Abstract
The haloalkaliphilic genus Thioalkalivibrio, widely used in bio-desulfurization, can oxidize H2S to So, which is excreted outside cells in the form of biosulfur globules. As by-product of bio-desulfurization, information on biosulfur globules is still very scant, which limits its high-value utilization. In this paper, the characteristics of biosulfur globules produced by Thioalkalivibrio versutus D301 and the possibility of cultivating sulfur-oxidizing bacteria as a high biological-activity sulfur source were studied. The sulfur element in the biosulfur globules existed in the form α-S8, which was similar to chemical sulfur. The biosulfur globule was wrapped with an organic layer composed of polysaccharides and proteins. The composition of this organic layer could change. In the formation stage of biosulfur globules, the organic layer was dominated by polysaccharides, and in later stage, proteins became the main component. We speculated that the organic layer was mainly formed by the passive adsorption of organic matter secreted by cells. The existence of organic layer endowed biosulfur with better bioavailability. Compared with those found using chemical sulfur, the growth rates of Acidithiobacillus thiooxidans ATCC 19377T, Thiomicrospira microaerophila BDL05 and Thioalkalibacter halophilus BDH06 using biosulfur increased several folds to an order of magnitude, indicating that biosulfur was a good sulfur source for cultivating sulfur-oxidizing bacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a toxic and harmful gas, H2S containing gas must be effectively desulfurized before being released or used (Sorokin et al. 2008). Biological desulfurization has been widely used due to their advantages of low energy consumption, mild conditions, no secondary pollution, and low cost (Chen et al. 2021). In particular, the biological desulfurization system with high salinity and high alkalinity has the advantages of high processing load and high efficiency and have become a most common biological desulfurization technique (Klok et al. 2012; Hao et al. 2021; Zhou et al. 2014). In the biological desulfurization process, reduced sulfur compounds (such as HS−, S2O32−) are oxidized under the action of sulfur-oxidizing bacteria to generate biosulfur (So) (Janssen et al. 1999; Kiragosyan et al. 2020). Biosulfur is the main by-product of biological desulfurization. Due to the lack of understanding of the physicochemical properties and structural characteristics of biosulfur, no ideal way to use it has been proposed to date. In some cases, the sulfur obtained from biological desulfurization is incinerated or landfilled, thus becoming a new source of sulfur environmental pollution. Therefore, it is necessary to conduct an in-depth study on the characteristics of biosulfur to find an ideal way to use this sulfur.
Although it is understood that the biosulfur produced in the biological desulfurization process has better hydrophilicity and is not easy to cause blockage of pipelines (van den Bosch et al. 2007), the detailed reason has not been clearly explained. However, fortunately, studies related to the biosulfur derived from other sulfur-oxidizing bacteria have been conducted (Maki 2013; Steudel 1996). Prange et al. studied the speciation of sulfur of metabolically different sulfur-accumulating bacteria in situ with X-ray absorption near edge structure (XANES) spectroscopy at the sulfur Kedge. It revealed at least three different forms of sulfur in biosulfur globules. Cyclooctasulfur dominates in the sulfur globules of Beggiatoa alba and Thiomargarita namibiensis. A second type of sulfur globules is present in Acidithiobacillus ferrooxidans: here the sulfur occurs as polythionates. In contrast, in purple and green sulfur bacteria the sulfur mainly consists of sulfur chains (Prange et al. 2002, 1999). However, it can be noted that there were different views on the speciation of biosulfur. For example, He et al. found that Acidithiobacillus ferrooxidans and Acidithiobacillus caldus can accumulate extracellular sulfur globules when grown on thiosulfate, and the major sulfur speciation of which were S8 for A. ferrooxidans and mixture of ring sulfur and polythionate for A. caldus, respectively (He et al. 2010).
Marnocha et al. studied the mineralogy and surface chemistry of extracellular biosulfur globules produced by Chlorobaculum tepidum. It was found that biosulfur globules produced by C. tepidum and abiotic sulfur sols are quite similar in terms of mineralogy and material properties, but the two are distinguished primarily by the properties of their surfaces. C. tepidum’s globules are enveloped by a layer of organics, which appears to slow the aging and crystallization of amorphous sulfur. (Marnocha et al. 2019, 2016). At present, the clearest research on the organic layer of sulfur particles is the intracellular sulfur particles from Allochromatium vinosum. The sulfur globules in the Allochromatium vinosum are enclosed by a protein envelope. This envelope is a monolayer of 2–5 nm consisting of three different hydrophobic Sgps of 10.5, 10.6 (SgpA and SgpB) and 8.5 kDa (SgpC) (Brune 1995; Pattaragulwanit et al. 1998). In bacteria forming extracellular sulfur globules, Sgps do not appear to be present (Pattaragulwanit et al. 1998, Dahl et al. 2006).
