Abstract
Fifty-six thermophilic strains including members of Caldanaerobacter, Caldicellulosiruptor, Caloramator, Clostridium, Thermoanaerobacter, and Thermoanaerobacterium, were investigated for branched-chain amino acid degradation in the presence of thiosulfate in batch culture. All of the Thermoanaerobacter and Caldanaerobacter strains (24) degraded the branched-chain amino acids (leucine, isoleucine, and valine) to a mixture of their corresponding branched-chain fatty acids and branched-chain alcohols. Only one Caloramator strain degraded the branched-chain amino acids to the corresponding branched-chain fatty acids. The ratio of branched-chain fatty acid production over branched-chain alcohol production for Thermoanaerobacter was 7.15, 6.61, and 11.53 for leucine, isoleucine, and valine, respectively. These values for Caldanaerobacter were 3.49, 4.13, and 7.31, respectively. This indicates that members within Caldanaerobacter produce proportionally more of the alcohols as compared with Thermoanaerobacter. No species within other genera investigated produced branched-chain alcohols from branched-chain amino acids in the presence of thiosulfate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermophilic species within Class Clostridia from the genera of Caldicellulosiruptor, Caldanaerobacter, Clostridium, Thermoanaerobacter, and Thermoanaerobacterium have been intensively investigated in the context of biofuel production due to their broad substrate spectrum, especially among the sugars present in lignocellulosic biomass, and because of their high ethanol and hydrogen yields (Taylor et al. 2009; Ren et al. 2009; Chang and Yao 2011; Scully and Orlygsson 2015). The protein and amino acid catabolism of thermophilic Clostridia has received considerably less attention likely due to the abundance of lignocellulosic biomass. However, several investigations have been performed mainly focusing upon the thermodynamics of the catabolism and end-product formation from specific amino acids (Elsden and Hilton 1978; Barker 1981; McInerney 1988; Orlygsson et al. 1994; 1995; Faudon et al. 1995; Fardeau et al. 1997). Thermoanaerobes can catabolize amino acids by oxidative and/or reductive deamination and decarboxylation (Elsden and Hilton 1978; Andreesen et al. 1989; Orlygsson et al. 1995). Thus, the oxidative fermentation of amino acids yields the corresponding α-keto acid in the first step which is thereafter decarboxylated to one carbon shorter volatile fatty acid. For instance, alanine is deaminated to pyruvate, which is then decarboxylated to acetate.
The amino acids that undergo oxidative deamination usually have reduced oxidation states, including the branched-chain amino acids (BCAAs), alanine, and glutamate (Andreesen et al. 1989; Fardeau et al. 1997). These amino acids can only be fermented if the electrons produced from the initial deamination step are scavenged due to the unfavorable thermodynamics involved (Orlygsson et al. 1995; Fardeau et al. 1997). For instance, the ∆G°′ for the degradation of the BCAAs is between +4.2 and +9.7 kJ/mol (Fardeau et al. 1997). The addition of thiosulfate (which can be reduced to H2S or S 0) to the medium or co-cultivating the amino acid degrader with hydrogen scavenging bacteria, such as a methanogen or sulfate reducer, drives the reaction forward resulting in the amino acids being degraded to their corresponding branched-chain fatty acids (BCFA) in addition to CO2 and ammonium (Fardeau et al. 1997).
The degradation of BCAAs to their corresponding BCFAs by Thermoanaerobacter brockii has been investigated in some detail (Fardeau et al. 1997). Under hydrogen scavenging conditions, leucine, isoleucine, and valine are degraded to 3-methylbutyrate, 2-methylbutyrate, and 2-methylpropionate, respectively. A recent investigation in our laboratory demonstrated that T. brockii (DSM 1457) and Caldanaerobacter subterraneus subsp. yonseiensis (DSM 13777) degrade BCAAs to a mixture of the corresponding BCFAs and branched chain alcohols (BCOHs) when cultivated in the presence of thiosulfate but only to the corresponding BCFAs when co-cultured with a hydrogenotrophic methanogen (Scully and Orlygsson 2014).
The majority of studies on the catabolism of the BCAAs have focused on aerobic bacteria such as species of Staphylococcus and Enterococcus (Beck et al. 2004; Ward et al. 1999), aerotolerant anaerobes including Lactobacillus sakei (Gutsche et al. 2012), or yeasts that use the so-called Ehrlich pathway (Ehrlich 1907; Hazelwood et al. 2008). These studies have often focused on the formation of compounds that contribute to the flavor profile of foods and beverages (branched-chain and aromatic aldehydes, alcohols, and acids) (Smit et al. 2005, 2009). Recently, some studies have focused on the production of branched-chain alcohols from protein-rich waste using genetically engineered Escherichia coli and Bacillus subtilis with the main focus being that BCOHs are promising biofuel candidates (Huo et al. 2011; Choi et al. 2014). Additionally, BCOHs can be used as building blocks for the production of higher chemicals (Sakuragi et al. 2011).
