Abstract
The extremely acidophilic, Fe(III)-reducing heterotrophic bacterium Acidocella aromatica strain PFBC was tested for its potential utility in bioreduction of highly toxic heavy metal, hexavalent chromium, Cr(VI). During its aerobic growth on fructose at pH 2.5, 20 µM Cr(VI) was readily reduced to Cr(III), achieving the final Cr(VI) concentration of 0.4 µM (0.02 mg/L), meeting the WHO drinking water guideline of 0.05 mg/L. Despite of the highly inhibitory effect of Cr(VI) on cell growth at higher concentrations, especially at low pH, Cr(VI) reduction activity was readily observed in growth-decoupled cell suspensions under micro-aerobic and anaerobic conditions. Strain PFBC was not capable of anaerobic growth via dissimilatory reduction of Cr(VI), such as reported for Fe(III). In the presence of both Cr(VI) and Fe(III) under micro-aerobic condition, microbial Fe(III) reduction occurred only upon complete disappearance of Cr(VI) by its reduction to Cr(III). Following Cr(VI) reduction, the resultant Cr(III), supposedly present in the form of cationic CrIII(OH2) 3+6 , was partially immobilized on the negatively charged cell surface through biosorption. When Cr(III) was externally provided, rather than microbially produced, it was poorly immobilized on the cell surface. Cr(VI) reducing ability was reported for the first time in Acidocella sp. in this study, and its potential role in biogeochemical cycling of Cr, as well as its possible utility in Cr(VI) bioremediation, in highly acidic environments/solutions, were discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is a widely used, important element having a diverse range of usages, such as in alloy production, leather processing, pigment development, catalyst production, and refractories (Jacobs and Testa 2005). On the other hand, Cr is considered a priority pollutant in many countries. Cr-containing effluents and solid wastes are a by-product in mining, metallurgical, and chemical industrial operations (Dhal et al. 2013). Although its valences from −2 to +6 are known, Cr generally exists primarily in the form of Cr(VI) and Cr(III). Except for the rarely found Cr(0), other oxidation states of Cr are unstable and thus not found in natural environments. The most common forms of Cr(VI) are oxyanions, hydrogen chromate (HCrVIO4 –) and chromate (CrVIO4 2–), with their relative abundance depending on pH (Hawley et al. 2005). In the absence of complexing agents, other than H2O or OH−, Cr(III) exists as \({\text{Cr}}^{III} \left( {{\text{H}}_{2} {\text{O}}} \right)_{6}^{3 + }\) and its hydrolysis products depending on pH (Cornelis et al. 2005). Cr(VI) oxyanions are known to be far more toxic and mobile than Cr(III), with the former classified as a human carcinogen by the US Environmental Protection Agency (EPA) (Hawley et al. 2005). Reduction of Cr(VI) to less toxic/mobile Cr(III) is, therefore, a favorable approach in Cr treatment strategy.
Conventional Cr treatment approaches are exemplified by the use of chemical reducing agents such as sulfur compounds (e.g. sulfur dioxide gas, SO2; sodium bisulfite, NaHSO3) and iron salts [e.g. iron(II) chloride, FeCl2; iron(II) sulfate, FeSO4] at acidic pHs, followed by neutralization to form Cr(III) precipitates, Cr(OH)3. However, the shortcomings encountered by these techniques include the generation of large amount of iron-sludge and the emission of secondary pollutants (e.g. SO2 gas). Moreover, both sulfur compounds and iron salts are generally not suitable for treatment of dilute Cr(VI) solutions (Barrera-Díaz et al. 2012).
Utilization of microbial Cr(VI) reduction ability is considered an alternative approach to Cr treatment. Cr(VI) can be microbially reduced either directly via metabolic (enzymatic) activity, or indirectly via production of reductants. The former direct mechanism is exemplified by aerobic Cr(VI) reduction by NADH-dependent, soluble chromate reductase activity found intracellularly in Bacillus spp. (Camargo et al. 2003; Soni et al. 2013), Pseudomonas (Ps.) putida (Ishibashi et al. 1990; Park et al. 2000), Arthrobacter spp. (Megharaj et al. 2003; Elangovan et al. 2010), Microbacterium sp. (Soni et al. 2013), Thermus scotoductus (Opperman and van Heerden 2007), Leucobacter sp. and Exiguobacterium sp. (Sarangi and Krishnan 2008), Pannonibacter phragmitetus (Xu et al. 2012), while involvement of constitutive membrane-bound enzyme was also suggested in Bacillus subtilis (Mangaiyarkarasi et al. 2011). Direct anaerobic Cr(VI) reduction involves activities of both soluble (e.g. hydrogenase) and membrane-associated (e.g. cytochrome c 3) enzymes in Desulfovibrio (D.) vulgaris and Desulfomicrobium norvegicum (Lovley and Phillips 1994; Michel et al. 2001; Chardin et al. 2002). Furthermore, dissimilatory Cr(VI) reducers include Shewanella spp. (Guha et al. 2003; Belchik et al. 2011), Enterobacter cloacae (Wang et al. 1989), Desulfotomaculum reducens (Tebo and Obraztsova 1998), Escherichia coli (Shen and Wang 1993) and Pantoea agglomerans (Francis et al. 2000). Other bacterial genera capable of Cr(VI) reduction include Pannonibacter (Chai et al. 2009), Ochrobactrum (He et al. 2009), Acinetobacter (Zakaria et al. 2007), Deinococcus (Fredrickson et al. 2000), Alcaligenes, Cupriavidus, Corynebacterium (Dey and Paul 2010), Rhodococcus (Patra et al. 2010), Achromobacter (Zhu et al. 2008), Serratia (Joutey et al. 2014), Amphibacillus (Ibrahim et al. 2012), Lysinibacillus (He et al. 2011), Geobacter, and Sulfurospirillum (Chovanec et al. 2012). Furthermore, indirect Cr(VI) reduction occurs through microbially generated reducing compounds, such as Fe(II) in Shewanella sp. (Wielinga et al. 2001; Fendorf and Li 1996) and sulfur compounds in Acidithiobacillus (At.) spp. (sulfite, thiosulfate, and polythionates; Sisti et al. 1996; QuiIntana et al. 2001; Allegretti et al. 2006).