Cron et al. demonstrated that soluble extracellular compounds produced by Sulfuricurvum kujiense are necessary to form and stabilize S(0) minerals outside of the cells. They proposed that sulfur-oxidizing bacteria could use soluble organics to stabilize stores of bioavailable S(0) outside the cells (Cron et al. 2019). This conjecture has been further confirmed by a process called ‘‘S(0) organomineralization”. Cosmidis et al. found that S(0) minerals are produced and stabilized following the oxidation of hydrogen sulfide in the presence of numerous types of dissolved organics. In this process, dissolved organics, such as monosaccharides and amino acids, will form an organic envelope on the surface of elemental sulfur particles through self-assembly, which helps to keep the elemental sulfur in a stable state (Cosmidis et al. 2016, 2019).
The haloalkaliphilic genus Thioalkalivibrio has been widely used in biological desulfurization (Muyzer et al. 2011; Mu et al. 2016, Pokorna et al. 2015). However, there has been no relevant report on whether the biosulfur globules produced by genus Thioalkalivibrio have the above-mentioned similar structure. In this study, Tav. versutus D301 was used as a research object (Mu et al. 2016; Sharshar et al. 2019, 2020), and the focus was on an analysis of the surface chemical characteristics and bioavailability of sulfur globules. We expected to obtain a more in-depth understanding of this by-product of industrial desulfurization in order to find a reasonable way to use it at commercial scale.
Materials and methods
Strains and medium
In this study, four species of bacteria were used, including haloalkaliphilic sulfur-oxidizing bacteria (Thioalkalivibrio versutus D301, Thiomicrospira microaerophila BDL05, and Thioalkalibacter halophilus BDH06) and acidophilic sulfur-oxidizing bacteria (Acidithiobacillus thiooxidans ATCC 19377 T). Haloalkaliphilic sulfur-oxidizing bacteria were cultured in TD medium or FTD medium. The composition of TD medium is as follows: Na2S2O3·5H2O 19.92 g/L, NaHCO3 58.8 g/L, NaOH 5 g/L, NH4Cl 0.268 g/L, KNO3 0.505 g/L, K2HPO4·3H2O 2 g/L, MgCl2·6H2O 0.1 g/L and a trace element solution as described in Pfenning and Lippert (Pfennig and Lippert 1966). Compared with TD medium, the only difference is that there is no Na2S2O3 in FTD medium. The pH of the medium was 9.5 ± 0.05 at 30 °C (Liu et al. 2021). A. thiooxidans was grown at 30 °C in the Starkey medium containing the following components: (NH4)2SO4 2.0 g/L, KH2PO4 3.0 g/L, MgSO4·7H2O 0.5 g/L, FeSO4·7H2O 0.001 g/L, and CaCl2·2H2O 0.25 g/L. The pH was adjusted to 2.5 with 2 N H2SO4 (Chen et al. 2013).
Experimental setup and biosulfur collection
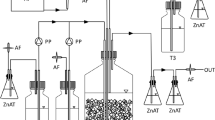
The laboratory setup consisted of a counter-current falling film gas absorber (15-L) for the removal of H2S, a bioreactor (50-L) for the production of elemental sulfur and a settling tower (10-L) for the separation of biosulfur globules (Fig. 1) (Mu et al. 2021). Tav. versutus D301 was firstly inoculated in the shake flasks with TD medium and cultured around 30 h at 30 °C and 180 rpm. Then, the cultures of 2 L were inoculated into bioreactor filled with FTD medium. The sulfur source used in the bioreactor came from the H2S absorbed. The feed gas was composed of 99.0% N2 (v/v) and 1.0% H2S (v/v) with 99.99 vol.% purity (Beijing Zhaoge Gas Technology Co., Ltd.). The dosing rate of feeding gas was regulated based on signals from an ORP sensor located in bioreactor. The ORP set-point was chosen at -380 mV to suppress sulfate formation (Mu et al. 2021; Klok et al. 2012). A digital gear pump was used to assure liquid recirculation between the settling tower and the gas absorber at a constant flow of 15 L h−1. The air compressor continuously provided oxygen to the bioreactor. The sulfur particles collected at 36 h and 84 h after inoculation were used to study the characteristics of the initial formation and aggregation period of sulfur particles, respectively. After stopping the supply of H2S for 40 h, the sulfur particles in the bioreactor were collected as samples in the degradation period. The collected sulfur particles were treated with ultrasound for 30 s and centrifuged at 2000 rpm for 1 min. The obtained sulfur particles were washed three times with FTD medium for analysis.
Bioavailability experiments of elemental sulfur
The ability of four strains (Tav. versutus D301, Tms. microaerophila BDL05, Tab. halophilus BDH06, and A. thiooxidans ATCC 19377 T) to use chemical sulfur or bio-sulfur as sulfur source was studied. Chemical sulfur here referred to sublimated sulfur, which was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. The biosulfur was the Tav. versutus D301 extracellular sulfur globules obtained from the above laboratory setup. The bacterial pellets of Tav. versutus D301, Tms. microaerophila BDL05 and Tab. halophilus BDH06 were inoculated into 100 ml FTD medium added with 1.0 g chemical sulfur or 1.0 g biosulfur, respectively. Similarly, A. thiooxidans ATCC 19377 T was inoculated into 100 ml Starkey liquid medium with 1.0 g chemical sulfur or 1.0 g biological sulfur. All experiments were carried out at 30 °C and 180 rpm. After 5 days, 2 ml cultures were taken and centrifuged at 1500 rpm for 1 min. The supernatants were used to determine the bacterial optical density with a spectrophotometer at 600 nm.
