment and its complex interactions with the tumor cells. Eventually a composite prognostic system can be built by combining tumor characteristics and its microenvironment characteristics, which can help in the development of therapy targeting the immune microenvironment. Therefore, large scale prospective studies with standardized protocols are recommended for identification of the role of the individual cells of the tumor microenvironment and its role in prognostication of DLBCL.
Conclusion
Our study highlights the role of immune cells, especially CTLs and TAMs in the microenvironment in determining clinical outcomes of DLBCL. Our study suggests that higher number of T-cells, especially cytotoxic T-cells in the tumor microenvironment of DLBCL were associated with complete response at the end of treatment. CD8 positive CTLs more than 6.5% proved to be an independent predictor of treatment outcome. In addition, higher number of CD68 positive macrophages in the tumor microenvironment of DLBCL lymph node biopsies correlated significantly with better overall survival suggesting that macrophages play a vital role in anti-tumor immunity. R-IPI remains to be the best prognostic tool for risk assessment. Further prospective studies with larger patient numbers, a wider immune panel with multiplex IHC can help in a more comprehensive understanding of the complexity of tumor microenvironment in DLBCL.
Data Availability
No sharing of data.
References
Swerdlow SH, Campo E, Harris NL (2017) WHO classification of tumors of haematopoietic and lymphoid tissues. 4th ed. IARC, Lyon, p.291–7
Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW et al (2008) Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319:1676–1679
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R et al (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235–242
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P et al (2007) The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109:1857–1861
Scott DW, Gascoyne RD (2014) The tumor microenvironment in B cell lymphomas. Nat Rev Cancer 14:517–534
de Jong D, Enblad G (2008) Inflammatory cells and immune microenvironment in malignant lymphoma. J Intern Med 264:528–536
Muris JJF, Meijer CJLM, Cillessen S a. GM, Vos W, Kummer JA, Bladergroen BA, et al (2004) Prognostic significance of activated cytotoxic T-lymphocytes in primary nodal diffuse large B-cell lymphomas. Leukemia 18:589–96.
Kridel R, Steidl C, Gascoyne RD (2015) Tumor-associated macrophages in diffuse large B-cell lymphoma. Haematologica 100:143–145
Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S (2008) Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica 93:193–200
Zou W (2006) Regulatory T cells, tumor immunity and immunotherapy. Nat Rev Immunol 6:295–307
Wang J, Ke X-Y (2011) The four types of Tregs in malignant lymphomas. J Hematol Oncol 4:50–50
Riihijärvi S, Fiskvik I, Taskinen M, Vajavaara H, Tikkala M, Yri O et al (2015) Prognostic influence of macrophages in patients with diffuse large B-cell lymphoma: a correlative study from a Nordic phase II trial. Haematologica 100:238–245
Hasselblom S, Hansson U, Sigurdardottir M, Nilsson-Ehle H, Ridell B, Andersson P-O (2008) Expression of CD68+ tumor-associated macrophages in patients with diffuse large B-cell lymphoma and its relation to prognosis. Pathol Int 58:529–532
Meyer PN, Fu K, Greiner T, Smith L, Delabie J, Gascoyne R et al (2011) The stromal cell marker SPARC predicts for survival in patients with diffuse large B-cell lymphoma treated with rituximab. Am J Clin Pathol 135:54–61
Olweny CL (1990) Cotswolds modification of the Ann Arbor staging system for Hodgkin’s disease. J Clin Oncol 8:1598–1598
Cheson BD (2015) Staging and response assessment in lymphomas: the new Lugano classification. Chin Clin Oncol 4:5
El-Galaly TC, Villa D, Alzahrani M, Hansen JW, Sehn LH, Wilson D et al (2015) Outcome prediction by extranodal involvement, IPI, R-IPI, and NCCN-IPI in the PET/CT and rituximab era: a Danish-Canadian study of 443 patients with diffuse-large B-cell lymphoma. Am J Hematol 90:1041–1046
Chang K-C, Huang G-C, Jones D, Lin Y-H (2007) Distribution patterns of dendritic cells and T cells in diffuse large B-cell lymphomas correlate with prognoses. Clin Cancer Res 13:6666–6672
Linderoth J, Edén P, Ehinger M, Valcich J, Jerkeman M, Bendahl P-O et al (2008) Genes associated with the tumor microenvironment are differentially expressed in cured versus primary chemotherapy-refractory diffuse large B-cell lymphoma. Br J Haematol 141:423–432
Lippman S, Spier C, Miller T, Slymen D, Rybski J, Grogan T (1990) Tumor-infiltrating T-lymphocytes in B-cell diffuse large cell lymphoma related to disease course. Mod Pathol 3:361–367
Hasselblom S, Sigurdadottir M, Hansson U, Nilsson-Ehle H, Ridell B, Andersson P-O (2007) The number of tumor-infiltrating TIA-1+ cytotoxic T cells but not FOXP3+ regulatory T cells predicts outcome in diffuse large B-cell lymphoma. Br J Haematol 137:364–373
Nam SJ, Go H, Paik JH, Kim TM, Heo DS, Kim CW et al (2014) An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 55:2466–2476
Wada N, Zaki MAA, Hori Y, Hashimoto K, Tsukaguchi M, Tatsumi Y et al (2012) Tumor-associated macrophages in diffuse large B-cell lymphoma: a study of the Osaka Lymphoma Study Group. Histopathology 60:313–319
Li Y-L, Shi Z-H, Wang X, Gu K-S, Zhai Z-M (2019) Tumor-associated macrophages predict prognosis in diffuse large B-cell lymphoma and correlation with peripheral absolute monocyte count. BMC Cancer 19:1049
Lee N-R, Song E-K, Jang KY, Choi HN, Moon WS, Kwon K et al (2008) Prognostic impact of tumor infiltrating FOXP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk Lymphoma 49:247–256
Serag El-Dien MM, Abdou AG, Asaad NY, Abd El-Wahed MM, Kora MAE-HM. (2017) Intratumoral FOXP3+ regulatory T cells in diffuse large B-cell lymphoma. Appl Immunohistochem Mol Morphol 25:534–42
Acknowledgements
The authors would like to acknowledge the support provided by Mr. Balamurali, Mrs. Angalaeswary, Mrs. Kasthuri, Mrs. Girija Natarajan, Mrs. Kalaivizhi and Mrs. Brindhavathi for their technical support in conducting the study.
Funding
The study was funded intramurally by the parent institution – JIPMER, Pondicherry, India (Grant number—(JIP/Res/Intramural/PHS 1/2018–19).
Author information
Authors and Affiliations
Contributions
SR and JSR have conceptualised, designed and executed the study. DB has given expert opinion for the scientific content of the study and SK has helped in the analysis of the data and the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Ethical approval was obtained from the Institute Ethics Committee (JIP/IEC/2018/0119).
Conflicts of Interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ravi, S., Rekha, J.S., Basu, D. et al. Prognostic Significance of T-Cells and Macrophages in the Tumour Microenvironment of Nodal DLBCL. Indian J Hematol Blood Transfus (2024). https://doi.org/10.1007/s12288-024-01770-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12288-024-01770-6