Considering that pollutions with heavy metals (including Cr) are often found in highly acidic waters, understanding roles played by acidophilic extremophiles on biogeochemical cycling of such metal pollutants is potentially important. While vast amount of studies on Cr(VI) bioreduction are available with neutrophils, the information about acidophilic extremophiles is yet highly limited. In fact, so far Acidiphilum (A.) cryptum is the only extreme acidophile reported to directly catalyze Cr(VI) reduction (Cummings et al. 2007); involvement of two potential Cr(VI) reduction mechanisms, namely, intracellular NADPH-dependent Cr(VI) reductase and periplasmic c-type cytochromes (ApcA) was suggested for this acidophilic bacterium (Magnuson et al. 2010).
Recently, a novel species of extremely acidophilic, mesophilic Alphaproteobacteria, Acidocella (Ac.) aromatica, was proposed for three obligately heterotrophic bacterial strains, WJB-3, LGS-3, and PFBC (Jones et al. 2013). The type strain PFBC grows aerobically on a limited range of substrates (such as fructose, acetate, and several aromatic compounds), and is not capable of Fe(II) oxidation. Strain PFBC catalyzes Fe(III) reduction under both micro-aerobic and anaerobic conditions, however, the strain does not grow anaerobically via Fe(III) respiration. Strains of Ac. aromatica were tolerant to nickel by about two orders of magnitude greater than those of other Acidocella spp., though similar levels of tolerance to other metals were reported (Jones et al. 2013).
Microbial activities in extremely acidic environments such as acid mine drainages are mostly recognized with chemolithotrophic prokaryotes which utilize Fe(II) or/and reduced inorganic sulfur compounds (RISCs). Nonetheless, heterotrophic acidophiles are ubiquitously present by interacting with chemolithotrophs in such environments, with Acidiphilium spp. being among the most frequently found Fe(III)-reducing heterotrophs (Johnson and McGinness 1991; Coupland and Johnson 2008). Among the four species recognized for the genus Acidocella, strains of Ac. aromatica were isolated from acid samples of different origins (Jones et al. 2013), indicating that this species may also account for the major heterotrophic constituent in highly acidic environments.
In this study, acidophilic extremophile, Ac. aromatica PFBC was tested for its Cr(VI) reduction ability and its subsequent immobilization to elucidate its role in Cr geobiochemical cycle in highly acidic environments.
Materials and methods
Microorganism
Ac. aromatica PFBC (NCCB 100456; DSM 27026) was routinely cultured aerobically in 300 mL Erlenmeyer flasks containing 100 mL of heterotrophic basal salts (HBS) media [per L; 450 mg (NH4)2SO4, 50 mg KCl, 50 mg KH2PO4, 500 mg MgSO4·7H2O, 14 mg Ca(NO3)2·4H2O and 142 mg Na2SO4: pH 3.0 with H2SO4] with 10 mM fructose and 0.025 % (w/v) tryptone soya broth (TSB). Flasks were incubated at 30 °C on a rotary shaker at 100 rpm.
Cr tolerance test and Cr(VI) reduction experiment during aerobic growth of Ac. aromatica PFBC
Cells were pre-grown (pH 3.0; as described above), harvested at the late-exponential phase by centrifugation (12,000 g, 10 min at 30 °C), washed twice, and inoculated in 300 mL flasks containing fresh media of the same composition (pH adjusted to 2.5 or 4.0, with H2SO4). The initial cell density was set to 1.0 × 107 cells/mL. Filter-sterilized Cr stock solutions [as Na2CrVIO4·4H2O or CrIII(NO3)3·9H2O] were added to the media to final Cr(VI) concentrations of 10, 20, 50 or 200 µM, or to final Cr(III) concentration of 1.0, 1.5, 2.0, 5.0, 10 or 25 mM. The flasks were aerobically incubated shaken at 100 rpm, 30 °C. Samples were regularly taken to monitor cell density (using bacterial counting chamber) and concentrations of Cr(VI) and total Cr. All experiments were conducted in duplicates.
