Abstract
Hexavalent chromate reductase was characterized and was found to be localized in the cytoplasmic fraction of a chromium-resistant bacterium Pannonibacter phragmitetus LSSE-09. The Cr(VI) reductase activity of cell-free extract (S12) was significantly improved by external electron donors, such as NADH, glucose, acetate, formate, citrate, pyruvate, and lactate. The reductase activity was optimal at pH 7.0 with NADH as the electron donor. The aerobic and anaerobic Cr(VI)-reduction enhanced by 0.1 mM NADH were respectively 3.5 and 3.4 times as high as that without adding NADH. The Cr(VI) reductase activity was inhibited by Mn2+, Cd2+, Fe3+, and Hg2+, whereas Cu2+ enhanced the chromate reductase activity by 29% aerobically and 33% anaerobically. The aerobic and anaerobic specific Michaelis–Menten constant K m of S12 fraction was estimated to be 64.95 and 47.65 μmol L−1, respectively. The soluble S150 fractions showed similar activity to S12 and could reduce 39.7% and 53.4% of Cr(VI) after 1 h of incubation aerobically and anaerobically while the periplasmic contents showed no obvious reduction activity, suggesting an effective enzymatic mechanism of Cr(VI) reduction in the cytoplasmic fractions of the bacterium. Results suggest that the enzymatic reduction of Cr(VI) could be useful for Cr(VI) detoxification in wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium and its compounds are widely used in industry, such as electroplating, leather tanning, water cooling, metal finishing, and wood preservation [1]. Hexavalent chromium [Cr(VI)] is generated as a by-product of these industrial processes [2]. Cr(VI)-containing wastewaters have become a well-recognized biohazard in water pollution control due to the extremely toxic, mutagenic, and carcinogenic effects of Cr(VI) on biological systems [3]. Cr(VI) is usually soluble under oxidizing conditions, and only limited removal can be achieved by conventional precipitation methods [4]. The major shortcomings of conventional treatments include poor removal efficiency, the production of large amounts of chemical sludge, and high cost of chemicals used for Cr (VI) reduction, especially for the removal of relatively low concentrations of Cr (VI) [5]. Several bacteria possess chromate reductase activity that can convert Cr(VI) to Cr(III), which is much less toxic and less soluble. Thus, biological reduction offers a viable alternative for Cr(VI) bioremediation [6–9].
Bioreduction of Cr(VI) can occur directly as a result of microbial metabolism [6, 10] or indirectly through a bacterial metabolite such as H2S [11, 12]. The enzymatic reduction of Cr(VI) mostly involves a soluble cytosolic chromate reductase under aerobic conditions [6, 10, 13–16] or a membrane-associated chromate reductase under anaerobic conditions [17, 18]. On the other hand, pseudomonad CRB5 [3] and Escherichia coli ATCC 33456 [19] possess a soluble reductase which can reduce Cr(VI) to Cr(III) both aerobically and anaerobically. It is suggested that the soluble chromate reductases found in chromate-reducing bacteria may have different primary roles [3]. Soluble chromate reductases are more amenable to protein engineering, which leads to increase the ability of the enzyme to function in the presence of other contaminants [6, 20]. In addition, other reductases such as hydrogenases [21] and nitroreductases [2] have been identified to function as chromate reductases.
Previous work has shown that a Cr(VI)-reducing strain, Pannonibacter phragmitetus LSSE-09 has the ability to reduce Cr(VI) under both aerobic and anaerobic conditions [22]. Strain LSSE-09 shows strong Cr (VI)-reduction potential under alkaline conditions, but the chromate reductase from Pannonibacter sp. has not been fully characterized to date. Elucidation of the enzymatic mechanisms involved in direct chromate reduction is crucial in designing an efficient Cr(VI) bioreduction process [16]. Hence, the objective of this study was to assess in vitro reduction of Cr(VI) by cytoplasmic fractions of this strain under both aerobic and anaerobic conditions. In addition, the present study evaluated the effects of electron donors and metal cations on Cr(VI)-reduction activity after 1 h of incubation.