Morphology analysis of sulfur globules
The biosulfur globules were taken out from the settling tower at 84 h. The sulfur globules were fixed overnight at 4 °C in 2.5% (w/v) glutaraldehyde solution. The samples were rinsed three times with 0.1 M phosphate buffer (pH 7.0) for 15 min each time. The samples were dehydrated with gradient concentrations of ethanol for 15 min, and then dehydrated with anhydrous ethanol twice for 20 min each time. After treated with gradient embedding agent and sliced with ultramicrotomy, the samples were observed by transmission electron microscope (TEM) JEM-2100F (JEOL, Japan) (Kang et al. 2019b). Take a small amount of dry biosulfur globules on the aluminum foil. The sulfur globules were sprayed with gold nanoparticles and observed by cold field emission scanning electron microscope (SEM) 6700F (JEOL, Japan). The wet sulfur globules samples can be directly observed under environmental SEM MLA 250 (FEI, USA). Particle size distribution of biosulfur globules was measured using nanoparticle analyzer DelsaNano C (Beckman Coulter, USA) (Rohit and Pal 2013).
X-ray diffraction (XRD) analysis
Dry chemical sulfur and biosulfur globules were ground into powder by a grinding bowl, and X-ray diffraction analysis (Empyrean, Panalytical B.V., Netherlands) was then performed through a 300-mesh screen. The scanning angle and speed were 10–90 degree and 0.05 degree/s, respectively. In addition, elemental analyses of the chemical sulfur particles and biosulfur globules were carried out using element analyzer EA 1108 (Carlo Erba, Italy).
Spectroscopic analysis
Raman spectroscopy was conducted using WITec, a scanning near-field optical microscope that incorporates a confocal Raman spectroscopy imaging (MonoVista CRS, S&I, Germany). Objective lenses used included a 100 × and 60 × long working distance. Spectra were collected over the range 0 to 3600 cm−1 and averaged using 1 s integration time. The lateral resolution of the instrument is about 380 nm, with a focal plane depth of ~ 700 nm.
Infrared spectra were obtained using a NICOLET iS 50 spectrometers (Thermo Scientific, USA) using an Attenuated Total Reflectance (ATR) attachment. Spectra were collected from 400 to 4000 cm−1 at a 1 cm−1 scan resolution. Contributions from atmospheric CO2 and H2O were subtracted from spectra using a correction within the Spectrum software. Sulfur particles suspended in deionized water was centrifuged into a pellet, then dried down onto a slide and collected as a powder for analysis on the instrument.
Extraction and characterization of the organic layer of the biosulfur globules
The organic layer was extracted from the biosulfur globules by mixing EDTA solution (150 mL of 68 mM EDTA solution) with 2.0 g biosulfur globules on an orbital shaker at 200 rpm for 3 h at 4 °C. The EDTA-globules solution was then centrifuged at 12000 rpm for 20 min at 4 °C. The supernatant, containing the released organic layer was collected and stored prior to analysis at -20 °C (Bourven et al. 2012). The organic layer sample was hydrolyzed in 2 mol trifluoroacetic acid at 100 °C for 4 h. The hydrolysate was analyzed by high performance anion exchange chromatography (Dionex ics-3000, USA) on PA-1 column.
Confocal laser scanning microscopy
The samples containing biological sulfur particles (1.0 ml) were centrifuged and the supernatant was removed. 200 μ l of 0.1 M sodium bicarbonate was added to the precipitation and mixed. The supernatant was removed by centrifugation. Then, 200 μ l of 0.85% (w/v) saline solution was added to the precipitation and mixed. The fluorescent dyes of 0.4 μL fluorescein isothiocyanate isomer (FITC; Sigma-Aldrich, Germany) or 1.0 μL tetramethyl rhodamine isothiocyanate-labeled lectin concanavalinA (TRITC-ConA; Biological life sciences, USA) or 0.6 μL 4,6-diamidino-2-Phenylindoldihydrochloride (DAPI; Invitrogen, USA) was added to the sample and incubated in a dark room for 30 min. After incubation, the supernatant was removed after centrifugation, and then washed three times with 0.85% (w/v) saline solution. The obtained sample can be used for CLSM observation. Microscopic analysis was performed using the epifluorescence microscope Zeiss (Carl Zeiss AG, Germany) with the appropriate filter blocks. From each sample, at least 10 digital images were taken using × 100 and × 60, 1.4 NA phase-contrast objective lens (Kimani et al. 2016).