Cr(VI) reduction experiments using cell suspension of Ac. aromatica PFBC under micro-aerobic condition
Cells were pre-grown (pH 3.0; as described above), harvested at the late-exponential phase by centrifugation (12,000 g, 10 min at 30 °C), washed twice, and re-suspended (to a final cell density of 1.0 × 109 or 1.0 × 1010 cells/mL) in 50 mL of fresh HBS media containing 10 mM fructose and 0.025 % (w/v) TSB (pH 2.5 with H2SO4). Stock solution of Cr(VI) [or Cr(III) as control] was added to the above media to a final concentration of 200 µM. To evaluate the effect of presence of Fe on Cr(VI) reduction, Fe stock solutions [as \({\text{Fe}}_{2}^{III} \left( {{\text{SO}}_{4} } \right)_{3} \cdot n{\text{H}}_{2} {\text{O}}\) or FeIISO4·7H2O] were added to the media to a final Fe(III) or Fe(II) concentration of 1,000 µM. To establish micro-aerobic conditions, Falcon tubes (50 ml) containing the above 50 ml cell suspensions were incubated at 30 °C without shaking. Samples were regularly taken to monitor concentrations of Cr(VI), total Cr, Fe(II) and total Fe. All of the experiments were conducted in duplicates.
Zeta-potential measurement
Ac. aromatica PFBC cells (1.0 × 1010 cells/mL), incubated with 200 µM Cr(VI), Cr(III), or without Cr for 200 h, were collected (12,000 g, 10 min), washed twice and re-suspended in 10 ml of 1 mM KCl solution (pH 2.0 with H2SO4) to final cell density of 1.0 × 108 cells/mL. The zeta-potential values of the Ac. aromatica PFBC cell surface were measured using ZETASIZER Nano series (Malvern), by automatically adjusting the pH values at 2.0, 3.0, 4.0, 5.0 and 6.0 with 0.25 M HCl and 0.25 M NaOH (MPT-2 Multi Purpose Titrator; Malvern).
Solution analysis
Liquid samples were filtered using a 0.20-µm cartridge filter to determine concentrations of total Cr (ICP-AES; SEIKO Vista-MPX), Cr(VI) (diphenylcarbazide method; Noroozifar and Khorasani-Motlagh 2003), and Fe(II)/total Fe (O-phenanthroline method with ascorbic acid as reducing agent).
X-ray absorption fine structure (XAFS)
Following the Cr(VI) reduction experiment (200 h) using 1.0 × 1010 cells/mL cell suspensions (as described above), cells were collected by centrifugation (12,000 g, 10 min), washed twice with HBS media (pH 2.5 with H2SO4) to avoid any residual Cr contamination from the original media, and freeze-dried overnight. Cell tablets for XAFS analysis were prepared using the same amount of cells by a tablet press machine at 10 MPa for 5 min. X-ray absorption spectra were collected on Kyushu University beam line (BL06) at Kyushu Synchrotron Light Research Center (SAGA-LS; 1.4 GeV storage ring with a circumference of 75.6 m). The measurements were conducted at the Cr K-edge and data were collected in transmission mode at the energy range from 5,660 to 7,500 eV. The data were also collected in fluorescence mode to compare the Cr-Kα1 peak intensity (5,418 eV) of different cell samples: for this purpose, cell tablets were prepared using the same amount of cells to exhibit the identical Zn-Kα3 peak intensity at 8,462.8 eV (no Zn was added in bacterial media and any other solutions throughout the experiment). Energy selection was accomplished by a double crystal Si (1, 1, 1) monochromator. Intensities of incident and transmitted X-ray were measured by ion chamber. Fluorescent intensities were measured by silicon drift X-ray detector. Standard chemicals, Na2CrVIO4 ·4H2O, \({\text{Cr}}_{2}^{III} {\text{O}}_{3}\) and CrIIIOOH were mixed with boron nitride (BN) at the ratios of 34.8, 73.0 and 65.3 times, respectively, for XAFS measurement.
Results and discussion
Cr(VI) tolerance and Cr(VI) reduction during aerobic growth of Ac. aromatica PFBC
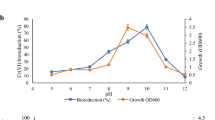
During aerobic growth on 10 mM fructose, strain PFBC readily reduced Cr(VI), especially at lower pH (Fig. 1). Since changes in total soluble Cr concentrations were only marginal (data now shown), Cr(VI) reduced by strain PFBC remained mostly in the soluble form of Cr(III). While the optimal pH for strain PFBC is reported to be 3.8 (with the growth pH range of 2.5–5.0; Jones et al. 2013), Cr(VI) reduction by strain PFCB was found more significant at sub-optimal pH of 2.5 than pH 4.0 (Fig. 1); though this observation is likely partly due to the chemical property of Cr(III) species being more stable under highly acidic solutions (Rai et al. 1989). At pH 2.5, the initial Cr(VI) concentration of 20 µM decreased effectively to 0.4 µM (0.02 mg/L) during aerobic cell growth (Fig. 1), satisfying the WHO guideline on Cr(VI) concentration in drinking water (0.05 mg/L; www.who.int/water_sanitation_health/dwq/chemicals/chromiumsum.pdf).