Materials and Methods
Microorganisms and Culture Conditions
P. phragmitetus LSSE-09 was isolated from industrial sludge of a chromate factory in Henan province, China [22]. This strain was inoculated into 300 mL of Luria–Bertani medium (10 g tryptone, 10 g NaCl, 5 g yeast extract, and 1 L de-ionized water), cultured aerobically at 37 °C and 200 rpm for 18 h.
Preparation of Cell-Free Extracts and Periplasmic Components
Cells were harvested by centrifugation (4,000×g) for 20 min at 4 °C, washed twice with de-ionized water, and then suspended in 20 mL Tris–HCl buffers (50 mM; pH 7.0). Suspensions of the cells were kept in an ice bath and disrupted with an ultrasonic probe (JY92-II, China) at 300 W with 6 s pulse and 12 s off mode for 99 times. The sonicate was centrifuged at 12,000×g and 4 °C for 20 min to pellet unbroken cells. The supernatant thus obtained was then filtered through 0.22 μm filters (Millipore, USA) and diluted to 60 mL to produce cell-free extract (S12). Thirty milliliters of the S12 was then spun at 150,000 g for 1 h at 4 °C to produce a soluble fraction (S150) and a membrane pellet. The membrane pellet fraction was re-suspended in 10 mL Tris–HCl buffer (50 mM; pH 7.0).
Extraction of periplasmic components was accomplished using the osmotic shock procedure [3, 23]. Harvested cells were washed twice with cold 10 mM Tris–HCl buffer (pH 7.1) with 30 mM NaCl. Cells were rapidly mixed into 40% (w/v) sucrose in 20 mL Tris–HCl buffers (33 mM; pH 7.1), followed by the addition of 0.1 M EDTA to a concentration of 0.1 mM EDTA. The suspension was placed on a rotary shaker at 25 °C for 10 min (180 rpm) and centrifuged at 12,000×g and 4 °C for 10 min to recover whole cells. After that, the obtained cell pellet was rapidly dispersed in 10 mL 0.5 mM MgCl2, gently stirred in an ice bath for 10 min and centrifuged. After centrifugation, the supernatant containing the osmotic shock proteins (periplasmic contents) was assayed for Cr(VI) reduction activity. Total protein in the supernatant fraction was measured before and after treatment with EDTA to ensure that the outer membrane was permeabilized.
Chromate Reductase Assay
Cr(VI)-reduction experiments by S12 were performed at 37 °C under both aerobic and anaerobic conditions, as a function of electron donors and metal cations. The electron donors evaluated were NADH, glucose, acetate, formate, citrate, pyruvate, and lactate at 0.1 mM. The metal ions tested were NaCl, MgCl2, MnCl2, CaCl2, CdCl2, CuCl2, FeCl3, and HgCl2 at 1.0 mM. The reaction system for the enzyme assay contained 5.6 mg L−1 of Cr(VI), 1.9 mL of S12 (2.0 mg mL−1), and 0.1 mM of the electron donor or 1.0 mM of the metal cation to be tested in 50 mM Tris–HCl buffer (pH 7.0) with a total volume of 8.0 mL. Meanwhile, the Michaelis constant (K m) and the maximum reaction rate (V max) for S12 were determined by varying the substrate concentration from 3.5 to 9.3 mg L−1 at pH 7.0 under aerobic and anaerobic conditions. Controls for all experiments were supplemented with all components except S12.
