Abstract
Bauxite residue (red mud), generated during the extraction of alumina from bauxite ore is characterized by high pH, high concentrations of soluble ions with low or virtually no organic matter. These extreme conditions along with numerous nutrient deficiencies, limit the microbial growth and vegetation establishment. In the present study, diversity of both cultivable and non-cultivable bacteria present in the red mud was investigated by 16S rDNA sequence analyses. The cultivable bacteria were identified as Agromyces indicus, Bacillus litoralis, B. anthracis, Chungangia koreensis, Kokuria flava, K. polaris, Microbacterium hominis, Planococcus plakortidis, Pseudomonas alcaliphila and Salinococcus roseus based on their 16S rDNA sequence analysis. These isolates were alkali tolerant, positive for one or more of the enzyme activities tested, able to produce organic acids and oxidize wide range of carbon substrates. For non-cultivable diversity of bacteria, DNA was extracted from the bauxite residue samples and 16S rDNA clone library was constructed. The 16S rDNA clones of this study showed affiliation to three major phyla predominant being betaproteobacteria (41.1 %) followed by gammaproteobacteria (37.5 %) and bacteroidetes (21.4 %). We are reporting for the first time about the bacterial diversity of this unique and extreme environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bauxite residue (red mud) is the alkaline by-product generated in the process of alumina extraction from bauxite ore with concentrated sodium hydroxide (NaOH) at elevated temperature in the Bayer process. Use of the NaOH in the Bayer process results in the bauxite residue being extremely alkaline having a pH range of 9–13. The disposal of bauxite residue is a major problem in alumina plants throughout the world. The production of bauxite residue is estimated at about 120 megatonnes (Mt) per annum (Graefe and Klauber 2011) and these residues are commonly deposited in nearby engineered impoundments. Generally, bauxite residue ponds are extreme environments characterized by high pH (pH > 10), high electrical conductivity (EC > 30 dSm−1), high exchangeable sodium percentage (>70) (Friesl et al. 2004; Snars et al. 2004). The major components in the red mud are iron oxide, silica, un-reacted alumina and residual NaOH as Na2CO3 as well as alkali bound in the form of sodalite, ferrite, etc. Elevated levels of soluble or exchangeable Al and Fe in residue have been observed particularly at high pH values (i.e., >9.5) (Jones and Haynes 2011). High pH, high concentration of soluble ions such as sodium and carbonate are toxic and competitively inhibit the uptake of nutrients in plants. Limited information is available on rehabilitation of these habitats especially changes in the key physico-chemical and microbiological characteristics and their impact on the vegetation cover (Babu and Reddy 2011; Banning et al. 2010).
In general, microbial populations are the major contributors to the transformation of organic carbon, sulfur, nitrogenous compounds and metals, and play an important role in nutrient cycling which results in vegetation establishment. Microbial communities are able to respond more rapidly to changes in environmental conditions than plant communities and may provide an early indication of the recovery trajectory (Harris 2009). Therefore, study of structure and functioning of microbial communities in such harsh environment is beneficial part in ecological restoration. Extreme alkalinity and salinity, poor water retention, no organic matter along with numerous nutrient deficiencies (Wehr et al. 2006) of red mud may limit the microbial growth and vegetation establishment.
The community composition of bacteria and archaea has been investigated in soda lakes and other alkaline environments which showed considerable phylogenetic diversity (Duckworth et al. 1996; Joshi et al. 2008; Tiago et al. 2004; Yang et al. 2010). In general, red mud differs with natural soda lakes and other alkaline environments mainly in their origin, chemical composition and productivity. The artificial environment of red mud is originating from the chemical Bayer process which uses high temperature and pressure. The chances of microbes occurring in this environment immediately after its disposal are very meager and if microbes are found, they might have entered the red mud later, being blown into the material or by human handling of this product. However, such environment exhibits a specific type of extreme habitat for the development of haloalkaliphilic and metal-tolerant microbes that grow at high electrical conductivity (EC), alkaline pH and heavy metals. Microbes in such harsh environment may possess unique adaptation mechanism for survival in multiple stressors at a time.
Therefore, study of diversity and functioning of microbes in red mud will explore valuable information about the predominant microbes, which could possibly be used in reclamation of the red mud ponds and other biotechnological applications. These conditions will also help in selecting oligotrophic bacteria that are adapted to alkaline conditions and tolerance to high metal concentrations. The microbial studies pertaining to the artificial alkaline environments such as bauxite residues are very scarce (Banning et al. 2010; Hamdy and Williams 2001). No published data is available on the cultivable and non-cultivable diversity of bacteria of bauxite residue. In the present investigation, we adapted culture and culture-independent methods to assess the bacterial diversity present in this unique environment and also studied their growth patterns and their role in remediation.