Changes in Cr(VI) concentrations (solid lines) and cell densities (broken lines) during aerobic growth of Ac. aromatica PFBC on 10 mM fructose, at pH 2.5 (filled circle, open circle) and pH 4.0 (filled square, open square). Solid (filled circle, filled square) and open (open circle, open square) symbols indicate inoculated and sterile control cultures, respectively. Data points are mean values from duplicate cultures; error bars depicting averages are not visible as these were smaller than the data point symbols in all cases
At different initial Cr(VI) concentrations tested, the inhibitory effect of Cr(VI) and Cr(III) on microbial growth was generally more significant at pH 2.5 than at pH 4.0. The presence of 200 µM Cr(VI) completely inhibited growth of strain PFBC, whereas elevated concentrations of Cr(III) (up to 1,000 µM) did not show any inhibitory effect on its growth at both pHs (Fig. 2). Similarly, it was reported that as low as 50 µM Cr(VI) completely inhibits aerobic growth of Acidiphilum sp., whereas Cr(III) at the same concentration has very little effect (Cummings et al. 2007). Strain PFBC (so as other Ac. aromatica strains) was reported to be tolerant to elevated concentrations of a variety of metals, with minimal inhibitory concentrations (MICs) of 300 mM Al(III), 172 mM Fe(II), 200 mM Mn(II), 200 mM Ni(II), and 300 mM Zn(II), and relatively sensitive to Cu(II) (MIC; 5 mM) at pH 3.0 (Jones et al. 2013). Compared to other metal species, Cr(VI) was shown indeed highly toxic to strain PFBC. Cr(VI) tolerance and Cr(VI) reduction ability are not necessarily interrelated, since Cr(VI) can be reduced by both Cr(VI)-sensitive and resistant strains and not all Cr(VI)-resistant bacteria are able to reduce Cr(VI) (Dhal et al. 2013). This trend was shown also the case with strain PFBC.
While strain PFBC was capable of Cr(VI) reduction during aerobic as well as micro-aerobic growth, it failed to grow under anaerobic condition using Cr(VI) as the sole electron acceptor (Y. M., unpublished result). Likewise, Cr(VI) reduction was shown not linked to energy conservation in Acidiphilum sp. (Cummings et al. 2007). The facts that anaerobic Cr(VI) reduction does not support the growth of strain PFBC and that Cr(VI) was reduced more readily at its sub-optimal growth pH of 2.5 [at which Cr(VI) exhibits greater inhibitory effect on cell growth], implies that Cr(VI)-reduction activity by strain PFBC may involve an inducible detoxification process responding to lower pHs [and thus higher Cr(VI) toxicity effect]. Cr(VI) reduction was also reported to be an inducible reaction in Shewanella (Sh.) oneidensis (Viamajala et al. 2002; Belchik et al. 2011).
Effect of Fe on Cr(VI) reduction in Ac. aromatica PFBC cell suspension under micro-aerobic condition
The initial Cr(VI) concentration was set to 200 µM (10 times greater than the previous experiment) to distinguish between different chemical and microbial Cr(VI) reduction effects in strain PFBC cell suspensions (1.0 × 109 cells/mL). In the absence of Fe, approximately 50 µM Cr(VI) was microbially reduced in about 70 h (Fig. 3a). Addition of Fe(II) instantly, chemically reduced 200 µM Cr(VI) completely, as shown in the Fe(II) oxidation-Cr(VI) reduction coupling reaction Eq. (1), based on the standard redox potential values for Eqs. (2) and (3) (Cornelis 2005).
Effect of Fe on Cr(VI) reduction in cell suspension (1.0 × 109 cells/mL) of Ac. aromatica PFBC at pH 2.5 using 10 mM fructose as electron donor. Changes in concentrations of Cr(VI) (a) and Fe(II) (b) are shown. Cultures contained Cr(VI) only (filled circle, open circle), Cr(VI) plus Fe(III) (filled square, open square), Cr(VI) plus Fe(II) (filled triangle, open triangle) or Cr(III) plus Fe(III) (filled diamond, open diamond). Solid or open symbols indicate cell suspensions or sterile control cultures, respectively. Data points are mean values from duplicate cultures
The amount of Fe(II) immediately oxidized to Fe(III) (370 µM) during complete reduction of 200 µM Cr(VI) was less than its theoretical value of 600 µM; this was probably caused by a slight difference in sampling time. After the complete chemical reduction of Cr(VI) to Cr(III) by Fe(II), the concentration of Fe(II) in PFBC cell suspensions started to re-increase. In contrast, Fe(II) concentration started to decrease in sterile controls, due to chemical oxidation of Fe(II) to Fe(III) (Fig. 3b). This observation indicates that strain PFBC initiated Fe(III) reduction, by responding to the disappearance of Cr(VI). When Fe(III) instead of Fe(II) was added, Cr(VI) reduction by PFBC cell suspensions was slightly facilitated (Fig. 3a). However, since slow chemical Cr(VI) reduction was seen by Fe(III) addition even in sterile controls (Fig. 3a), this was likely caused by the presence of a trace amount of Fe(II) in the Fe(III) solution added. Since no apparent increase in Fe(II) concentration was observed in this case, strain PFBC was shown not to be able to reduce Fe(III) in the presence of Cr(VI). When Cr(III) instead of Cr(VI) was initially added in the media together with Fe(III), microbial reduction of Fe(III) to Fe(II) initiated from the beginning (Fig. 3b). The results obtained here under micro-aerobic condition thus suggest the following: Fe(III) reduction by strain PFBC is strictly suppressed by the presence of Cr(VI), and thus Cr(VI) reduction initiates only upon complete disappearance of Cr(VI) by its reduction to Cr(III).