For aerobic experiments, the reaction mixtures were incubated in 20-mL glass bottles. Anaerobic experiments were performed using 20 mL anaerobic glass bottles. Each bottle was sparged with nitrogen for 3 min, and introduced by a syringe needle through the self-sealing rubber septum. Air displacement was achieved by inserting another syringe needle as the outlet. Samples were taken to analyze residual Cr(VI) concentrations after 1 h of incubation. Cr(VI)-reduction experiments by cell extracts (S12, S150, membrane fraction, and periplasmic contents) were performed under aerobic and anaerobic conditions, with 0.1 mM of NADH as the electron donor. Experiments were conducted in duplicate as described above. One unit of enzyme activity was derived as amount of enzyme that reduced 1.0 nmol of Cr(VI) per min at 37 °C. Protein was determined using Coomassie blue at 595 nm, with bovine serum albumin as the standard. The analysis of Cr(VI) in aqueous samples was performed using a UV–vis spectrophotometer (UNICO7200, USA) at 540 nm after complexation with 1,5-diphenylcarbazide [24].
Results and Discussion
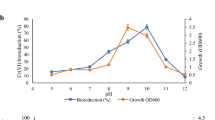
Effect of pH
Since Cr (VI) reduction is enzyme-mediated, changes in pH will affect the degree of ionization of the enzyme, the protein’s conformation, and the enzyme activity [25]. To find a suitable pH for effective chromium reduction by S12, experiments were performed at different initial pH values (pH 6.0, 7.0, 7.5, 8.0, and 9.0) with NADH as the external electron donor. As shown in Fig. 1, chromate reductase activity was observed in a wide pH range with an optimum at 7.0, and the relative activity was much lower at pH 9.0 after 1 h of incubation. However, growing cells of the alkaline-tolerant strain LSSE-09 show a strong potential to intracellularly reduce Cr(VI) to Cr(III) at pH 9.0 [22]. It could be deduced that strain LSSE-09 maintains a cytoplasmic pH lower than the ambient pH under alkaline conditions. This could contribute to an alkaline pH homeostasis which plays an essential and dominant role in strain LSSE-09 to lower the cytoplasmic pH, because bacteria must maintain an optimal cytoplasmic pH that supports growth [26]. In addition, the reductase activity proceeded at a higher rate under anaerobic conditions, which was similar to pseudomonad CRB5 [3]. The anaerobic reduction would be advantageous for bacterial removal of chromate due to the low energy requirement and small excess sludge production [27].
Effect of External Electron Donors
Figure 2a shows the effect of different electron donors on chromate reductase activity of P. phragmitetus LSSE-09 after 1 h of incubation. The blank in Fig. 2a is only S12 without electron donors. The reductase activity increased on supplementation in the reaction mixtures with all tested electron donors, suggesting that external electron donors were essential to improve the reductase activity. Glucose has been reported to act as an electron donor to increase Cr(VI) reduction rate by Bacillus sphaericus AND 303 [13]; also, formate-dependent Cr(VI) reductases have been reported in Shewanella putrefaciens MR-1 [28]. Among all tested electron donors, S12 incubated with NADH showed the most significant enhanced effect on dichromate reduction activity. The aerobic and anaerobic Cr(VI)-reduction enhanced by 0.1 mM NADH were respectively 3.5 and 3.4 times as high as that without the addition of NADH after 1 h of incubation. Other researchers have also reported that Cr(VI)-reduction by the soluble fractions of Bacillus sp. [10, 29], Pseudomonas putida PRS2000 [14], and MK1 [6] required NADH as an electron donor for activity. In comparison, pseudomonad CRB5 [3], B. sphaericus AND 303 [13], and E. coli ATCC 33456 [19] can use endogenous electron donor reserves and do not require the presence of an external electron donor.