Materials and methods
Site description and sample collection
Red mud pond of National Aluminium Company Limited (NALCO) is situated near Damanjodi (18o 46′ 22″N 082o 53′ 23″E), Orissa, India. This region is characterized by humidity with mean minimum and maximum temperature range of 27.0 and 39.6 °C, respectively. The waste of the refinery is dumped in a large open area (red mud pond) for the last 20 years. This impoundment has inclined surface due to which some portions are immersed in water and some are exposed. The exposed surface of the red mud desiccates after sun drying which eventually hardened to form the crack and the white soda flakes also appear on the outer surface of the dried red mud. These upper flakes of soda were removed and samples were collected (0–30 cm) from three different locations of the red mud pond using alcohol-sterilized Auger drillers. These samples were transported in ice and stored at 4 °C until their use. The stored sample was used for the bacterial isolation and extraction of DNA for culture-independent studies. Red mud samples were dried, sieved (<200 mesh), and ground. Finely crushed red mud samples were vacuum air-dried and analyzed qualitatively and quantitatively using an energy dispersive X-ray (EDX) Phoenix Microanalyzer, which was equipped with a Hitachi Scanning Electron Microscopy (SEM). The pH and electrical conductivity (EC) were determined in 1:2.5 (w/v) water extract using Deluxe Water and Soil Analysis Kit (Model 191 E). For chemical and anion analysis, standard methods were applied (Aichberger et al. 1986).
Isolation and characterization of bacterial strains
For isolation and enumeration of oligotrophic bacteria, red mud samples were diluted in saline [0.85 % of NaCl (w/v)] and plated on 10× diluted alkaline nutrient agar (g/L) (Casein hydrolysates 5.0, Peptone 5.0, NaCl 5.0 and Agar 20.0), alkaline tryptic soy agar (Casein enzyme hydrolysates 17.0, Papaic digest of soyabean meal 3.0, NaCl 5.0, K2HO4 2.5, Dextrose 2.5 and Agar 20.0) and alkalophilic Horikoshi agar medium (1999) (Glucose 10.0, Yeast extract 1.0, K2HPO4 0.2, Mg2SO4.7H2O 0.2, Na2CO3 10 and Agar 20.0) and the plates were incubated at 37 °C for 1 week. The final pH of media was adjusted to 8.0 using 0.1 M NaOH. The grown bacterial colonies from the plates were subcultured and maintained on the same medium. After incubation, morphologically distinct bacterial colonies were picked and repeatedly streaked on the same medium until the colony morphology of each isolate reached homogenous.
REP-PCR analysis
To study the variation among the isolates of bacteria, repetitive extragenic palindrome (REP) PCR technique was used. Genomic DNA was extracted from the bacterial isolates as described by Sambrook et al. (1989) and purified with a wizard column (Promega, USA). Reaction mixture for the REP-PCR consisted of 1× PCR buffer, 1.5 mM MgCl2, 200 µM of each dNTPs, 0.1 µM of each primer and 2.5 U units of Taq DNA polymerase (Invitrogen, USA) in a final volume of 100 µl. DNA amplification was performed with Genamp PCR system (Applied Biosystems, USA) using the following program: initial denaturation 95 °C for 2 min, 35 cycles of 92 °C for 30 s, 38 °C for 1.5 min and 60 °C for 3 min and a final extension at 68 °C for 8 min. The primers used were REP F 5′ - III ICG ICG ICA TCI GGC -3′ and REP R 5′-ICG ICT TAT CIG GCC TAC-3′.