In neutrophilic bacteria, the presence of Cr(VI) was reported to affect dissimilatory nitrate reducing activity differently in three nitrate reduces possessing different nitrate reductases [Geobacter (G.) metallireducens, D. desulfuricans, and Sulfurospirillum (S.) barnesii]: growth of G. metallireducens on nitrate was completely inhibited by Cr(VI). D. desulfuricans growth on nitrate media was initially delayed in the presence of Cr(VI), but ultimately reached comparable to the Cr(VI)-free control, following the transformation of Cr(VI) to Cr(III). In contrast, S. barnesii growth on nitrate was not affected by Cr(VI) (Chovanec et al. 2012). In the case of acidophilic Fe(III)-reducing heterotrophs, Acidocella sp. and Acidiphilum sp., they share similar characteristics in that; (i) they are both aerobes, (ii) Fe(III) reduction is observed under both micro-aerobic and anaerobic conditions, (iii) though anaerobic growth via Fe(III) respiration has not been substantiated (Jones et al. 2013). Nonetheless, as was the case with three neutrophilic nitrate reducers (Chovanec et al. 2012), it is possible that the two acidophilic Fe(III)-reducers possess different Fe(III) reduction mechanisms, since the presence of Cr(VI) affected differently on their Fe(III)-reducing activities. In A. cryptum, addition of Fe(III) resulted in much more significant Cr(VI) reduction compared to direct enzymatic Cr(VI) reduction, showing its capability in Fe(III) reduction in the presence of Cr(VI). The presence of oxygen hardly affected its Cr(VI) reduction (Cummings et al. 2007). In strain PFBC, reduction of either Fe(III) or Cr(VI) progressed in the presence of oxygen. However, strain PFBC preferentially utilized Cr(VI) as electron acceptor until depletion before Fe(III) reduction initiated, though the bacterium was shown incapable of growth via Fe(III) or Cr(VI) respiration.
Cr(VI) reduction and the following Cr(III) immobilization in Ac. aromatica PFBC high cell density suspension
In PFBC cell suspensions, there was an immediate drop in Cr(VI) concentration by 17 %, accompanied with a sudden decrease in total Cr concentration by approximately 10 % (Fig. 4). After that, microbial Cr(VI) reduction continued readily until 48 h after the incubation, followed by a slow, chemical Cr(VI) reduction by the presence of fructose (Fig. 4; as was also seen with sterile controls, García et al. 2006; Leita et al. 2011). Along with Cr(VI) reduction, total Cr was continuously removed from the solution in PFBC cell suspensions, eventually up to 35 % in 140 h (Fig. 4). Since no precipitates were visible throughout the experiment, it was anticipated that Cr was immobilized through biosorption on the cell surface, either in the form of Cr(VI) or Cr(III). To investigate the Cr immobilization mechanism, PFBC cells incubated with either Cr(VI) or Cr(III) were collected to be analysed by XAFS. Cr K X-ray absorption edges were measured in Cr(VI) standard (Na2CrVIO4 4H2O), Cr(III) standards (\({\text{Cr}}_{2}^{III} {\text{O}}_{3}\), CrIIIOOH), cells incubated with either Cr(VI) or Cr(III), and Cr-free cells (Fig. 5). Cr(VI) standard was readily differentiated from Cr(III) standard, based on the presence of a distinct pre-edge peak at 5,993 eV with the former (Fig. 5a–c; Sparks 2002). None of the cell samples tested showed the Cr(VI) pre-edge peak in their XANES spectra (Fig. 5d–f). Accordingly, it was evident that not only cells incubated with Cr(III), but also those incubated with Cr(VI), immobilized solely Cr(III) on the cell surface through biosorption. The amount of Cr(III) immobilized onto the cell surface was, however, significantly different depending on if Cr(III) was microbially produced or externally provided: Only 5 % of externally provided Cr(III), whereas 40 % of microbially produced Cr(III), were adsorbed onto the cell surface in 140 h (Fig. 4). This trend also corresponded to the results obtained from fluorescence XANES spectra (Fig. 6). The three cell samples measured by XANES were shown to contain the same amount of cells, since the Zn-Kα3 peak intensities at 8,462.8 eV were almost identical (Fig. 6a). However, a significant difference in the Cr-Kα1 peak intensities at 5,418 eV was apparent (Fig. 6b), confirming that microbially produced Cr(III), but not externally provided Cr(III) was readily adsorbed onto the cell surface.
