Abstract
Alkaliphilic and halophilic Bacillus sp. BG-CS10 was isolated from Zabuye Salt Lake, Tibet. The gene celB, encoding a halophilic cellulase was identified from the genomic library of BG-CS10. CelB belongs to the cellulase superfamily and DUF291 superfamily, with an unknown function domain and less than 58% identity to other cellulases in GenBank. The purified recombinant protein (molecular weight: 62 kDa) can hydrolyze soluble cellulose substrates containing beta-1,4-linkages, such as carboxylmethyl cellulose and konjac glucomannan, but has no exoglucanase and β-glucosidase activities. Thus, CelB is a cellulase with an endo mode of action and glucomannanase activity. Interestingly, the enzyme activity was increased approximately tenfold with 2.5 M NaCl or 3 M KCl. Furthermore, the optimal temperatures were 55°C with 2.5 M NaCl and 35°C without NaCl, respectively. This indicates that NaCl can improve enzyme thermostability. The K m and k cat values of CelB for CMC with 2.5 M NaCl were 3.18 mg mL−1 and 26 s−1, while the K m and k cat values of CelB without NaCl were 6.6 mg mL−1 and 2.1 s−1. Thus, this thermo-stable, salt and pH-tolerant cellulase is a promising candidate for industrial applications, and provides a new model to study salt effects on the structure of protein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulose is a polymer composed of glucopyranose units linked by β-1,4-glucosidic bonds. It is the most abundant polysaccharide in plant biomass. It also provides a regenerable energy resource, which can be enzymatically converted into useful products, such as soluble sugar, alcohol and other industrially important chemicals (Cho et al. 2008). Cellulose was hydrolyzed by multi-component cellulase enzymes, including endoglucanase, β-glucosidase and exoglucanase (Voget et al. 2006). Firstly, cellulose was hydrolyzed by endoglucanase into a large number of small molecules of cellulose. Then, the exoglucanase cleaved the non-reduced end of cellobiose from the cellulose chain. Finally, cellobiose was transformed into glucose by β-glucosidase.
Cellulases show a wide range of applications in the food industry, in the field of feed additives, in biomedical science and the chemical industry. In particular, cellulase systems can transform cellulose into glucose, which can be fermented to fuel ethanol. Prior to a cellulase reaction, lignocelluloses are pretreated with alkalis or acids to release cellulose (Klinke et al. 2004). The alkalis or acids can be removed with large amounts of water, or neutralized with acid/alkaline solutions, which produces large amounts of salt. Halophilic or halostable cellulase works well in high-salt and high osmotic pressure environments. Thus, water consumption is profoundly reduced by utilizing halostable or halophilic cellulase compared to regular cellulase.
A large number of microorganisms are found in soda lake areas of western China (Ma et al. 2004), contributing to maintenance of the ecological cycle in these settings. These extremophiles also are important resources to obtain novel enzymes. Recently, Bacillus sp. BG-CS10 was isolated from Zabuye Salt Lake in Tibet, China. We found that BG-CS10 has an optimum pH 10 for growth and produces significant amounts of extracellular glycoside hydrolyases, such as cellulase, xylanase, mannanase, pectate lyase and amylase. BG-CS10 can grow in a wide range of NaCl concentrations from 0 to 18%. It may also play an important role in the ecological cycle of soda lakes. In this report, we investigate the functions and properties of cellulase CelB from BG-CS10. Our results show that it is a different halophilic cellulase from other cellulases.
Materials and methods
Bacterial strains, plasmids, and media
Strains, plasmids, culture conditions and chemicals
Bacillus sp. BG-CS10, grown at 37°C in modified Horikoshi-II medium (Horikoshi 1971), was deposited at the China General Microbiological Culture Collection Center (CGMCC), with the registration number CGMCC No. 1081. E. coli Rosetta (DE3) and XL10-Gold were obtained from Stratagene (Santa Clara, USA), and cultivated on LB medium. Ampicillin 100 μg mL−1 or kanamycin 50 μg mL−1 was added to select plasmid transformants. Plasmids pHBM803 (Hu et al. 2008) and pET-28a (Novagen, Darmstadt, Germany) were used as cloning and expression vectors, respectively. Cellulase activity of transformants were screened on LAK medium (LB medium supplemented with 100 μg mL−1 ampicillin, 1% konjac, 0.025% trypan blue), and induced by 0.1 mM IPTG (isopropyl β-d-1-thiogalactopyranoside).