In this study, the production of BCOHs from the BCAAs was investigated within various genera of thermophilic bacteria to gain a broader knowledge whether this phenomenon is genus specific within the genera of Thermoanaerobacter and Caldanaerobacter. Thus, the present investigation examines the ability of thermophilic anaerobes from several genera to produce BCOHs from BCAAs. Forty-eight (48) thermophilic anaerobic bacteria within Class Clostridia isolated from various hot springs in Iceland were investigated. Additionally, eight type strains from other thermophilic genera within Class Clostridia were used for comparison.
Materials and methods
Medium and cultivation
The BM medium consisted of (per liter): NaH2PO4 2.34 g, Na2HPO4 3.33 g, NH4Cl 2.2 g, NaCl 3.0 g, CaCl2 8.8 g, MgCl2·6H2O 0.8 g, yeast extract 2.0 g, resazurin 1 mg, trace element solution 1 ml, vitamin solution (DSM141) 1 ml, and NaHCO3 0.8 g. The trace element solution consisted of the following on a per liter basis: FeCl2·4H2O 2.0 g, EDTA 0.5 g, CuCl2 0.03 g, H3BO3, ZnCl2, MnCl2·4H2O, (NH4)Mo7O24, AlCl3, CoCl2·6H2O, NiCl2, and 0.05 g, Na2S·9H2O 0.3 g, and 1 mL of concentrated HCl. Carbon and energy sources were 20 mM. The medium was prepared by adding the buffer to distilled water containing resazurin and then boiled for 10 min and cooled under nitrogen flushing. The mixture was then transferred to serum bottles using the Hungate technique (Hungate 1969) and autoclaved for 60 min. All other components of the medium were added separately through filter (0.45 µm) sterilized solutions. All experiments were conducted at 65 °C and at pH 7.0. In all cases, experiments were performed in duplicate.
Bacterial strains
Forty-eight (48) strains from our culture collection (hereafter called AK strains) used in this investigation were isolated as previously described on various carbon sources (Orlygsson and Baldursson 2007; Orlygsson et al. 2010). Eleven strains belong to the genus Thermoanaerobacter, 9 to Caldanaerobacter, 14 to Thermoanaerobacterium, 7 to Clostridium, 4 to Caloramator and 3 to Caldicellulosiruptor. Additionally, the following strains were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ): Thermoanaerobacter wiegelii (DSM 10319), Thermoanaerobacter thermohydrosulfuricus (DSM 567), Thermoanaerobacter ethanolicus (DSM 2246), Caldanaerobacter subterraneus subspecies subterraneus (DSM 13054), Caldicellulosiruptor saccharolyticum (DSM 8903), Caldicellulosiruptor owensis (DSM 13100), Thermoanaerobacterium saccharolyticum (DSM 571), and Caloramator viterbiensis (DSM 13723).
Screening for branched-chain fatty acid and branched-chain alcohol formation
All strains (AK and DSM) were screened for branched-chain amino acid degradation. The strains were inoculated from frozen (−20 °C) cultures stored in 30 % (v/v) glycerol and reactivated on BM medium containing glucose (20 mM). Reactivated cultures were inoculated (2 % v/v) from exponential growth phase to 25 mL serum bottles (liquid–gas ratio was 1:1) containing a mixture of leucine, isoleucine, and valine (9 mM each) in the presence of thiosulfate (20 mM). Cultures were grown for 5 days and thereafter 1 mL of liquid and 0.2 mL of gases were removed for analysis of end-products.
Production of branched-chain fatty acids and branched-chain alcohols from individual branched-chain amino acids in the presence of thiosulfate
All strains showing positive growth on BCAAs in the presence of thiosulfate from the initial screening experiment (mixture of BCAAs) were cultivated in 25 mL serum bottles (L–G ratio 1:1) on 20 mM of individual BCAAs (leucine, isoleucine, and valine) containing 20 mM of thiosulfate.