Cr(VI) reduction (broken lines) and total Cr removal (solid lines) in Ac. aromatica PFBC high cell density suspensions (1.0 × 1010 cells/mL) (filled circle, filled triangle), and in sterile controls (open circle, open triangle). Initially 200 µM of Cr(VI) (filled circle, open circle) or Cr(III) (filled triangle, open triangle) were added in the media and incubated under micro-aerobic condition at pH 2.5, using 10 mM fructose as electron donor. Data points are mean values from duplicate cultures; errors bars depicting averages are not visible as these were smaller than the data point symbols in all cases
Based on the zeta-potential measurement, the surface of PFBC cells was negatively charged (−3.9 eV) at pH 2.5 in the absence of Cr (Fig. 7). Since Cr(VI) and Cr(III) supposedly exist in solution as HCrVIO4 − and CrIII(H2O) 3+6 , respectively, at pH 2.5 (Hawley et al. 2005; Cornelis et al. 2005), negatively charged cells likely absorb the cation, CrIII(H2O) 3+6 preferentially to anion, HCrVIO4 −. After incubation with Cr(VI), cells readily reduced Cr(VI) to Cr(III), followed by immobilization of Cr(III) on the cell surface through biosorption, whereas immobilization of externally provided Cr(III) was only negligible (Fig. 4). The results obtained from the zeta-potential measurement also support the finding: The surface charge of PFBC cells shifted to more positive after incubation with Cr(VI) (−0.66 eV), compared to after incubation with externally provided Cr(III) (−3.0 eV) at pH 2.5. The overall trend was consistent at the pH range tested (Fig. 7).
Although bio-reduced Cr(III) was reported to precipitate as Cr(OH)3 in cultures of some neutrophils (e.g. Ps. synxantha; McLean et al. 2000), a more complex fate of Cr(III) has been also revealed as an integral part of the biogeochemical cycle of chromium: Cr(VI) reduction in the presence of cellular organic metabolites formed both soluble and insoluble organo-Cr(III) complexes and it was suggested that produced Cr(III) is primarily complexed to NAD+, DNA, and other cellular components inside bacteria (Puzon et al. 2005). Deleterious effect caused by elevated concentrations of Cr(III) on Shewanella sp. MR-4 was alleviated by formation of less toxic and soluble organo-Cr(III) complexes, resulted in increased cell survival and extended Cr(VI) reduction activity of the bacterium, although which in turn precluded precipitation of Cr(III) (Bencheikh-Latmani et al. 2007). Based on such previous observations about organo-Cr(III) complex formation, it is well likely that in the case of strain PFBC, microbially reduced Cr(III) species were able to readily from complex with organic ligands available on the cell surface including extracellular polymeric substances (EPS), to result in Cr(III) immobilization. Externally provided Cr(III), on the other hand, was shown not well-immobilized on the cell surface, indicating that complexation between Cr(III) and organic ligands on the cell surface were promoted through the microbial Cr(VI) reduction process.
Conclusion
This study reports the presence of direct Cr(VI) reduction ability and its subsequent immobilization in Acidocella sp. Contribution of other Fe(III)-reducing microbes in indirect Cr(VI) reduction is expected to be significant via production of highly reactive Fe(II) ions, accounting for the dominant Cr(VI) reduction mechanism compared to direct enzymatic Cr(VI) mechanism. However, an insight of this study found that reduction of Fe(III) is strictly suppressed in the presence of Cr(VI) in Ac. aromatica: In this case, production of Fe(II) does not contribute to Cr(VI) reduction when both electron acceptors are simultaneously present in the environment. However, under the conditions where availability of Fe(III) and Cr(VI) alternates, both direct (enzymatic) and indirect [via production of Fe(II)] Cr(VI) reduction processes should come into effect accordingly.
The rate of Cr(VI) reduction maybe generally slower with acidophiles than with neutrophiles. Nonetheless, together with the observations from A. cryptum, the results of this study suggests that Cr(VI) reduction and its subsequent immobilization via biosorption may widely take place among acidophilic herterotrophs, playing important roles in Cr biogeochemistry in highly acidic environments, also showing their potential applicability in Cr(VI) bioremediation in highly acidic environments.
References
Allegretti P, Furlong J, Donati E (2006) The role of higher polythionates in the reduction of chromium(VI) by Acidithiobacillus and Thiobacillus cultures. J Biotechnol 122:55–61
Barrera-Díaz CE, Lugo-Lugo V, Bilyeu B (2012) A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J Hazard Mater 223:1–12
Belchik SM, Kennedy DW, Dohnalkova AC, Wang YM, Sevinc PC, Wu H, Lin YH, Lu HP, Fredrickson JK, Shi L (2011) Extracellular reduction of hexavalent chromium by cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl Environ Microbiol 77:4035–4041