Results
Characteristic analysis of biosulfur globules
The shape and particle size of the biosulfur globules collected from the settling out of a biological desulfurization system were studied. Due to the ultrasonic dispersion of the sample during pretreatment, the biosulfur globules exhibited good dispersibility (Fig. 2a). In fact, the biosulfur globules could aggregate to form millimeter-sized particles, allowing the biosulfur to have a good settling performance. As shown from observation by TEM, the biosulfur globules had an approximately circular shape, with different sizes, and the particle size distribution was over a wide range (0.2–5 μm) (Fig. 2b). As the volume of the sulfur globules increased, their quantity decreased. It was speculated that the large-volume sulfur globules were formed by the aggregation of small-volume sulfur globules, which could be observed by TEM imaging with higher magnification (Fig. 2c). Moreover, it was found that during the aggregation process, the small sulfur globules had obvious morphological changes, indicating that the biosulfur globules in the solution were very deformable, which was contrary to the chemical sulfur.
X-ray diffraction (XRD) and Raman spectroscopy were used to analyze the existing form of sulfur in biosulfur globules. The XRD pattern matched well with the JCPDS database diffraction pattern. The highest peaks of the XRD patterns of biosulfur and chemical sulfur both appeared at a diffraction angle of 22.94°-23°. The difference between the two was that the peak values of biosulfur at all positions were all slightly lower than those of chemical sulfur (Fig. 3a). These results indicated that the sulfur element in the biosulfur globules mainly existed in the form α-S8, which was consistent with chemical sulfur, but its crystallinity was lower (Marnocha et al. 2019). In the Raman spectra, the biosulfur globules were characterized by two strong peaks at 152 cm−1 and 216 cm−1, corresponding to the bending and stretching modes of a folded 8 membered ring, respectively, and the third strong peak at 473 cm−1 corresponding to the vibration of an S–S bond (Fig. 3b), indicating that the S8 form was present in biosulfur globules (Marnocha et al. 2019; Nims et al. 2019), which was consistent with the XRD results mentioned above.
Characterization of the organic layer of biosulfur globules
Environmental SEM and cold-field emission SEM were used to study the surface characteristics of biosulfur globules. As can be seen from the observations, a layer was found on the surface of both wet and dry biosulfur globules (Fig. 4a, b). The surface of the wet biosulfur globules seemed to be covered with a layer, and the surface layer formed wrinkles after drying (Fig. 4b). Thin slices of these biosulfur globules were next observed. There were clear boundaries between the aggregated small sulfur globules (Fig. 2c), which should have been caused by the presence of the layer. It was also found that sulfur globules of different particle sizes have layer, and it could be inferred that the layer of the biosulfur globules was gradually formed outside the cell. This was the first time that the layer of extracellular sulfur globules produced by haloalkaliphilic sulfur-oxidizing bacteria have been observed.
In order to study the composition of the layer of biosulfur globules, firstly, the proportions of C, N, S, and H in these biosulfur globules and chemical sulfur particles were compared. The results indicated that compared to chemical sulfur with more than 98% sulfur, the elemental composition of biosulfur was more abundant. Among them, the proportions of S, N, C, and H were 82.110%, 3.539%, 12.696%, and 0.924%, respectively. The above findings showed that, in addition to being composed of sulfur, the biosulfur globules also contained a certain amount of organic matter. Fourier infrared spectroscopy was used to analyze the chemical groups on the surface of these biosulfur globules. The surface of chemical sulfur was covered by few functional groups (Fig. 4c). The surface of the biosulfur globules contained a large number of functional groups, such as hydroxyl (-OH, 3471 cm−1, 3421 cm−1, 3275 cm−1), amide I (1616 cm−1, 1634 cm−1), amide II (1398–1542 cm−1), alkyl groups (–CH3 and –CH2, 2921 cm−1, 2853 cm−1, 1457 cm−1), and carboxylic groups (COO–, 1398 cm−1), as well as groups related to polysaccharides (C–C and C–O, 900–1130 cm−1). This finding indicates that proteins and polysaccharides may be the main components of the organic layer of these sulfur globules.
Subsequently, the composition and changes of the organic layer of Tav. versutus D301 extracellular sulfur globules were studied with protein, polysaccharide, and nucleic acid specific fluorescent probes. Using aggregated biosulfur globules as a research object, the samples during the initial period, accumulation period, and degradation period of biosulfur globules were taken for staining and observation. The results showed that at the initial period of the formation of biosulfur globules, the globules showed yellow fluorescence, indicating that polysaccharides were dominant in the organic layer of these sulfur globules (Fig. 5a). With the aggregation of sulfur globules and the increase in particle size, most of the small globules aggregated to form large globules of different sizes. Polysaccharides and proteins components covered the outer layer of the sulfur globules and overlapped, causing a certain interference between the two types of fluorescence signals and producing the fluorescence suppression phenomenon (Fig. 5b). In the late period of cultivation, a large number of sulfur particles were formed by aggregation, and there were more biological macromolecules attached to the surface, and in particular, the proteins content increased (Fig. 5c). In addition, with the accumulation of biosulfur globules, the fluorescence intensity became stronger, indicating that the organic layer continued to accumulate with the extension of culture time, and the organic layer gradually became thicker. The combination of DAPI and nucleic acid showed a blue color. According to the images, there was a very low amount of nucleic acid on the surface of these biosulfur globules. We identified the polysaccharide components in the organic layer of the sulfur globules and found that they were mainly composed of D-( +)-glucuronic acid (1.8323 mg/g), D-( +)-glucose (0.0043 mg/g), and D-(-)-arabinose (0.0016 mg/g), among which glucuronic acid was dominant, accounting for 64% of the total sugar content.