16S rRNA sequence analysis of cultivable bacteria
Isolates with different REP-PCR profiles were selected and 16S rRNA genes of the representative isolates were amplified using the following primers: forward primer 5′-AGAGTTTGATCCTGGCTCAG-3 and reverse primer 5′-ACGGGCGGTGTGTTC-3. Reaction mixture for PCR contained 1x PCR buffer, 1.5 mM MgCl2, 200 µM of each dNTPs, 0.1 µM of each primer and 2.5 U units of Taq DNA polymerase in a final volume of 100 µl. Amplification was performed with an initial denaturation of 95 °C for 2 min, 36 cycles of 92 °C for 1 min, 48 °C for 30 s and 72 °C for 2 min and final extension at 72 °C for 8 min. The purified PCR products were cloned separately into pGMT-T Easy Vector and transformed into competent Escherichia coli DH5α as described by Krishna et al. (2008). The sequences were generated by chain termination method using Applied Biosystems automated DNA sequencer (Delhi University South Campus, Delhi). The 16S rRNA gene sequences were compared against the available DNA sequences in Ez Taxon-e database (http://eztaxon-e.ezbiocloud.net) (Kim et al. 2012a). The sequences of closely related strains were aligned using ClustalW program. The evolutionary distances were calculated by Kimura-2 parameter; phylogenetic tree was constructed by neighbor-joining method using MEGA 5 software (Tamura et al. 2011). For analysis, 1000 bootstrap replicates were performed to assess the statistical support for the tree.
Characterization of bacterial isolates
Bacterial isolates were subjected to Gram stain and different biochemical tests. Catalase and oxidase activities, nitrate reduction, citrate utilization, DNase, and acid production from carbohydrates were determined as described by Barrow and Feltham (1993). A total of 35 carbohydrate fermentation tests were performed according to the manufacturer’s instructions (Hi-Media, Mumbai, India). Enzyme activities such as acid and alkaline phosphatase (Tabatabai and Bremner 1969), urease (McGarity and Myers 1967), xylanase (Bailey et al. 1985) and CMCase (Tabatabai 1982) were also determined. Initial pH of the media was adjusted to 10 except for acid phosphatase where pH was adjusted to 6.0. The growth at different temperatures (25, 30, 37 and 45 °C), pH (5, 6, 7, 8, 9, 10.5, 11.3 and 12.0), and tolerance to various salt concentrations (2, 5, 7, 10, 12 and 15 %) was checked. Salt tolerance was monitored at 37 °C and pH 10.5, and temperature optimum was done at pH 10.5 in the absence of any added salt. To test the growth of bacteria at different pH, following buffer systems (final concentration 0.1 M) were used: phosphate buffer (pH 7.0), Tris buffer (pH 8–9) and carbonate buffer (pH 10–11.0) and disodium hydrogen phosphate buffer (pH 11–12).
The pH value of each medium was measured prior to use. To determine the organic acids released by bacterial isolates, 100 μl of bacterial suspension (≥0.5 OD at 620 nm) was aseptically inoculated into 250-ml Erlenmeyer flasks containing 100 ml of sterilized alkaline nutrient broth (pH 10) and incubated at 37 °C for 3 days on rotary shaker (100 rpm). Supernatants were collected from one ml of culture by centrifugation followed by passage through a 0.22-µm filter to remove bacteria. The concentrations of organic acids in the cell-free supernatants were detected using HPLC (Perkin Elmer, USA), equipped with Polypore-H column (Brownlee Column, Perkin Elmer, USA) and a Micro-Guard column (Perkin Elmer, USA).
Microcosm and nursery studies
To assess the survival abilities of bacterial isolates in red mud, 5.0 ml (107 cells/ml) of the bacterial suspensions was aseptically inoculated in 50-ml flask containing 20 g of red mud. Red mud and bacterial suspensions were thoroughly mixed aseptically and the flasks were incubated at 30 ◦C for 15 days. During the experiment, the samples were weighed and the water loss due to evaporation was replenished with distilled water every 4 days. Bacterial growth in red mud was determined by recording the colony forming units (CFU) by dilution plating on alkaline nutrient agar medium.
A nursery experiment was conducted to evaluate the potential role of bacterial isolates in establishing the vegetation cover on bauxite residue. Bacterial consortium was prepared by mixing equal volumes of the bacterial isolates (O.D600 1.0), centrifuging and mixing the pellet with soil rite. An alkali-tolerant bermudagrass (Cynodon dactylon) adapted to local conditions was grown in bauxite residue by inoculating the bacterial consortium (~350 log cfu/plot) in different plots (20 cm × 20 cm). The grass was irrigated without adding any fertilizer. After 6 months of plantation, the above-ground grass was harvested to evaluate the growth. The physico-chemical and biochemical properties of rhizosphere soils were also analyzed.