Analytical methods
Hydrogen was analyzed using a Perkin Elmer gas chromatograph equipped with a thermo conductivity detector. Nitrogen was used as carrier gas at a rate of 3 ml/min, with another 10 ml/min as make-up gas in the detectors. The separation was performed on a Supelco 1010 Carboxen CC Plot Capillary Column. The oven temperature was 80 °C and the injector and detector temperatures were 200 °C. Volatile fatty acids and alcohols were analyzed by gas chromatograph (Perkin Elmer Clarus 580) using a FID detector with 30 m DB-FFAP capillary column (Agilent Industries Inc, Palo Alto, CA, US). Samples (1 mL) were centrifuged for 20 min at 6000g. The supernatants were acidified with 25 % formic acid, and crotonic acid was used as the internal standard. Thiosulfate was analyzed by the method of Westley (1987) with modifications as described by Scully and Orlygsson (2014); the background interference was measured by adding 10 µL of 0.25 M KCN and 10 µL of dH2O to 180 µL of sample; after mixing at 1000 rpm for 20 s, 100 µL of Sorbo’s ferric nitrate reagent (10 % w/v Fe(NO3)3·9H2O and 20 % v/v HNO3) was added. Samples were allowed to clarify and the absorbance read at 460 nm against a water blank. The analyte was then determined by repeating the procedure and substituting 10 µL of 0.20 M CuSO4 for dH2O. Thiosulfate concentrations were calculated as the background absorbance–analyte against a standard curve generated using thiosulfate concentrations between 0.1 and 10 mM. Amino acids were analyzed using the ninhydrin method by adding 100 µL of a sample and 100 µL of 1 % (w/v) ninhydrin reagent (60 % v/v 2-propanol and 40 mM acetate buffer, pH 5.5) in a microtiter plate and incubated at 100 °C for 20 min; relevant amino acids (leucine, isoleucine, and valine) were used as standards. After cooling to ambient temperature, 200 µL of 50 % (v/v) 2-propanol was added and the absorbance read at 580 nm on a Bioscreen C (Oy Growth Curves Ab, Finland). Hydrogen sulfide was quantified as described by Cord-Ruwisch (1985). Briefly, the pH of the medium was adjusted to 10 with the addition of 6 M NaOH; samples were then allowed to stand for 60 min with periodic mixing. 50 µL of sample was then transferred into a cuvette containing 1.95 mL of 5 mM CuSO4 in 50 mM HCl under rapid stirring. After 5 s of stirring, the cuvette was transferred to a Perkin Elmer Lambda 25 UV–Vis spectrophotometer and the absorbance read at a wavelength of 480 nm against a dH2O blank. Other sulfur species were not analyzed in the present investigation.
Molecular methods
DNA was extracted from the strains and used as templates in 16S rRNA PCR reactions. 16S rRNA genes were amplified with primers F9 and R1544, which are specific for bacterial genes (Skirnisdottir et al. 2000). The PCR products were sequenced with the universal 16S rRNA primers F9, F515, F1392, R357, R805, R1195, and R1544 (Skirnisdottir et al. 2000) using a Big Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems). Subsequently, the DNA was analyzed with a 3730 DNA analyzer from Applied Biosystems. The nucleotide sequence was displayed and analyzed with Sequencer (Gene Code Corporation). Sequences from 16S rRNA analysis were submitted to the NCBI database (National Center for Biotechnology Information) using the nucleotide–nucleotide BLAST tool (Altschul et al. 1997). The most similar sequences obtained were aligned with sequencing results in MEGA6 (Tamura et al. 2013) and the maximum likelihood method based on the Tamura–Nei model was used to generate a phylogenetic tree (Tamura and Nei 1993). Evolutionary analyses were conducted in MEGA6 (Tamura et al. 2013).
Results
Screening of the culture collection for branched-chain amino acid degradation
Fifty-six strains of thermophilic bacteria, either isolated from various hot springs in Iceland (48) or obtained from culture collections (8) were investigated in present study. The origin of AK strains showing temperature and pH at site of isolation, together with end product formation from glucose is showed in Supplementary Table 1. Additionally, substrate spectra of strains are presented. All of the Thermoanaerobacter (14) and Caldanaerobacter (10) strains were positive for BCFA and BCOH formation (Table 1). Only one other strain was positive for BCFA formation, Caloramator strain AK49, but it did not produce BCOHs. Other strains from the genera Clostridium, Caldicellulosiruptor and Thermoanaerobacterium did not degrade the BCAAs and thus did not produce BCFAs or BCOHs (are thus not included in Table 1). The relative amount of BCFAs and BCOHs produced by the 25 strains that were positive on BCAA degradation is shown in Table 1 together with phylogenetic data showing the closest relative of each strain. In general, the highest amounts of BCFAs (>20 mM of all three fatty acids) and BCOHs (>3 mM of all three alcohols) were produced by the genera of Caldanaerobacter. Most of the Thermoanaerobacter species produced between 5 and 13 mM of the BCFA and between 1 and 3 mM of the BCOH. The best alcohol producer, Caldanaerobacter strain AK72, most closely related to C. subterraneus subsp. yonseiensis, produced 24.5 ± 1.3 mM of the BCFAs (sum of 3-methylbutyrate, 2-methylbutyrate, and 2-methylpropionate) and 5.7 ± 0.3 mM of BCOH (summary of 3-methylbutanol, 2-methylbutanol, and 2-methylpropanol).