Fluorescence staining analysis of the organic layer of biosulfur globules in different cultivation stages. Figure 4a, b, and c correspond to the initial period, the accumulation period, and the degradation period of sulfur particles, respectively. The dye c860 combines with polysaccharides, in yellow; FITC reacts with proteins, in green; DAPI combines with nucleic acids, in blue. The scale bar is 2 μm
Bioavailability of Tav. versutus D301 biosulfur globules
With chemical sulfur particles as a control, the utilization of Tav. versutus D301 biosulfur globules by four species of sulfur-oxidizing bacteria as sulfur sources were studied. The optical density (OD) value after 96 h of cultivation showed that all four sulfur-oxidizing bacteria could use the biosulfur globules as electron donors to obtain growth energy, as shown in Fig. 6a. In comparison, only A. thiooxidans ATCC 19377T could efficiently use chemical sulfur, but the growth rate was only about half of that using biosulfur globules. Haloalkaliphilic sulfur-oxidizing bacteria showed a poor capability to use chemical sulfur, especially Tms. microaerophila BDL05 and Tab. halophilus BDH06, both of which could hardly use chemical sulfur. We observed the morphological changes of two sulfur sources during Tav. versutus D301 cultivation. After the sulfur element in the biosulfur globules were almost completely utilized, only the organic layer of the sulfur globule remained, which also confirmed the existence of the organic layer of the sulfur globules (Fig. 6b). Meanwhile, the chemical sulfur particles only showed some shallow pits on the surface. As can be seen from the magnified image, bacteria could aggregate in the pits, indicating that these pits were formed by bacteria using sulfur (Fig. 6c). Thus, biosulfur globules can be used as a sulfur source that is easily used to cultivate sulfur-oxidizing bacteria.
Bioavailability analysis of biosulfur globules. a The growth of Tav. versutus D301, Tms. microaerophila BDL05, Tab. halophilus BDH06 and A. thiooxidans ATCC 19377 T using chemical sulfur and biosulfur. In each group, the light color represents the use of chemical sulfur, and the dark color represents the use of biosulfur. b SEM imaging of residues after biosulfur was utilized. c, d SEM images of the residue after chemical sulfur was utilized (scale bar, 1 μm)
Discussion
Existing forms of sulfur in biosulfur globules
Although haloalkaliphilic sulfur-oxidizing bacteria have been used in biological desulfurization systems and have achieved good results, there is still a lack of understanding of the extracellular biosulfur globules. This paper carried out a study on the characteristics of the extracellular sulfur globules produced by Tav. versutus D301. To date, numerous forms of sulfur have been found in various sulfur-oxidizing bacteria, such as Acidithiobacillus ferrooxidans (He et al. 2010), Chlorobaculum tepidum (Marnocha et al. 2019), and Allochromatium vinosum (Franz et al. 2009). Among them, sulfur atoms are easily linked into linear or cyclic molecules, including S8, Sn2−, –O3S–Sn–SO3–, and R–Sn–R (Kleinjan et al. 2003; George et al. 2008). This study used Raman spectroscopy combined with XRD to confirm that the sulfur in the biosulfur globule produced by Tav. versutus D301 existed in the form α-S8, which was similar to chemical sulfur. Due to the significantly better bioavailability of these biosulfur globules, the crystallinity of the biosulfur globules may be lower than that of chemical sulfur, which could be confirmed by the changeable shape of the biosulfur globules. It has been reported that the crystallinity of biosulfur globules will continue to change (Hanson et al. 2015, Marnocha et al. 2019, Cosmidis et al. 2016). Fresh biosulfur globules will gradually age, and the sulfur crystallinity gradually increases. After drying or aging, they will be converted into crystalline orthorhombic sulfur (S8).