Extraction of total DNA from red mud, amplification of 16S rRNA gene, construction of library and sequence analysis
Total genomic DNA was extracted from red mud samples pooled together collected from three different sites with little modification to the method described by Mau (1997). In brief, 1 gm red mud sample was suspended in 560 μl TE buffer (pH 8.0) and 10 μg of crystalline lysozyme was added and incubated at 37 °C for approximately 60 min. After addition of 6 μl Proteinase-K (10 mg/ml), and 30 μl SDS (10 %), the mixture was incubated again at 37 °C for 60 min. Subsequently, 100 μl NaCl (5 M) was added and the tubes were incubated for 2 min at 65 °C. Then, 80 μl preheated CTAB solution (10 % CTAB in 0.7 M NaCl) was added and the tubes were incubated another 10 min at 65 °C followed by a series (three times) of bead beating at 2-min intervals. This was extracted with organic solvents: first, with an equal volume of chloroform: isoamyl alcohol (24:1) solution, and centrifuged 5 min at 15000×g. The upper aqueous phase was extracted with an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1) and, finally, with an equal volume of chloroform:isoamyl alcohol (24:1). DNA was precipitated by the addition of 0.3 volume ammonium acetate and 0.7 volume isopropanol to the aqueous phase, followed by 30-min centrifugation at 15000×g. DNA pellet was suspended in TE buffer and again precipitated a second time by adding 0.1 volume 3 M sodium acetate (pH 5.2) and 2.5 volumes ethanol and washed with 70 % ethanol, dried and resuspended in a final volume of 40-μl TE buffer.
The DNA was purified with a wizard column (Promega, USA) and subjected to whole genome amplification (GenomiPhi DNA Amplification Kit, GE Healthcare, USA) as per manufacturer’s direction. Amplification and cloning of 16S rRNA gene has been done as described above. About one-fifth of clones were randomly selected for amplification by PCR with vector-specific primers T7 and SP6. PCR products were sequenced using bacteria specific 16S forward primer. Vector-based sequences and chimeric sequences were eliminated using Gene Tool version 2 (http://www.biotools.com). Sequences were then subjected to BLAST to identify the nearest taxa and these sequences were aligned using CLUSTALW. Phylogenetic tree was constructed using neighbor-joining method. Bootstrap analysis, based on 1,000 replicate datasets, was performed to assess stability among the clades.
Clones were considered to belong to the same operational taxonomic Unit (OTU) if they were >97 % identical over the region of the 16S rRNA gene sequenced (Stackebrandt et al. 1993). Coverage was defined to be C = 1 − (n/N) × 100, where n is the number of OTUs, N is the number of clones examined and C is the percentage coverage (Good 1953). Shannon index (H′) was calculated with the equation H′ = −Σp i ln(pi), pi was the proportion of clones in the OTU (Hill et al. 2003). Richness estimates and diversity indices were computed using SPADE program. The extent of diversity covered by culture-independent approaches has been analyzed by rarefaction analysis using the program RarefactWin (Holland 2003).
Accession numbers of nucleotide sequences
Nucleotide sequences of the 16S rRNA genes of the cultivable bacteria and partial 16S rRNA sequences of non-cultivable bacteria have been deposited in the GenBank of NCBI under the accession numbers of EF675619–EF675628 for cultivable bacteria and EU665639–EU665677 and JX266016–JX266055 for non-cultivable bacteria, respectively.
Results and discussion
Characteristics of bauxite residue
Red mud is characterized by the dominance of iron oxide (47.2 %) and aluminum oxide (6.6 %). The pH of the bauxite residue was 11.1 and electric conductivity of 4.9 mS/cm. Organic carbon (0.0036 mg/g) and available phosphorus (0.0014 mg/g) was very low, and the total nitrogen was not detected by Kjeldahl method (Table 1).
Characterization of bacterial isolates
Colonies isolated from different media became visible after 3–4 days of incubation and number of colonies varied from 10 to 100 CFU per gram of red mud sample. Based on differences in morphology and color, bacterial colonies were picked and repeatedly streaked on the same medium until the colony morphology of each isolate reached homogenous. Finally, a total of 57 bacterial isolates were selected and subjected to Rep-PCR fingerprinting. Rep-PCR analysis clustered all these bacteria into 10 different groups. One bacterium from each group was taken as representative strain for further analysis and these strains were designated as RM9P, RM10E, RM10D, RM11R, RM12 W, RM13Y, RM1, RM1A, RM6 and RM8.