Effect of electron donors on chromate reductase activity of S12 at initial pH 7.0 under aerobic and anaerobic conditions: a effect of different electron donors (100% relative activity—aerobic, 1.31 ± 0.12 units mg−1 protein; anaerobic, 1.79 ± 0.06 units mg−1 protein); b Cr (VI)-reduction by S12 with or without NADH
Time course of Cr(VI)-reduction by S12 at an initial Cr(VI) concentration of 5.6 mg L−1 Cr(VI) is shown in Fig. 2b. There was only a slight level of Cr(VI)-reduction without external electron donors under both aerobic and anaerobic conditions after 4 h of incubation, whereas the reductase activity was significantly improved by NADH. When supplemented with 0.1 mM NADH, the extent of reduction was rapid during the first 1 h of incubation, and S12 at a concentration of 2.0 mg mL−1 completely reduced 5.6 mg L−1 Cr(VI) within 2 h anaerobically, compared with 71.6% aerobically. Approximately 84.1% reduction was observed aerobically after 4 h of incubation with NADH.
Effect of Metal Cations
The effect of different metal cations on the chromate reductase activity of P. phragmitetus LSSE-09 after 1 h of incubation was determined as exhibited in Fig. 3. The blank in Fig. 3 is S12 added with 0.1 mM NADH without metal cations. Among the metal ions tested, Na+, Mg2+, and Ca2+ did not exhibit any significant effect on the reductase activity, whereas the presence of Mn2+, Cd2+, Fe3+, and Hg2+ inhibited Cr(VI) reduction at various levels. Hg2+ strongly inhibited the enzyme with 59% and 45% reduction in relative activity aerobically and anaerobically, presumably because mercury could bind to a variety of enzyme systems, with a particular affinity for ligands containing SH groups [10]. The negative effect of Hg2+ on Cr(VI) reduction by other chromate reductases was also reported [10, 29].
It is important to note that Cu2+ enhanced the chromate reductase activity by 29% aerobically and 33% anaerobically. Similarly, enhancement of chromate reductase activity by Cu2+ has been observed in case of Bacillus sp. ES 29 [10], Bacillus sp. RE [29], and Pseudomonas sp. G1DM21 [16] under aerobic conditions. It has been reported that Cu2+ is a transition metal that is a prosthetic group for many reductase enzymes. The role of Cu2+ in stimulation of Cr(VI) reductase could be attributed to its action as a protective agent for electron-transport, as a single electron redox center and as a shuttle for electrons between protein subunits [10, 30]. In addition, for the oxygen-sensitive chromate reductase of strain LSSE-09 which shows higher activity under anaerobic conditions, it is also possible that Cu2+ is indirectly involved in the protection of chromate reductase from O2 [10]. On the other hand, Cu2+ has been known to inhibit the chromate reductase from P. putida [6] and B. sphaericus AND 303 [13].
Kinetic Analysis
Cr(VI)-reduction by chromate reductase showed strong dependence on substrate concentration, and the aerobic and anaerobic apparent K m and the maximum specific reduction rate V max values for S12 were determined. The kinetic constants were calculated by fitting the initial rate data to a double-reciprocal Lineweaver–Burk plot of 1/V [micromoles Cr(VI) per minute per gram protein] versus 1/[Cr (VI)] (micromoles per liter) derived from a linear transformation of the Michaelis–Menten equation. This allowed the estimation of the specific K m and V max for cell-free extract reduction. The total protein in the S12 fraction was measured and then added at a concentration of 2.0 mg mL−1.
As shown in Fig. 4a, aerobic reduction by S12 resulted in an apparent K m of 64.95 and a maximum specific velocity, V max, of 2.07 nmol Cr(VI) min−1 mg−1 protein. Accordingly, the anaerobic K m and V max was 47.65 μmol L−1 and 2.83 nmol Cr(VI) min−1 mg−1 protein, as shown in Fig. 4b. The aerobic and anaerobic K m values are similar to that reported for Arthrobacter rhombi RE without the presence of NADH under aerobic conditions [31]. In this study, the anaerobic K m value is less than the aerobic one, suggesting that the crude reductase has higher activity under anaerobic condition.