The organic layer characteristics of biosulfur globules
Tav. versutus D301 biosulfur globules will aggregate to form millimeter-sized particles, allowing the biosulfur globules to have a good settling performance that can be easily separated from a desulfurization solution (Sharshar et al. 2020). After ultrasonic treatment, it was observed that the individual biosulfur globules of Tav. versutus D301 were an approximately circular shape, with different sizes, and micron-sized sulfur globules accounted for the majority these. We found that large-volume sulfur globules were formed by the aggregation of small-volume sulfur globules, and from the cross section of the sulfur globules, it could be observed that the outer layer of the small particles had clear boundaries with a dark color. Therefore, we speculate that the surface of the biosulfur globules were covered with an organic layer. Li et al. proposed the “outer membrane vesicles (OMVs) sulfur secretion” hypothesis (Li et al. 2020). This hypothesis assumes that elemental sulfur is formed in the periplasmic space of sulfur-oxidizing bacteria, and the outer membrane of the cell wall envelopes elemental sulfur to form vesicles, and finally, these vesicles fall off from the cell wall. Based on this, it has been inferred that when being secreted from a cell, these extracellular biosulfur globules already have the structure of an outer membrane.
Using FT-IR analysis, it was found that the surface of these biosulfur globules contained functional groups, such as carboxyl and amide groups. We speculated that the organic layer was composed of organic matter such as proteins or polysaccharides. The organic layer of Tav. versutus D301 sulfur globules were labeled with polysaccharide, protein, and nucleic acid specific fluorescent probes. Unlike in the literature which has reported that the surface of intracellular sulfur particles formed by A. vinosum were covered with SgpABC protein (Dahl and Prange 2006), the organic layer of Tav. versutus D301 extracellular sulfur globules contained large amounts of polysaccharides. In addition, it was found that the composition of the organic layer of Tav. versutus D301 sulfur globules could change with an extension of the cultivation time. In the early stage of sulfur globules formation, the organic layer was dominated by polysaccharides, while in the late stage, protein became the main component of sulfur globules. With an extension of the cultivation time, the organic matter in the organic layer continued to accumulate, which also seems to indicate that the organic layer of sulfur particles was mainly formed by the passive adsorption of organic matter secreted by cells. This seemed to be similar to the organomineralization process of elemental sulfur discovered by Cosmidis (Cosmidis et al. 2016, 2019).
Bioavailability of biosulfur globules
The difference in the utilizations of Tav. versutus D301 biosulfur globules and chemical sulfur particles by haloalkaliphilic sulfur-oxidizing bacteria and acidophilic sulfur-oxidizing bacteria was also compared here. The four species of sulfur-oxidizing bacteria could efficiently use these biosulfur globules, and they did not have any species differences in the source of biosulfur globules. Therefore, the biosulfur produced by this haloalkaliphilic biological desulfurization system was an ideal sulfur source, which could be potentially used in the pre-cultivation of sulfur-oxidizing bacteria to greatly increase the growth rate of these strains. For the utilization of chemical sulfur particles, the four species of sulfur-oxidizing bacteria studied all showed poor vitality, especially Tms. microaerophila BDL05 and Tab. halophilus BDH06, both of which could hardly use chemical sulfur particles. Through observing the process of Tav. versutus D301 using two sulfur sources, we found that the bacteria did not firmly adhere to the biosulfur particles during the utilization process. As reported in the literature, the process of bacteria using biosulfur globules can proceed without contact or with only intermittent contact with sulfur globules (Marnocha et al. 2016). However, Tav. versutus D301 must first adsorb before using the chemical sulfur particles, which was demonstrated by the accumulation of cells in the pits on the surface. The reason why Tav. versutus D301 was slow to use chemical sulfur may be related to its dense structure and high crystallinity. In contrast, the formation of an organic layer on the surface of the biosulfur globule hinders the crystallization process of sulfur particles, and endowing these sulfur globules with a hydrophilic surface, which improves the bioavailability of these sulfur particles (Hanson et al. 2015).
Conclusion
The sulfur element in the biosulfur globules produced by Tav. versutus D301 existed in the form α-S8, which was similar to chemical sulfur. Micron-sized biosulfur globules were formed by the aggregation of smaller globules. The biosulfur globule was wrapped with an organic layer composed of polysaccharides and proteins. The composition of the organic layer of Tav. versutus D301 sulfur globules could change with an extension of the cultivation time. In the early stage of the formation of these sulfur globules, the organic layer was dominated by polysaccharides, and in later stages, protein became the main component of the layer of these sulfur globules. With an extension of the cultivation time, the organic matter in the layer continued to accumulate. It was speculated that the organic layer of the sulfur globules was mainly formed by the passive adsorption of organic matter secreted by cells. The organic layer formed on the surface of these biosulfur globules improved the bioavailability of sulfur particles. The biosulfur produced by Tav. versutus D301 was an ideal sulfur source, which could be potentially used in the pre-cultivation of sulfur-oxidizing bacteria to greatly increase the growth rate of these strains.