Bencheikh-Latmani R, Obraztsova A, Mackey MR, Ellisman MH, Tebo BM (2007) Toxicity of Cr(III) to Shewanella sp. strain MR-4 during Cr(VI) reduction. Environ Sci Technol 41:214–220
Camargo FAO, Okeke BC, Bento FM, Frankenberger WT (2003) In vitro reduction of hexavalent chromium by a cell-free extract of Bacillus sp. ES 29 stimulated by Cu2+. Appl Microbiol Biotechnol 62:569–573
Chai LY, Huang SH, Yang ZH, Peng B, Huang Y, Chen YH (2009) Hexavalent chromium reduction by Pannonibacter phragmitetus BB isolated from soil under chromium-containing slag heap. J Environ Sci Health Part A 44:615–622
Chardin B, Dolla A, Chaspoul F, Fardeau ML, Gallice P, Bruschi M (2002) Bioremediation of chromate: thermodynamic analysis of the effects of Cr(VI) on sulfate-reducing bacteria. Appl Microbiol Biotechnol 60:352–360
Chovanec P, Sparacino-Watkins C, Zhang N, Basu P, Stolz JF (2012) Microbial reduction of chromate in the presence of nitrate by three nitrate respiring organisms. Front Microbiol 3:1–12
Cornelis R, Caruso JA, Crews H, Heumann KG (2005) Handbook of elemental speciation II: species in the environment, food, medicine and occupational health. Wiley, Chichester
Coupland K, Johnson DB (2008) Evidence that the potential for dissimilatory ferric iron reduction is widespread among acidophilic heterotrophic bacteria. FEMS Microbiol Lett 279:30–35
Cummings DE, Fendorf S, Singh N, Sani RK, Peyton BM, Magnuson TS (2007) Reduction of Cr(VI) under acidic conditions by the facultative Fe(III)-reducing bacterium Acidiphilium cryptum. Environ Sci Technol 41:146–152
Dey S, Paul AK (2010) Occurrence and evaluation of chromium reducing bacteria in seepage water from chromite mine quarries of Orissa, India. J Water Resource Prot 2:380–388
Dhal B, Thatoi HN, Das NN, Pandey BD (2013) Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a review. J Hazard Mater 250:272–291
Elangovan R, Philip L, Chandraraj K (2010) Hexavalent chromium reduction by free and immobilized cell-free extract of Arthrobacter rhombi-RE. Appl Biochem Biotechnol 160:81–97
Fendorf SE, Li GC (1996) Kinetics of chromate reduction by ferrous iron. Environ Sci Technol 30:1614–1617
Francis CA, Obraztsova AY, Tebo BM (2000) Dissimilatory metal reduction by the facultative anaerobe Pantoea agglomerans SP1. Appl Environ Microbiol 66:543–548
Fredrickson JK, Kostandarithes HM, Li SW, Plymale AE, Daly MJ (2000) Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl Environ Microbiol 66:2006–2011
García S, Ciullo L, Olivera MS, Gonzalez JC, Bellu S, Rockembauer A, Korecz L, Sala LF (2006) Kinetics and mechanism of the reduction of chromium(VI) by d-fructose. Polyhedron 25:1483–1490
Guha H, Jayachandran K, Maurrasse F (2003) Microbiological reduction of chromium(VI) in presence of pyrolusite-coated sand by Shewanella alga Simidu ATCC 55627 in laboratory column experiments. Chemosphere 52:175–183
Hawley EL, Deep RA, Kavanaugh MC, Jacobs JA (2005) Treatment technologies for chromium(VI). In: Guertin J, Jacobs JA, Avakian CP (eds) Chromium(VI) handbook. CRC Press, Boca Raton, pp 275–310
He ZG, Gao FL, Sha T, Hu YH, He C (2009) Isolation and characterization of a Cr(VI)-reduction Ochrobactrum sp. strain CSCr-3 from chromium landfill. J Hazard Mater 163:869–873
He MY, Li XY, Liu HL, Miller SJ, Wang GJ, Rensing C (2011) Characterization and genomic analysis of a highly chromate resistant and reducing bacterial strain Lysinibacillus fusiformis ZC1. J Hazard Mater 185:682–688
Ibrahim ASS, El-Tayeb MA, Elbadawi YB, Al-Salamah AA, Antranikian G (2012) Hexavalent chromate reduction by alkaliphilic Amphibacillus sp. KSUCr3 is mediated by copper-dependent membrane-associated Cr(VI) reductase. Extremophiles 16:659–668
Ishibashi Y, Cervantes C, Silver S (1990) Chromium reduction in Pseudomonas putida. Appl Environ Microbiol 56:2268–2270
Jacobs JA, Testa SM (2005) Overview of chromium(VI) in the environment: background and history. In: Guertin J, Jacobs JA, Avakian CP (eds) Chromium(VI) handbook. CRC Press, Boca Raton, pp 275–310
Johnson DB, McGinness S (1991) Ferric iron reduction by acidophilic heterotrophic bacteria. Appl Environ Microbiol 57:207–211
Jones RM, Hedrich S, Johnson DB (2013) Acidocella aromatica sp nov.: an acidophilic heterotrophic alphaproteobacterium with unusual phenotypic traits. Extremophiles 17:841–850
Joutey NT, Bahafid W, Sayel H, Ananou S, Ghachtouli NE (2014) Hexavalent chromium removal by a novel Serratia proteamaculans isolated from the bank of Sebou River (Morocco). Environ Sci Pollut Res 21:3060–3072
Leita L, Margon A, Sinicco T, Mondini C (2011) Glucose promotes the reduction of hexavalent chromium in soil. Geoderma 164:122–127
Lovley DR, Phillips EJP (1994) Reduction of chromate by Desulfovibrio vulgaris and its c 3 cytochrome. Appl Environ Microbiol 60:726–728
Magnuson TS, Swenson MW, Paszczynski AJ, Deobald LA, Kerk D, Cummings DE (2010) Proteogenomic and functional analysis of chromate reduction in Acidiphilium cryptum JF-5, an Fe(III)-respiring acidophile. Biometals 23:1129–1138