Localization and Characterization of Chromate Reductase Activity
The cellular distribution of the soluble enzyme was investigated by monitoring the Cr(VI)-reducing activity in the cell-free extract fractionated by ultracentrifugation and the isolated periplasmic contents of P. phragmitetus LSSE-09. As shown in Table 1, the soluble S150 fractions showed similar activity to S12 and could reduce 39.7% and 53.4% of Cr(VI) after 1 h of incubation aerobically and anaerobically. No obvious reduction of Cr(VI) using the membrane fraction was observed under aerobic and anaerobic conditions. The results indicated that the enzyme activity was mainly associated with the soluble fraction (S150), not with the re-suspended membrane fraction as reported for pseudomonad CRB5 [3]. For Cr(VI) reduction by E. coli ATCC 33456 [19], under aerobic and anaerobic conditions, Shen and Wang have indicated that there was minor Cr(VI) reductase activity associated with the membrane electron transport chain, although Cr(VI) reduction this strain was largely due to soluble reductase activity. However, whether Cr(VI) reduction in strain LSSE-09 is carried out by the respiratory-chain-associated electron transport activity remains unclear. On the other hand, most soluble cytosolic chromate reductases reported could only reduce Cr(VI) under aerobic conditions [6, 10, 13–16].
Since reduction is associated with the soluble fraction, the enzyme responsible could be either cytoplasmic or periplasmic in origin [3]. After EDTA treatment to permeabilize the outer membrane, the amount of protein in solution outside the cells increased significantly, indicating that periplasmic proteins were released. However, there was only slight Cr(VI)-reduction using the periplasmic fraction under aerobic and anaerobic conditions when the periplasmic protein concentration was approximately the same as the concentration in the S150 fraction. It could be deduced that the soluble chromate reductase was present in cytoplasmic fractions of P. phragmitetus LSSE-09. Further investigation is being undertaken to purify and characterize the soluble chromate reductase from P. phragmitetus LSSE-09.
Conclusions
The present study presented the localization and characterization of the Cr(VI) reductase of P. phragmitetus LSSE-09. External electron donors were essential to improve the Cr(VI) reductase activity, and NADH showed the most significant increase in activity under both aerobic and anaerobic conditions. The Cr(VI) reductase activity which processed at a higher anaerobic rate was not stable in presence of Mn2+, Cd2+, Fe3+, and Hg2+, whereas the activity was obviously enhanced by Cu2+ under both aerobic and anaerobic conditions, presumably because of its electron-transport protection, acting as a single electron redox center and as a shuttle for electrons between protein subunits. Cu2+ could also be indirectly involved in the protection of chromate reductase from O2 for oxygen-sensitive reductase of strain LSSE-09. Chromate reductase assays of the soluble fraction (S150) have shown a high Cr(VI) reductase activity while the periplasmic contents showed no obvious reduction activity, implicating the localization of enzyme in the cytoplasmic fraction. Characterization of the chromate reductase present in strain LSSE-09 indicates that it may be useful for Cr(VI) bioremediation.
References
Patterson, J. W. (1975). Wastewater treatment technology (1st ed.). New York: Ann Arbor Science Publishers Inc.
Ackerley, D. F., Gonzalez, C. F., Keyhan, M., Blake, R., & Matin, A. (2004). Environmental Microbiology, 6, 851–860.
McLean, J., & Beveridge, T. J. (2001). Applied and Environmental Microbiology, 67, 1076–1084.
Wang, Y. T., & Shen, H. (1995). Journal of Industrial Microbiology, 14, 159–163.
Kratochvil, D., Pimentel, P., & Volesky, B. (1998). Environmental Science and Technology, 32, 2693–2698.
Park, C. H., Keyhan, M., Wielinga, B., Fendorf, S., & Matin, A. (2000). Applied and Environmental Microbiology, 66, 1788–1795.
Ackerley, D. F., Gonzalez, C. F., Park, C. H., Blake, R., II, Keyhan, M., & Matin, A. (2004). Applied and Environmental Microbiology, 70, 873–882.