Phylogenetic relationship of selected Caldanaerobacter and Thermoanaerobacter species
Figure 1 shows the phylogenetic relationship between all Thermoanaerobacter and Caldanaerobacter species with standing in nomenclature as well as the twenty AK strains belonging to these two genera. Eleven (11) of the AK strains belong to the genus Thermoanaerobacter and nine (9) belong to Caldanaerobacter. Most of the Thermoanaerobacter AK strains were most closely related to T. thermohydrosulfuricus and T. ethanolicus (8 strains) and T. uzonensis (3 strains). Six of the Caldanaerobacter strains were most closely related to Caldanaerobacter subterraneus subsp. subterraneus and three to Caldanaerobacter subterraneus subsp. yonseiensis. It is noteworthy that Caldanaerobacter AK113 is phylogenetically distant from its nearest neighbor Caldanaerobacter subterraneus subsp. yonseiensis. The reason could be that this strain has a relatively short 16S sequenced (406 nt). Table 1 shows the most closely related species assigned for each AK strain together with accession numbers obtained from NCBI.
End product formation from individual branched-chain amino acids
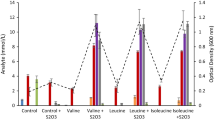
All strains positive for growth on a mixture of BCAAs were tested on for growth and end product formation in the presence of thiosulfate on individual BCAAs. Data showing end product formation from these experiments are shown for Thermoanaerobacter and Caldanaerobacter species in Supplementary Tables 2 and 3, respectively. In all cases, thiosulfate was nearly completely utilized (data not shown). All strains were positive for BCAA degradation although some variations in amounts of end products were observed. For simplicity, data is presented for three representative Thermoanaerobacter strains (T. wiegelii, AK15, and AK68; Fig. 2a–c) and three Caldanaerobacter strains (C. subterraneus subsp. subterraneus, AK102, and AK112; Fig. 3a–c).
Thermoanaerobacter wiegelii (DSM 10319) produced between 12.0 and 14.0 mM of the BCFAs and 1.8 to 3.0 mM of the BCOHs (Fig. 2a). Hydrogen concentrations were from 5.3 to 7.8 mmol/L for the three amino acids and hydrogen sulfide from 7.8 to 12.6 mmol/L. In all cases, more than 90 % of the BCAAs were degraded and carbon balances for leucine, isoleucine, and valine were 80.5, 88.5, and 84.0 %, respectively (Fig. 2a; Supplementary Table 2). Thermoanaerobacter strain AK 68 (most closely related to T. uzonensis) almost completely degraded all three amino acids and produced similar amounts of BCFA (12.2 to 13.0 mM) and BCOH (2.3 to 4.0 mM) (Fig. 2b). Carbon balances ranged from 83.3 to 93.8 % (Supplementary Table 2). Hydrogen concentrations were very low or between 0.2 and 0.6 mmol/L and more than 11 mmol/L of H2S were produced in all three cases. Thermoanaerobacter strain AK15 (most closely related to T. uzonensis) also almost completely degraded all three amino acids with carbon recoveries ranging from 82.0 % (isoleucine) to 88.4 % (leucine) as summarized in Supplementary Table 2. Similar amounts of BCFAs and BCOHs were observed as for T. wiegelii, except for BCOH formation from isoleucine which was considerable lower (Fig. 2c). Hydrogen was between 0.8 and 1.8 mmol/L and H2S produced between 11.2 and 12.1 mmol/L.
Caldanaerobacter subterraneus subsp. subterraneus (DSM 13054) showed similar fermentation spectrum on all three amino acids (Fig. 3a); with the production of BCFAs between 14.0 and 15.6 mM and the BCOHs formation between 3.4 and 5.7 mM with carbon recoveries between 91.1 to 99.0 % (Supplementary Table 3). Very low concentrations of both H2 (<1 mmol/L) and H2S (<1.3 mmol/L) were observed. Figure 3b shows end product formation for Caldanaerobacter strain AK102 (most closely related to Caldanaerobacter subterraneus subsp. subterraneus). This strain produces lower amounts of both BCFAs and BCOHs as compared to Caldanaerobacter subterraneus subsp. subterraneus (DSM 13054) but between 5.7 and 11.0 mM of the amino acids was not degraded by this strain but carbon balances were between 85.5 and 88.5 % (Supplementary Table 3). Hydrogen concentrations were between 4.1 and 8.4 mmol/L and H2S concentrations were between 5.8 and 8.0 mmol/L. Caldanaerobacter strain AK112 is most closely related to C. subterraneus subsp. yonseiensis produced between 11.7 and 12.9 mM and between 1.4 and 4.0 of the BCFA and BCOHs, respectively (Fig. 3C) and carbon balances were between 82.8 and 89.3 (Supplementary Table 3). Hydrogen and H2S concentrations ranged from 9.6 to 15.4 mmol/L and 7.5 to 10.2 mmol/L, respectively.