References
Bourven I, Costa G, Guibaud G (2012) Qualitative characterization of the protein fraction of exopolymeric substances (EPS) extracted with EDTA from sludge. Bioresour Technol 104:486–496
Brune DC (1995) Isolation and characterization of sulfur globule proteins from Chromatium vinosum and Thiocapsa roseopersicina. Arch Microbiol 163:391–399
Chen LX, Lin JQ, Liu XM, Pang X, Lin HB, Lin JQ (2013) Transposition of IS elements induced by electroporation of suicide plasmid in Acidithiobacillus caldus. Enzyme Microb Technol 53(3):165–169
Chen Z, Yang G, Hao XM, Nadia AS, Jia YP, Sumit P, Mu TZ, Yang MH, Xing JM (2021) Recent advances in microbial capture of hydrogen sulfide from sour gas via sulfur-oxidizing bacteria. Eng Life Sci 21:693–708
Cosmidis J, Templeton AS (2016) Self-assembly of biomorphic carbon/sulfur microstructures in sulfidic environments. Nat Commun 7:12812
Cosmidis J, Nims CW, Diercks D, Templeton AS (2019) Formation and stabilization of elemental sulfur through organomineralization. Geochim Cosmochim Ac 247:59–82
Cron B, Henri P, Chan CS, Macalady JL, Cosmidis J (2019) Elemental sulfur formation by Sulfuricurvum kujiense is mediated by extracellular organic compounds. Front Microbiol 10:2710
Dahl C, Prange A (2006) Bacterial sulfur globules: occurrence, structure and metabolism. Inclusions in Prokaryotes. Springer, New York, pp 21–51
Franz B, Gehrke T, Lichtenberg H, Hormes J, Dahl C, Prange A (2009) Unexpected extracellular and intracellular sulfur species during growth of Allochromatium vinosum with reduced sulfur compounds. Microbiology 155:2766–2774
George GN, Gnida M, Bazylinski DA, Prince RC, Pickering IJ (2008) X-ray absorption spectroscopy as a probe of microbial sulfur biochemistry: the nature of bacterial sulfur globules revisited. J Bacteriol 190(19):6376–6383
Hanson TE, Bonsu E, Tuerk A, Marnocha CL, Powell DH, Chan CS (2015) Chlorobaculum Tepidum growth on biogenic S(0) as the sole photosynthetic electron donor. Environ Microbiol 18(9):2856–2867
Hao XM, Mu TZ, Sharshar MM, Yang MH, Zhong W, Jia YP, Chen Z, Yang G, Xing JM (2021) Revealing sulfate role in empowering the sulfur-oxidizing capacity of Thioalkalivibrio versutus D301 for an enhanced desulfurization process. Bioresour Technol 337(3):125367
He H, Xia JL, Jiang HC, Yan Y, Liang CL, Ma CY, Zheng L, Zhao YD, Qiu GZ (2010) Sulfur species investigation in extra- and intracellular sulfur globules of Acidithiobacillus ferrooxidans and Acidithiobacillus caldus. Geomicrobiol J 27:707–713
Janssen AJH, Lettinga G, de Keizer A (1999) Removal of hydrogen sulphide from wastewater and waste gases by biological conversion to elemental sulphur: colloidal and interfacial aspects of biologically produced sulphur particles. Colloids Surf A Physicochem Eng Aspects 151(1–2):389–397
Kimani VN, Chen L, Liu YP, Raza W, Zhang N, Mungai LK, Shen QR, Zhang RF (2016) Characterization of extracellular polymeric substances of Bacillus amyloliquefaciens SQR9 induced by root exudates of cucumber. J Basic Microbiol 56:1–11
Kiragosyan K, Picard M, Sorokin DY, Dijkstra J, Klok JBM, Roman P, Janssen AJH (2020) Effect of dimethyl disulfide on the sulfur formation and microbial community composition during the biological H2S removal from sour gas streams. J Hazard Mater 386:121916
Kleinjan WE, Keizer AD, Janssen AJH (2003) Biologically produced sulfur. Top Curr Chem 230:167–188
Klok JBM, van den Bosch PLF, Buisman CJN, Stams AJM, Keesman KJ, Janssen AJH (2012) Pathways of sulfide oxidation by haloalkaliphilic bacteria in limited-oxygen gas lift bioreactors. Environ Sci Technol 46(14):7581–7586
Li BJ, Zhang M, Kang D, Chen WD, Yu T, Xu DD, Zeng Z, Li YY, Zheng P (2020) Mechanisms of sulfur selection and sulfur secretion in a biological sulfide removal (BISURE) system. Environ Int 137:105549
Liu ZX, Yang MH, Mu TZ, Liu JL, Zhang X, Xing JM (2021) Transcriptional response of Thialkalivibrio versutus D301 to different sulfur sources and identification of the sulfur oxidation pathways. J Biotechnol 329:160–169
Maki JS (2013) Bacterial intracellular sulfur globules: structure and function. J Mol Microbiol Biotechnol 23:270–280
Marnocha CL, Levy AT, Powell DH, Hanson TE, Chan CS (2016) Mechanisms of extracellular S(0) globule production and degradation in Chlorobaculum tepidum via dynamic cell-globule interactions. Microbiology 162(7):1125–1134