The bacterial isolates had diverse morphological appearance, pigmentation and growth patterns (Tables 2, 3). RM9P, RM11R and RM1 strains showed pink pigmentation. RM10D and RM8 were yellowish in color and other isolates were whitish to cream whitish. RM9P, RM10D, RM10E, RM11R, RM1A, RM6 and RM8 were Gram positive, while RM13Y and RM1 showed variable Gram reaction and RM12 W was Gram negative. All isolates were catalase positive except RM11R, which was weak positive. RM10E, RM11R, RM1A and RM6 were only oxidase positive. All isolates were positive for nitrate reduction whereas only RM10E was able to utilize citrate. RM8 and RM10D showed positive for urease production. RM10E, RM11R, RM12W, RM1, RM1A, RM6 and RM8 were able to produce extracellular acid phosphatase. RM1 and RM8 were able to hydrolyze xylan and carboxy methyl cellulose (CMC). All the isolates were found to be negative for the hydrolysis of lysine, ornithine, ONPG and TDA, and H2S production (Table 2).
RM9P was able to grow in 15 % of NaCl-amended media, while other isolates were grown either at 3 or 6 % of NaCl. All isolates were grown at 25–37 °C, whereas four isolates (RM9P, RM10E, RM12W and RM8) had shown the growth up to 45 °C (Table 2). The isolates were grown in nutrient media amended with different pH buffers to study alkali tolerance. RM9P, RM11R, RM12W and RM8 were able to grow at pH 10.5 and two isolates (RM9P and RM8) grew at pH 11.3, and others were grown well at pH 8 buffered media (Table 3). All bacterial isolates could grow well in un-buffered media, where sodium hydroxide was used to adjust the pH at 10.5. These isolates exuded organic acids mainly acetic acid and succinic acid and were able to oxidize different carbon sources (Table 3).
16S rDNA sequence analyses led to the identification of these bacteria as Agromyces indicus (RM8), Bacillus litoralis (RM6), B. anthracis (RM10E), Chungangia koreensis (RM1A), Kokuria flava (RM13Y), K. polaris (RM1), Microbacterium hominis (RM10D), Planococcus plakortidis (RM9P), Pseudomonas alcaliphila (RM12W) and Salinococcus roseus (RM11R). Most of the bacteria were related to the phylum Firmicutes and Actinobacteria except one isolate which belong to Proteobacteria. Phylogenetic analysis clustered these sequences into three groups with high bootstrap values. There were five isolates in group A, which belong to Phylum Firmicutes and the genera of Bacillus, Chungangia, Planococcus and Salinococcus. The isolates RM6 and RM10E were clustered with Bacillus species and RM1A with Chungangia. RM9P has phylogenetic affiliation with Planococcus and RM11R with Salinococcus. Four isolates belong to Actinobacteria related to genus Microbacterium, Kocuria and Agromyces were clustered in group B. RM1 and RM13Y clustered with Kocuria spp., RM10D with Microbacterium and RM8 with Agromyces spp. Group C consisted of only one isolate (RM12W) which clustered with Pseudomonas species (Fig. 1; Table 2).
Unrooted phylogenetic tree based on a comparison of the 16S rRNA sequences of bauxite residue isolates and some of their closest phylogenetic relatives. The tree was created by neighbor-joining method. Bar represents 2 substitutions per 100 nucleotides. Numerical values indicate bootstrap percentile from 1000 replicates
To study the effect of red mud on bacterial growth, microcosm experiment was conducted by inoculating the bacterial isolates into red mud. Figure 2 shows the total bacterial counts in the range of 46 × 104 to 157 × 104 cfu/g of red mud. Though there is a variation among the isolates in their growth, the results suggest that these strains are well adapted to red mud conditions.
Results of the nursery experiment revealed that the growth of the bermudagrass was very poor and unable to grow in some control plots, whereas the growth of grass significantly increased due to bacterial inoculation. Inoculation of bacteria significantly increased the physico-chemical properties compared to the control. pH was reduced from 11.01 to 10.5 and the EC value was significantly reduced due to bacterial inoculation. Inoculation of bacterial consortia significantly enhanced the organic carbon, available P and total nitrogen (Table 4).