Statistical tests were done to reveal the difference between the ratio of BCFA production versus the BCOH production for all alcohol forming species of Thermoanaerobacter and Caldanaerobacter. The difference proved statistically significant. The data points used for the test are presented in Supplementary Tables 2 and 3. A preliminary test for equality of variances for the ratio of 3-methylbutanol and 3-methylbutanol (products from leucine degradation) indicates that there is a clear difference between the strains within the two genera (F = 4.11, p = 0.015). Therefore, a two-sample t test (95 % confidence interval) was performed that does not assume equal variances. The mean score for Thermoanaerobacter (M = 7.15, SD = 2.31, N = 14) was significantly higher than the scores for Caldanaerobacter (M = 3.28, SD = 1.14, N = 11). Similarly, t test showed a clear difference between the ratio of 2-methylbutyrate and 2-methylbutanol (from isoleucine) and the ratio of 2-methylpropionate and 2-methylpropanol (from valine).
Discussion
The present investigation examines the distribution of BCAA metabolism among selected thermophilic Clostridia with the main emphasis of the genera Thermoanaerobacter and Caldanaerobacter. The degradation of the BCAAs to their corresponding BCFAs been shown to be possible by Thermoanaerobacter species when grown under electron scavenging systems (Fardeau et al. 1997; Scully and Orlygsson 2014). Although bacteria have the enzymes responsible for the degradation of these amino acids, hydrogen accumulation from initial deamination, and decarboxylation steps inhibits further degradation. By adding thiosulfate to the cultures, Thermoanaerobacter and Caldanaerobacter species can use it as an electron acceptor producing elemental S, H2S, or possibly other sulfur species (Lee et al. 1993; Cann et al. 2001; Kozianowski et al. 1997). Other means of scavenging electrons is by co-cultivating the amino acid degraders either with hydrogenotrophic methanogens (Fardeau et al. 1997; Scully and Orlygsson 2014) and sulfate reducers (Stams and Hansen 1984).
A recent investigation in our laboratory demonstrated that Thermoanarobacter brockii and Caldanaerobacter subterraneus subsp. yonseiensis produce a mixture of BCFAs and BCOHs from BCAAs in the presence of thiosulfate (Scully and Orlygsson 2014). Thus, these strains produced a mixture of 3-methylbutyrate and 3-methylbutanol from leucine, 2-methylbutyrate and 2-methylbutanol from isoleucine and 2-methyl-propionate and 2-methylpropanol from valine. No BCOH formation occurred when the strains were co-cultivated with the hydrogenotrophic methanogen Methanothermobacter strain M39. The present investigation focused on other well-known species within these two genera as well as with other species within other genera to see if this ability of BCOH was genus specific or not.
Phylogenetic considerations
Recently, two species within Thermoanaerobacter were moved to a new genus, Caldanaerobacter. Thus, currently, the Thermoanaerobacter genus has 15 species and five sub-species (Euzéby 1997; Parte 2014) and Caldanaerobacter (Fardeau et al. 2004) has two species and 4 sub-species. The phylogenetic tree of most of the type species within Thermoanaerobacter and Caldanaerobacter together with all AK strains positive for BCAA degradation is presented in Fig. 1. Many of the Thermoanaerobacter AK strains were closely related with T. thermohydrosulfuricus, T. ethanolicus, and T. pseudoethanolicus. Comparing data from Table 1 with phylogenetic relationship of good BCOH producers the only four strains producing more than 20 mM of BCFA and more than 5 mM of BCOH belong to the genus Caldanaerobacter. Additionally, only three strains that produced less than 1 mM of BCOH all belong to the genus Thermoanaerobacter. Thus, in general species within Caldanaerobacter produce higher concentrations of the alcohol as compared to the Thermoanaerobacter species investigated. This was further analyzed statistically with a t test in present study. Interestingly, there was also a clear difference between the ratio of BCFAs over BCOHs from different amino acids degraded. Thus, from leucine and isoleucine, this ratio was between 7.15 ± 2.31 and 6.61 ± 2.77 for Thermoanaerobacter, but 3.49 ± 1.18 to 4.13 ± 1.30 for Caldanaerobacter. This value for valine was, however, much higher (11.53 ± 4.65 and 7.31 ± 1.74 for Thermoanaerobacter and Caldanaerobacter, respectively) indicating proportionally more alcohol formation from the 6 carbon amino acids (leucine and isoleucine) as compared to valine (5 carbons). This could be due to the enzyme specificity of the different enzymes used for the degradation of the BCAAs and needs further investigation.