Mangaiyarkarasi MSM, Vincent S, Janarthanan S, Rao TS, Tata BVR (2011) Bioreduction of Cr(VI) by alkaliphilic Bacillus subtilis and interaction of the membrane groups. Saudi J Biol Sci 18:157–167
Mclean JS, Beveridge TJ, Phipps D (2000) Isolation and characterization of a chromium-reducing bacterium from a chromated copper arsenate-contaminated site. Environ Microbiol 2:611–619
Megharaj M, Avudainayagam S, Naidu R (2003) Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47:51–54
Michel C, Brugna M, Aubert C, Bernadac A, Bruschi M (2001) Enzymatic reduction of chromate: comparative studies using sulfate reducing bacteria—key role of polyheme cytochromes c and hydrogenases. Appl Microbiol Biotechnol 55:95–100
Noroozifar M, Khorasani-Motlagh M (2003) Specific extraction of chromium as tetrabutylammonium-chromate and spectrophotometric determination by diphenylcarbazide: speciation of chromium in effluent streams. Anal Sci 19:705–708
Opperman DJ, van Heerden E (2007) Aerobic Cr(VI) reduction by Thermus scotoductus strain SA-01. J Appl Microbiol 103:1907–1913
Park CH, Keyhan M, Wielinga B, Fendorf S, Matin A (2000) Purification to homogeneity and characterization of a novel Pseudomonas putida chromate reductase. Appl Environ Microbiol 66:1788–1795
Patra RC, MalikS Beer M, Megharaj M, Naidu R (2010) Molecular characterization of chromium(VI) reducing potential in gram positive bacteria isolated from contaminated sites. Soi Biol Biochem 42:1857–1863
Puzon GJ, Roberts AG, Kramer DM, Xun LY (2005) Formation of soluble organo-chromium(III) complexes after chromate reduction in the presence of cellular organics. Environ Sci Technol 39:2811–2817
QuiIntana A, Curutchet G, Donati E (2001) Factors affecting chromium(VI) reduction by Thiobacillus ferrooxidans. Biochem Eng J 9:11–15
Rai D, Eary LE, Zachara JM (1989) Environmental chemistry of chromium. Sci Total Environ 86:15–23
Sarangi A, Krishnan C (2008) Comparison of in vitro Cr(VI) reduction by CFEs of chromate resistant bacteria isolated from chromate contaminated soil. Bioresour Technol 99:4130–4137
Shen H, Wang YT (1993) Characterization of enzymatic reduction of hexavalent chromium by Escherichia coli Atcc-33456. Appl Environ Microbiol 59:3771–3777
Sisti F, Allegretti P, Donati E (1996) Reduction of dichromate by Thiobacillus ferrooxidans. Biotechnol Lett 18:1477–1480
Soni SK, Singh R, Awasthi A, Singh M, Kalra A (2013) In vitro Cr(VI) reduction by cell-free extracts of chromate-reducing bacteria isolated from tannery effluent irrigated soil. Environ Sci Pollut Res 20:1661–1674
Sparks DL (2002) Environmental soil chemistry, 2nd edn. Academic Press, San Diego
Tandon RK, Crisp PT, Ellis J, Baker RS (1984) Effect of pH on chromium(VI) species in solution. Talanta 31:227–228
Tebo BM, Obraztsova AY (1998) Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiol Lett 162:193–198
Viamajala S, Peyton BM, Apel WA, Petersen JN (2002) Chromate reduction in Shewanella oneidensis MR-1 is an inducible process associated with anaerobic growth. Biotechnol Progr 18:290–295
Wang PC, Mori T, Komori K, Sasatsu M, Toda K, Ohtake H (1989) Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl Environ Microbiol 55:1665–1669
Wielinga B, Mizuba MM, Hansel CM, Fendorf S (2001) Iron promoted reduction of chromate by dissimilatory iron-deducing bacteria. Environ Sci Technol 35:522–527
Xu L, Luo MF, Jiang CY, Wei XT, Kong P, Liang XF, Zhao JM, Yang LR, Liu HZ (2012) In vitro reduction of hexavalent chromium by cytoplasmic fractions of Pannonibacter phragmitetus LSSE-09 under aerobic and anaerobic conditions. Appl Biochem Biotechnol 166:933–941
Zakaria ZA, Zakaria Z, Surif S, Ahmad WA (2007) Hexavalent chromium reduction by Acinetobacter haemolyticus isolated from heavy-metal contaminated wastewater. J Hazard Mater 146:30–38
Zhu WJ, Chai LY, Ma ZM, Wang YY, Mao HJ, Zhao K (2008) Anaerobic reduction of hexavalent chromium by bacterial cells of Achromobacter sp. strain Ch1. Microbiol Res 163:616–623
Acknowledgments
This work was partly supported by grants from the Japan Society for the Promotion of Science (JSPS: No. 23810024), Kurita Water and Environment Foundation (KWEF: No. 23001), and the Kyushu University P and P program. The XAFS experiments were performed at Kyushu University Beamline (SAGA-LS/BL06: No. 2013IIK018). Y. Masaki is grateful for financial assistance provided by the Kyushu University Advanced Graduate Program in Global Strategy for Green Asia. Ac. aromatica PFBC was kindly provided by Prof. D. B. Johnson (University of Bangor, UK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
Rights and permissions
About this article
Cite this article
Masaki, Y., Hirajima, T., Sasaki, K. et al. Bioreduction and immobilization of hexavalent chromium by the extremely acidophilic Fe(III)-reducing bacterium Acidocella aromatica strain PFBC. Extremophiles 19, 495–503 (2015). https://doi.org/10.1007/s00792-015-0733-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-015-0733-6