Bauxite residue is unique and artificially induced environment and ill-characterized substrate for microbial growth and nutrient availability. Presence of low organic carbon, nitrogen and available phosphorus are limiting conditions to grow any heterotrophic population of microbes. We have isolated a total of 57 bacterial colonies from the different media used and Rep-PCR fingerprinting clustered all these bacteria into 10 different groups which were further identified based on 16S rDNA sequence analysis. A comparison of the bacterial diversity observed in this study with that of other studies is difficult because of lack of similar environments. Most of the bacteria isolated from the red mud are either halophilic or alkali-tolerant. Bacillus litoralis has been reported as halophilc bacteria isolated from a tidal flat of the yellow sea of Korea and is able to tolerate 3 % NaCl (Yoon and Oh 2005). Kim et al. (2012b) reported Chungangia koreensis from marine sediment and able to grow well at pH 8.0. Kaur et al. (2012) isolated Planococcus plakortidis from the marine sponge collected at a depth of 30 m from the Bay of Bengal. Salinicoccus roseus isolated from salty environments of Iran able to grow in wide range of pH (5.0–10.5), temperature (25–45 °C) and high concentration of NaCl (4.0 M) and able to degrade tellurite (Amoozegar et al. 2008). The Firmicute group of bacteria isolated in the present study is also able to grow at high pH and salt concentrations. Dastager and Damare (2013) reported that Agromyces indicus isolated from the sediment of marine environment is able to solubilize insoluble phosphates. Achal et al. (2011) reported that Kokuria flava isolated from mining area was able to remediate copper contaminated sites. Yumoto et al. (2001) isolated Pseudomonas alcaliphila from sea water and this bacteria is also known to remove nickel from Ni–citrate complexes.
The bacteria isolated in the present study showed more similarity with the bacteria isolated from marine environments and are either halophilic or alkali-tolerant. To maintain the homeostasis of the cytoplasm and the acidic environment near the cell wall, exudation of the organic acids is an important aspect of the microbial physiology. Organic acids also help in chelation of the metals present in the medium (Gadd and Griffiths 1977). This bacterial trait highlights the potential use of these bacteria in pH reduction of red mud. In the present study, majority of the bacterial isolates produced enzymes like cellulase, xylanase and phosphatase at alkaline pH. The stability of these enzymes at alkaline pH attributed to their habitat and growth profile in wide range of pH. Inoculation of bacterial consortia significantly improved the growth of bermudagrass and also the physio-chemical properties of the residue. Babu and Reddy (2011) reported that inoculation of arbuscular fungi (AM) fungi significantly increased the plant growth, nutrient uptake and improvement of soil physico-chemical and biochemical properties. They also reported that gypsum and sludge amendment to the residue along with AM fungi improved the biomass and nutrient uptake and reduction of pH of the residue. Further studies are required to carry out the influence of bacterial consortia with different amendments such as gypsum, sludge, fly ash and top soil for remediation of bauxite residue ponds.
Non-cultivable bacterial diversity
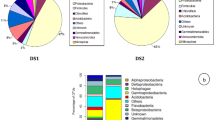
We have screened 154 clones and 60 clones were randomly selected and sequenced partially. From these, four clones were detected as chimeric sequences and 56 clones were analyzed and the closest matches were identified. Table 5 depicts the summary of the sequence analysis of the clones. The clone library can be grouped into 16 OTUs based on a cutoff value of 97 % or greater sequence similarity. The 16S rRNA clones examined in this study showed affiliation with three major phyla, predominant being gammaproteobacteria (41.1 %), followed by betaproteobacteria (37.5 %) and bacteroidetes (21.4 %) group. Out of these 56 clones analyzed, 21 turned out to be betaproteobacteria in which 18 clones showed sequence similarity with Cupriavidus while 3 clones with Achromobacter. The 23 clones which belong to gammaproteobacteria showed sequence similarities with different bacteria which include species of Acinetobacter, Pseudomonas, Serratia, Stenotrophomonas, and some uncultured bacterial clones. The bacteroidetes group consisted of 12 clones which showed similarities with Sphingobacteria and some uncultured bacterial clones.
A neighbor-joining tree was reconstructed using 16S rRNA sequences of representative clones of each OTU along with related sequences available in the database. Phylogenetic analysis clearly separated these clones into 3 groups which belong to betaproteobacteria, gammaproteobacteria and bacteroidetes (Fig. 3). The coverage value, Simpson and Shannon indices of the clone library are 0.92, 0.10 and 2.65, respectively. The rarefaction curve shown plateau phase for the bacterial populations in red mud sample (Fig. 4) indicating the richness of the bacterial diversity. The rarefaction curve analysis implied that these are likely to be minimal estimates of diversity.