Degradation on single branched-chain amino acids
A great variation was observed on end product formation between strains using single BCAAs as substrates with 20 mM of thiosulfate (Supplementary Tables 2 and 3). Selected data are presented for only six strains to emphasize this variation (Figs. 2, 3). For instance, Caldanaerobacter subterraneus subsp. subterraneus showed good yields in BCFAs and BCOHs (between 91.1 and 99.0 in carbon balances) and most of the hydrogen was scavenged by thiosulfate reduction. However, the balances for sulfur species are incomplete since very little H2S was detected at the end of cultivation. Lee and co-workers stated that Thermoanaerobacter species reduce thiosulfate to hydrogen sulfide, whereas Thermoanaerobacterium reduce it to elementary sulfur (Lee et al. 1993). Later, it became clear that this is not a unified characteristic of these genera when two species within Thermoanaerobacterium were shown to be unable to reduce thiosulfate to sulfur (Cann et al. 2001). Additionally, it is known that Thermoanaerobacter italicus produces a mixture of sulfur and hydrogen sulfide from thiosulfate (Kozianowski et al. 1997) while Thermoanaerobacter sulfurigignens produces only sulfur (Lee et al. 2007). In all cases, only trace amounts of thiosulfate were detected after end of cultivation in present study suggesting that all thiosulfate was reduced to other sulfur species. Sulfur was not analyzed but a strong yellow color and microscopic observation showed the presence of sulfur in some of the culture bottles, especially when H2S was found in low concentrations. Theoretically, 20 mM of a BCAA should yield 40 mmol/L of hydrogen and if thiosulfate (20 mM) is the only electron acceptor it should produce 20 mmol/L of hydrogen sulfide. These yields are in good correlation for most of the strains tested; usually the H2S was little lower as compared with the amount of BCFA produced (Figs. 2a–c, 3b, c; Supplementary Tables 2 and 3). However, in some cases this was not the case and very low H2S concentrations were indeed observed. Clearly, the different species use different sulfur metabolism for scavenging the electrons and this can also been seen by different hydrogen concentrations observed. Earlier data from Caldanaerobacter subterraneus subsp. yonseiensis and Thermoanarobacter brockii showed H2S yields varying from 11.9 to 17.0 mmol/L from 20 mM of BCAA in the presence of 40 mM of thiosulfate and from 7.5 to 9.3 mmol/L for C. subterraneus subsp. yonseiensis and T. brockii, respectively (Scully and Orlygsson 2014). Clearly, there is a need for further studies to better explore the differences in the sulfur metabolism of these two genera.
Most Thermoanaerobacter and Caldanaerobacter strains grow optimally at neutral pH and between 65 and 70 °C. Thus, for simplicity reasons, in present investigation, all strains belonging to these two genera were cultivated at the same pH and temperature (pH 7.0 and at 65 °C). This may, however, not be their optimum growth conditions and results should be taken with caution concerning yields end product formation. For the Thermoanaerobacter and Caldanaerobacter strains, a more detailed study using not only variation in the liquid–gas phase ratios and the concentration of thiosulfate, but also an investigation into the role of culture conditions such as temperature and pH is needed to better understand conditions leading to the production of BCOHs rather than the corresponding BCFAs. From the study of Caldanaerobacter subterraneus subsp. yonseiensis, it was clear that both the concentration of thiosulfate as well as the ratio of liquid gas phases was of great importance for BCOH formation (Scully and Orlygsson 2014). Thus, using a high liquid–gas ratios increases the relative amount of the BCOH over the BCFA for this strain.
The majority of studies on the catabolism of the BCAAs have focused on aerobic bacteria such as species of Staphylococcus and Enterococcus (Beck et al. 2004; Ward et al. 1999), aerotolerant anaerobes including Lactobacillus sakei (Gutsche et al. 2012), or yeasts that use the Ehrlich pathway as shown in Fig. 4 (Ehrlich 1907; Hazelwood et al. 2008). Here, we report the production of these products using species of Thermoanaerobacter. The phenomenon may be common among other members of class Clostridia, although the production of BCOHs may have escaped detection, as culture conditions require electron scavenging systems and low pH2. The degradation of amino acids presents a renewable route to potentially important feedstock chemicals. The interest in the production of BCOHs has often been directed to the formation of flavor compounds (branched- and aromatic chain aldehydes, alcohols, and acids) in food and beverage products (Hazelwood et al. 2008). Additionally, (S)-2-methylbutanol is a potential biofuel (Peralta-Yahya and Keasling 2010) and some of the BCOHs may serve as building blocks (Sakuragi et al. 2011). A crude protein extract from fish waste or whey protein might be used as a good source of BCAAs for the production of these compounds.
Conclusions
The production of BCOHs during the fermentation of BCAAs by thermophilic Clostridia appears to be limited to the genera of Thermoanaerobacter and Caldanaerobacter. The amount of BCOH was found to be proportionally higher within the genus Caldanaerobacter compared with Thermoanaerobacter and more profound from leucine and isoleucine as compared with valine. Clearly, there is a difference in thiosulfate reduction between investigated species. The role of pH2 and the enzymes involved requires further investigations to elucidate the pathway these bacteria use for BCOH formation.