Okeke, B. (2008). Journal of Industrial Microbiology and Biotechnology, 35, 1571–1579.
Okeke, B., Laymon, J., Crenshaw, S., & Oji, C. (2008). Biological Trace Element Research, 123, 229–241.
Camargo, F. A. O., Okeke, B. C., Bento, F. M., & Frankenberger, W. T. (2003). Applied Microbiology and Biotechnology, 62, 569–573.
Michel, C., Brugna, M., Aubert, C., Bernadac, A., & Bruschi, M. (2001). Applied Microbiology and Biotechnology, 55, 95–100.
Cheung, K. H., & Gu, J.-D. (2003). Chemosphere, 52, 1523–1529.
Pal, A., Dutta, S., & Paul, A. K. (2005). Current Microbiology, 51, 327–330.
Ishibashi, Y., Cervantes, C., & Silver, S. (1990). Applied and Environmental Microbiology, 56, 2268–2270.
Megharaj, M., Avudainayagam, S., & Naidu, R. (2003). Current Microbiology, 47, 51–54.
Desai, C., Jain, K., & Madamwar, D. (2008). Process Biochemistry, 43, 713–721.
Wang, P. C., Mori, T., Toda, K., & Ohtake, H. (1990). Journal of Bacteriology, 172, 1670–1672.
Bopp, L. H., & Ehrlich, H. L. (1988). Archives of Microbiology, 150, 426–431.
Shen, H., & Wang, Y. T. (1993). Applied and Environmental Microbiology, 59, 3771–3777.
Timmis, K. N., Steffan, R. J., & Unterman, R. (1994). Annual Review of Microbiology, 48, 525–557.
Chardin, B., Giudici-Orticoni, M. T., Luca, G., Guigliarelli, B., & Bruschi, M. (2003). Applied Microbiology and Biotechnology, 63, 315–321.
Xu, L., Luo, M., Li, W., Wei, X., Xie, K., Liu, L., Jiang, C., & Liu, H. (2011). Journal of Hazardous Materials, 185, 1169–1176.
Nossal, N. G., & Heppel, L. A. (1966). Journal of Biological Chemistry, 241, 3055–3062.
Greenberg, A. E., Trussell, R. R., & Clesceri, L. S. (1985). Standard methods for the examination of water and wastewater (16th ed.). New York: APHA.
Camargo, F. A. O., Bento, F. M., Okeke, B. C., & Frankenberger, W. T. (2003). Journal of Environmental Quality, 32, 1228–1233.
Padan, E., Bibi, E., Ito, M., & Krulwich, T. A. (2005). Bba-Biomembranes, 1717, 67–88.
Komori, K., Wang, P. C., Toda, K., & Ohtake, H. (1989). Applied Microbiology and Biotechnology, 31, 567–570.
Myers, C. R., Carstens, B. P., Antholine, W. E., & Myers, J. M. (2000). Journal of Applied Microbiology, 88, 98–106.
Elangovan, R., Abhipsa, S., Rohit, B., Ligy, P., & Chandraraj, K. (2006). Biotechnology Letters, 28, 247–252.
Abe, F., Miura, T., Nagahama, T., Inoue, A., Usami, R., & Horikoshi, K. (2001). Biotechnology Letters, 23, 2027–2034.
Elangovan, R., Philip, L., & Chandraraj, K. (2010). Applied Biochemistry and Biotechnology, 160, 81–97.
Acknowledgments
This work was financially supported by the National Basic Research Program of China (no. 2007CB613507).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, L., Luo, M., Jiang, C. et al. In Vitro Reduction of Hexavalent Chromium by Cytoplasmic Fractions of Pannonibacter phragmitetus LSSE-09 under Aerobic and Anaerobic Conditions. Appl Biochem Biotechnol 166, 933–941 (2012). https://doi.org/10.1007/s12010-011-9481-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9481-y