It is widely believed that there is a limitation to provide a more comprehensive picture of bacterial diversity in alkaline environmental sample due to low DNA recovery (Lepere et al. 2011). In the present study, to enhance the low copy number template sequences from red mud, a pre-PCR was applied to whole genome amplification (WGA) with GenomiPhi DNA amplification Kit. Shannon index, rarefaction and coverage were used to analyze the bacterial diversity within the library. In the present study, Shannon index was 2.65 which indicated the richness of bacterial diversity. Rarefraction analysis also demonstrated similar trend of bacterial diversity in this habitat. In the present study, most of the sequences of clone library showed similarity with beta- and gammaproteobacteria while few clones represented bacteroidetes. Most of the betaproteobacteria found in red mud showed similarity with Cupriavidus species which possesses the unique ability to survive in extremely toxic environments. These bacteria are well known to live in extreme environments that are heavily contaminated with toxic metal ions (Nies 2000). The clone library sequences of red mud showed no overlap with those of isolates which is a common phenomenon in many environments. Majority of the clones, which have isolated from red mud were not found previously in alkaline environment. Moreover, because of oligotrophic and alkaline condition these microbes could not grow well and hence could not be cultivated in laboratory media.
In conclusion, the present work gives the first insight of bacterial diversity of red mud pond. Red mud might be considered as a model system having a unique habitat of alkaline, saline, sodic condition, contaminated by heavy metals and low organic carbon and nitrogen for investigating biological interactions to their surroundings and their survival mechanism. The biogeochemical cycle of the red mud is little understood and need to be worked out. Isolation of novel alkaliphilic bacteria from this environment may result in the finding of enzymes with novel properties that could be useful for diverse industrial applications. The 16S rRNA of uncultured clones suggests that bacteria affiliated with gammaproteobacteria, betaproteobacteria and bacteroidetes are unique habitat for alkaline, saline, sodic conditions. Further studies are needed to explore and exploit the cultivable bacteria present in this environment for diverse biotechnological applications.
References
Achal V, Pan X, Zhang D (2011) Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol Eng 37:1601–1605
Aichberger K, Eibelhuber A, Hofer G (1986) In: Gomez et al (eds) Sampling problems for the chemical analysis of sludge, soils and plants. Elsevier Science, New York, pp 38–44
Amoozegar MA, Ashengropha M, Malekzadeha F, Razavib MR, Naddafb S, Kabiria M (2008) Isolation and initial characterization of the tellurite reducing moderately halophilic bacterium, Salinicoccus sp. strain QW6. Microbiol Res 163:456–465
Babu GA, Reddy MS (2011) Influence of arbuscular mycorrhizal fungi on the growth and nutrient status of bermudagrass grown in alkaline bauxite processing residue. Environ Pollut 159:25–29
Bailey P, Markovic O, Mislovicova D (1985) Sensitive detection of endo-1,4-β-glucanases and endo-1,4-β-xylanases in gels. Anal Biochem 144:147–151
Banning NC, Phillips IR, Jones DL, Murphy DV (2010) Development of microbial diversity and functional potential in bauxite residue sand under rehabilitation. Restor Ecol 17:350–358
Barrow GI, Feltham RKA (1993) Cowan and steel’s manual for the identification of medical bacteria, 3rd edn. Cambridge University Press, Cambridge
Dastager SG, Damare S (2013) Marine actinobacteria showing phosphate-solubilizing efficiency in Chorao Island, Goa, India. Curr Microbiol 66:421–427
Duckworth AW, Grant WD, Jones BE, Steenbergen RV (1996) Phylogenetic diversity of soda lake alkaliphiles. FEMS Microbiol Ecol 19:181–191
Friesl W, Horak O, Wenze W (2004) Immobilization of heavy metals in soil by the application of bauxite residues: pot experiments under field conditions. J Plant Nutr Soil Sci 167:54–59
Gadd GM, Griffiths AJ (1977) Microorganisms and heavy metal toxicity. Microb Ecol 4:303–317
Good IJ (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40:237–264
Graefe M, Klauber C (2011) Bauxite residue issues: IV. Old obstacles and new pathways for in situ residue bioremediation. Hydrometallurgy 108:46–59
Hamdy MK, Williams FS (2001) Bacterial amelioration of bauxite residue waste of industrial alumina plants. J Ind Microbiol Biotechnol 27:228–233