References
Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W, Lipman D (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Andreesen JR, Bahl H, Gottschalk G (1989) Introduction to the physiology and biochemistry of the genus Clostridium. In: Minton NP, Clarke DJ (eds) Clostridia. Plenum Press, New York, pp 27–62
Barker HA (1981) Amino acid degradation by anaerobic bacteria. Annu Rev Biochem 50:23–40
Beck HC, Hansen AM, Lauritsen FR (2004) Catabolism of leucine to branched-chain fatty acids in Staphylococcus xylosus. J Appl Microbiol 96:1185–1193
Cann IKO, Stroot PG, Mackie KR, White BA, Mackie RI (2001) Characterization of two novel saccharolytic, anaerobic thermophiles, Thermoanaerobacterium polysaccharolyticum sp. nov. and Thermoanaerobacterium zeae sp. nov., and emendation of the genus Thermoanaerobacterium. Int J Sys Evol Microbiol 51:293–302
Chang T, Yao S (2011) Thermophilic, lingocellulolytic bacteria for ethanol production: current state and perspectives. Appl Micorbiol Biotechnol 92:13–27
Choi KY, Wernick DG, Tat CA, Liao JC (2014) Consolidated conversion of protein waste into biofuels and ammonia using Bacillus subtilis. Metab Eng 23:53–61
Cord-Ruwisch R (1985) A quick method for determination of dissolved and precipitated sulphides in cultures of sulphate-reducing bacteria. J Microbiol Methods 4:33–36
Ehrlich F (1907) Uber die Bedingungen der Fuselöbildung und uber ihren Zusammenhang mit dem Eiweissaufbau der Hefe. Ber Dtsch Chem Ges 40:1027–1047
Elsden SR, Hilton MG (1978) Volatile acid production from threonine, valine, leucine and isoleucine by clostridia. Arch Microbiol 117:165–172
Euzéby JP (1997) List of bacterial names with standing in nomenclature: a folder available on the internet. Int J Syst Evol Microbiol 47:590–592
Fardeau M-L, Patel BKC, Magot M, Ollivier B (1997) Utilization of serine, leucine, isoleucine, and valine by Thermoanaerobacter brockii in the presence of thiosulfate or Methanobacterium sp. as electron acceptors. Anaerobe 3:405–410
Fardeau M-L, Salinas MB, L’Haridon S, Jeanthon C, Verhé F, Cayol J-L, Patel BKC, Garcia J-L, Ollivier B (2004) Isolation from oil resevoirs of novel thermophilic anaerobes phylogenetically related to Thermoanaerobacter subterraneus: reassignment of T. subterraneus, Thermoanaerobacter yonseiensis, Thermoanaerobacter tengcongensis and Carboxydibrachium pacificum to Caldanaerobacter subterraneous gen. nov., sp. nov., com. nov. as four novel subspecies. Int J Syst Evol Microbiol 54:467–474
Faudon C, Fardeau ML, Heim J, Patel B, Magot M, Ollivier B (1995) Peptide and amino acid oxidation in the presence of thiosulfate by members of the genus Thermoanaerobacter. Curr Microbiol 31:152–157
Gutsche KA, Tran TBT, Vogel RF (2012) Production of volatile compounds by Lactobacillus sakei from branched chain alpha-keto acids. Food Microbiol 29:224–228
Hazelwood LA, Daran J-M, van Maris AJA, Pronk JT, Dickinson JR (2008) The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisae. Appl Environ Microbiol 74:2259–2266
Hungate RE (1969) A roll tube method for cultivation of strict anaerobes. In: Norris JR, Ribbons DW (eds) Methods in microbiology, vol 3B. Academic Press, New York, pp 117–132
Huo YX, Cho KM, Rivera JGL, Monte E, Shen CR, Yan YJ, Liao JC (2011) Conversion of proteins into biofuels by engineering nitrogen flux. Nat Biotechnol 29:346–351
Kozianowski G, Canganella F, Rainey FA, Hippe H, Antranikian G (1997) Purification and characterization of thermostable pectate-lyases from a newly isolated thermophilic bacterium, Thermoanaerobacter italicus sp. nov. Extremophiles 1:171–182
Lee YE, Jain MK, Lee CY, Lowe SE, Zeikus JG (1993) Taxonomic distinction of saccharolytic thermophilic anaerobes—description of Thermoanaerobacterium xylanolyticum gen-nov, sp-nov, and Thermoanaerobacterium saccharolyticum gen-nov, sp-nov - reclassification of Thermoanaerobium brockii, Clostridium thermosulfurogenes, and Clostridium thermohydrosulfuricum E100-69 as Thermoanaerobacter brockii comb-nov, Thermoanaerobacterium thermosulfurigenes comb-nov, and Thermoanaerobacter thermohydrosulfuricus comb-nov, respectively—and transfer of Clostridium thermohydrosulfuricum 39E to Thermoanaerobacter ethanolicus. Int J Syst Bacteriol 43:41–51