Marnocha CL, Sabanayagam CR, Modla S, Powell DH, Henri PA, Steele AS, Hanson TE, Webb SM, Chan CS (2019) Insights into the mineralogy and surface chemistry of extracellular biogenic S(0) globules produced by Chlorobaculum tepidum. Front Microbiol 10:271
Mu TZ, Zhou JM, Yang MH, Xing JM (2016) Complete genome sequence of Thialkalivibrio versutus D301 isolated from soda lake in northern China, a typical strain with great ability to oxidize sulfide. J Biotechnol 227:21–22
Mu TZ, Yang MH, Xing JM (2021) Performance and characteristic of a haloalkaliphilic bio-desulfurizing system using Thioalkalivibrio versutus D301 for efficient removal of H2S. Biochem Eng J 165:107812
Muyzer G, Sorokin DY, Mavromatis K, Lapidus A, Clum A, Ivanova N, Pati A, d’Haeseleer P, Woyke T, Kyrpides NC (2011) Complete genome sequence of “Thialkalivibrio sulfidophilus” HL-EbGr7. Stand Genomic Sci 4(1):23–35
Nims C, Cron B, Wetherington M, Macalady J, Cosmidis J (2019) Low frequency Raman spectroscopy for micron-scale and in vivo characterization of elemental sulfur in microbial samples. Sci Rep 9(1):7971
Pattaragulwanit K, Brune DC, Trüper HG, Dahl C (1998) Molecular genetic evidence for extracytoplasmic localization of sulfur globules in Chromatium vinosum. Arch Microbiol 169:434–444
Pfennig N, Lippert KD (1966) Vitamin B12-Bedürfnis phototropher Schwefelbakterien. Arch Mikrobiol 55(3):245–256
Pokorna D, Zabranska J (2015) Sulfur-oxidizing bacteria in environmental technology. Biotechnol Adv 33(2):1246–1259
Prange A, Arzberger I, Engemann C, Modrow H, Schumann O, Trüper HG, Steudel R, Dahl C, Hormes J (1999) In situ analysis of sulfur in the sulfur globules of phototrophic sulfur bacteria by X-ray absorption near edge spectroscopy. BBA-Gen Subjects 1428:446–454
Prange A, Chauvistré R, Modrow H, Hormes J, Trüper H, Dahl C (2002) Quantitative speciation of sulfur in bacterial sulfur globules: X-ray absorption spectroscopy reveals at least three different species of sulfur. Microbiology 148:267–276
Rohit B, Pal KI (2013) Pharmacokinetics, tissue distribution and relative bioavailability of isoniazid-solid lipid nanoparticles. Int J Pharm 441:202–212
Sharshar MM, Nadia AS, Hao XM, Mu TZ, Zhong W, Yang MH, Sumit P, Ambreen S, Xing JM (2019) Enhanced growth-driven stepwise inducible expression system development in haloalkaliphilic desulfurizing Thioalkalivibrio versutus. Bioresour Technol 288:121486
Sharshar MM, Nadia AS, Ambreen S, Hao XM, Mu TZ, Zheng C, Gao Y, Liu ZX, Jia YP, Li XY, Zhong W, Sumit P, Yang MH, Xing JM (2020) Improving confirmed nanometric sulfur bioproduction using engineered Thioalkalivibrio versutus. Bioresour Technol 317:124018
Sorokin DY, van den Bosch PLF, Abbas B, Muyzer G (2008) Microbiological analysis of the population of extremely haloalkaliphilic sulfur-oxidizing bacteria dominating in lab-scale sulfide-removing bioreactors. Appl Microbiol Biotechnol 80(6):965–975
Steudel R (1996) Mechanism for the formation of elemental sulfur from aqueous sulfide in chemical and microbiological desulfurization processes. Ind Eng Chem Res 35(4):1417–1423
van den Bosch PLF, van Beusekom OC, Buisman CJN, Janssen AJH (2007) Sulfide oxidation at halo-alkaline conditions in a fed-batch bioreactor. Biotechnol Bioeng 97(5):1053–1063
Zhou JM, Song ZY, Yan DJ, Liu YL, Yang MH, Xing CHB, JM, (2014) Performance of a haloalkaliphilic bioreactor and bacterial community shifts under different COD/SO42− ratios and hydraulic retention times. J Hazard Mater 274:53–62
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFA0906800) and National Science Foundation of China (31872633, 21878307, 31800030).
Author information
Authors and Affiliations
Contributions
ZL: methodology, data curation, and writing. MY: conceptualization, writing- review and editing, supervision, and funding acquisition. TM: methodology and data curation; JL: conceptualization, supervision, writing-review and editing; LC: methodology and data curation; DM: methodology and data curation; JX: conceptualization, supervision and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Communicated by Oren.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Yang, M., Mu, T. et al. Organic layer characteristics and microbial utilization of the biosulfur globules produced by haloalkaliphilic Thioalkalivibrio versutus D301 during biological desulfurization. Extremophiles 26, 27 (2022). https://doi.org/10.1007/s00792-022-01274-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00792-022-01274-z