Harris J (2009) Soil microbial communities and restoration ecology: facilitators or followers? Science 325:573–574
Hill TCJ, Walsh K, Harris JA, Moffett BF (2003) Using ecological diversity measures with bacterial communities. FEMS Microbial Ecol 43:1–11
Holland SM (2003) Analytical rarefaction 1.3 program. University of Georgia, Athens. http://www.uga.edu/*strata/software/
Horikoshi K (1999) Akaliphiles: some applications of their products for biotechnology. Microbiol Mol Biol Rev 63:735–750
Jones BEH, Haynes RJ (2011) Bauxite processing residue: a critical review of its formation, properties, storage, and revegetation. Crit Rev Environ Sci Technol 41:271–315
Joshi AA, Kanekar PP, Kelkar AS, Shouche YS, Vani AA, Borgave SB, Sarnaik SS (2008) Cultivable bacterial diversity of alkaline Lonar Lake, India. Microb Ecol 55:163–172
Kaur I, Das AP, Acharya M, Sree A, Mayliraj S (2012) Planococcus plakortidis sp. nov., isolated from the marine sponge Plakortis simplex (Schulze). Int J Sys Evol Microbiol 62:883–889
Kim OS, Cho YJ, Lee K, Yoon SH (2012a) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Micr 62:716–721
Kim W, Traiwan J, Park MH, Jung MY, Oh SJ, Yoon JH, Sukhoom A (2012b) Chungangia koreensis gen. nov., sp. nov., isolated from marine sediment. Int J Sys Evol Microbiol 62:1914–1920
Krishna P, Arora A, Reddy MS (2008) An alkaliphilic and xylanolytic strain of actinomycetes Kocuria sp. RM1 isolated from extremely alkaline bauxite residue sites. World J Microbiol Biotechnol 24:3079–3085
Lepere C, Demura M, Kawachi M, Romac S, Probert I, Vaulot D (2011) Whole-genome amplification (WGA) of marine photosynthetic eukaryote populations. FEMS Microbiol Ecol 76:513–523
Mau MK (1997) 16S rDNA-sequenzanalyse und sondendesign zur charakterisierung der bakterienpopulation im sediment eines hochbelasteten gewässers. Doctoral Thesis, Technische Universität Braunschweig
McGarity JW, Myers MG (1967) A survey of urease activity in soils of northern New South Wales. Plant Soil 27:217–238
Nies DH (2000) Heavy metal resistant bacteria as extremophiles: molecular physiology and biotechnological use of Ralstonia sp. CH34. Extremophiles 4:77–82
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd Edn. Cold Spring Harbor, New York
Snars K, Hughes JC, Gilkes RJ (2004) The effects of addition of bauxite red mud to soil on P uptake by plants. Aust J Agri Res 55:25–31
Stackebrandt E, Liesack W, Goebel BM (1993) Bacterial diversity in a soil sample from a subtropical Australian environment as determined by SSU rDNA analysis. FASEB J 7:232–236
Tabatabai MA (1982) Soil enzymes. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, microbiological and biological properties. Soil Science Society of America, Madison, pp 903–947
Tabatabai MA, Bremner JM (1969) Use of p-Nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tiago I, Chun AP, Verissimo A (2004) Bacterial diversity in a nonsaline alkaline environment: heterotrophic aerobic populations. Appl Environ Microbiol 70:7378–7387
Wehr JB, Fulton I, Menzies NW (2006) Revegetation strategies for bauxite refinery residue: a case study of Alcan Gove in Northern Territory, Australia. Environ Manag 37:297–306
Yang C, Niu Y, Su H, Wang Z, Tao F, Wang X, Tang H, Ma C, Xu P (2010) A novel microbial habitat of alkaline black liquor with very high pollution load: microbial diversity and the key members in application potentials. Bioresource Technol 101:1737–1744
Yoon JH, Oh TK (2005) Bacillus litoralis sp. nov., isolated from a tidal flat of the Yellow Sea in Korea. Int J Sys Evol Microbiol 55:1945–1948
Yumoto I, Yamazaki K, Hishinuma M, Nodasaka Y, Suemori A, Nakajima K, Inoue N, Kawasaki K (2001) Pseudomonas alcaliphila sp. nov., a novel facultatively psychrophilic alkaliphile isolated from seawater. Int J Sys Evol Microbiol 51:349–355
Acknowledgments
We would like to thank Department of Biotechnology (DBT), GoI, for their financial support to this project (BT/PR-4697/BCE/08/327/2004). The authors are thankful to Mr. S. K. Patnaik, NALCO, Damjodi for his help while collecting the samples and also to TIFAC-CORE, Thapar University, Patiala for providing lab facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. da Costa.
Rights and permissions
About this article
Cite this article
Krishna, P., Babu, A.G. & Reddy, M.S. Bacterial diversity of extremely alkaline bauxite residue site of alumina industrial plant using culturable bacteria and residue 16S rRNA gene clones. Extremophiles 18, 665–676 (2014). https://doi.org/10.1007/s00792-014-0647-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0647-8