Lee Y-J, Dashti M, Prange A, Rainey FA, Rohde M, Whitman WB, Wiegel J (2007) Thermoanaerobacter sulfurigignens sp. nov., an anaerobic thermophilic bacterium that reduces 1 M thiosulfate to elemental sulfur and tolerates 90 mM sulfite. Int J Syst Evol Microbiol 57:1429–1434
McInerney MJ (1988) Anaerobic hydrolysis and fermentation of fats and proteins. In: Zehnder AJB (ed) Biology of anarobic microorganisms. Wiley, New York, pp 373–415
Orlygsson J, Baldursson SRB (2007) Phylogenetic and physiological studies of four hydrogen-producing thermoanaerobes from Icelandic geothermal areas. Icelandic Agric Sci 20:93–106
Orlygsson J, Houwen FP, Svensson BH (1994) Influence of hydrogenotrophic methane formation on the thermophilic anaerobic degradation of proteins and amino acids. FEMS Microbiol Ecol 13:327–334
Orlygsson J, Houwen FP, Svensson BH (1995) Thermophilic anaerobic amino acid degradation–deamination rates and end product formation. Appl Microbiol Biot 43:235–241
Orlygsson J, Sigurbjornsdottir MA, Bakken HE (2010) Bioprospecting thermophilic ethanol and hydrogen producing bacteria from hot springs in Iceland. Icelandic Agricult Sci 23:73–85
Parte AC (2014) LPSN—list of prokaryotic names with standing in nomenclature. Nucl Acid Res 42:D613–D616
Peralta-Yahya PP, Keasling JD (2010) Advanced biofuel production in microbes. Biotechnol J 5:147–162
Ren N, Wang A, Cao G, Xu J, Gao L (2009) Bioconversion of lignocellulosic biomass to hydrogen: potential and challenges. Biotechnol Adv 27:1051–1060
Sakuragi H, Kuroda K, Ueda M (2011) Molecular breeding of advanced microorganism for biofuel production. J Biomed Biotechnol. doi:10.1155/2011/416931
Scully SM, Orlygsson J (2014) Branched-chain alcohol formation from branched-chain amino acids by Thermoanaerobacter brockii and Thermoanaerobacter yonseiensis. Anaerobe 30:82–84
Scully SM, Orlygsson J (2015) Recent advances in second generation ethanol production by thermophilic bacteria. Energies 8:1–30
Skirnisdottir S, Hreggvidsson GO, Hjorleifsdottir S, Marteinsson VT, Petursdottir SK, Holst O, Kristjansson JK (2000) Influence of sulfide and temperature on species composition and community structure of hot spring microbial mats. Appl Environ Microbiol 66:2835–2841
Smit G, Smit A, Engels WJM (2005) Flavour formation by lactic acid bacteria and biochemical flavor profiling of cheese products. FEMS Microbiol Rev 29:591–610
Smit BA, Engels WJM, Smit G (2009) Branched chain aldehydes: production and breakdown pathways and relevance for flavor in foods. Appl Microbil Biot 81:987–999
Stams AJM, Hansen JA (1984) Fermentation of glutamate and other compounds by Acidaminobacter hydrogenoformans gen. nov. sp. nov., an obligate anaerobe isolated from black mud. Studies with pure cultures and mixed cultures with sulfate-reducing and methanogenic bacteria. Arch Microbiol 137:329–337
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Bio Evol 10:512–526
Tamura K, Stecher G, Peterson A, Kumar S (2013) MEGA6: molecular evolutionary genetic analysis. Version 6.0. Mol Biol Evol 30:2725–2729
Taylor MP, Eley KL, Martin S, Tuffin M, Burton SG, Cowan DA (2009) Thermophilic ethanologenesis: future prospects for second-generation bioethanol production. Trends Biotechnol 27:398–405
Ward DE, Ross P, van der Weijden CC, Snoep JL, Claiborne A (1999) Catabolism of branched-chain alpha-keto acids in Entercoccus faecalis: the bkd gene cluster, enzymes, and metabolic route. J Bacteriol 181:5433–5442
Westley J (1987) Thiocyanate and thiosulfate. Methods in Enzym 143:22–25
Acknowledgments
The authors would like to thank Margret Auður Sigurbjörnsdóttir for her assistance constructing the phylogenetic tree and Eva María Ingvadóttir for her helpful feedback during the preparation of this manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Atomi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scully, S.M., Iloranta, P., Myllymaki, P. et al. Branched-chain alcohol formation by thermophilic bacteria within the genera of Thermoanaerobacter and Caldanaerobacter . Extremophiles 19, 809–818 (2015). https://doi.org/10.1007/s00792-015-0756-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-015-0756-z