Carboxylmethyl cellulose (CMC), methyl cellulose, pNP-β-1,4-glucopyranoside, curdlan from Alcaligenes faecalis, β-d-glucan from barley, β-1,3-glucan from Euglena gracilis, laminarin from Laminaria digitata, Guar Gum, locust bean gum, birchwood xylan, cellobiose, cellotriose, cellulose powder and pectin were purchased from Sigma Chemical Co. (St. Louis, MO). Konjac glucomannan was purchased from Megazyme (Wicklow, Ireland). Soluble starch and cellulose microcrystalline were purchased from Shanghai Chemicals division, and cellulose CM-52 and dextran were purchased from Guoyao Group Chemical Co. (Beijing, China). Konjac was purchased from Wuhan Qingjiang Konjac Production Co. (Wuhan, China).
Construction of a genomic library and screening of cellulase gene
Genomic library of BG-CS10 was constructed based on the plasmid pHBM803 (Zhang et al. 2006; Hu et al. 2008) by digestion with EarI. All transformants were replicated onto LAK medium plates supplemented with 0.1 mM IPTG, and cultured overnight at 37°C. Then, the cultures were transferred to 4°C for 2 days. The target colony showing blur zone was inoculated on LAK medium with IPTG and cultured in the conditions above described. The plasmid in the target colony showing a clear zone was named pHBM803-BG, and the inserting fragment was sequenced.
Sequencing and computer analysis of DNA and protein sequences
DNA sequencing was carried out by SinoGenoMax Co., Ltd, China. Sequence comparison was performed with updated BLAST programs online (Altschul et al. 1997). The resulting 1710 bp ORF region proposed to encode a cellulase was obtained and designated as CelB. The signal peptide was identified using the SignalP 3.0 server (http://www.cbs.dtu.dk/services/SignalP), and the promoter sequence was predicted online (http://linux1.softberry.com/berry.phtml).
Construction of the expression plasmid
Amplification of the cellulase gene celB by PCR was performed with plasmid pHBM803-BG as template and the primers with BamHI and SalI restriction sites:
-
celB1: 5′-cgcggatccgatgaaggtgctaagcaaacagatattc-3′;
-
celB2: 5′-agcgtcgacttatcgtctaccttgaacatggtttcc-3′.
The PCR product was subcloned into pET28a using two restriction sites BamHI and SalI, and the resultant plasmid was named pET28a-celB.
Expression and purification of recombinant cellulase CelB
Plasmid pET28a-celB was transformed into E. coli Rosetta (DE3). A single colony was inoculated into 30 mL LB-kanamycin medium, and the preculture was incubated at 37°C and 250 rpm overnight. Then, 10 mL of overnight culture was diluted 100-fold with fresh LB-kanamycin medium and further incubated at 37°C and 250 rpm for 3 h until OD600 reached 0.6–0.9. After IPTG induction, the culture was continued overnight at 18°C. Cells were harvested by centrifugation, followed by lysis using sonication. Purification of His-tagged cellulase was achieved using the Histrap-BindTM Kits (Novagen). Then, the fractions were further purified by Superdex 200 in 50 mM Tris–HCl buffer (pH 7.0). The molecular weight and purity of cellulase were analyzed by 12% SDS-PAGE and visualized by staining with Coomassie brilliant blue R-250. The concentration of protein was measured by the method of Bradford with a protein assay kit from Bio-Rad, using bovine serum albumin as the standard.
Enzymatic characterization of recombinant cellulase CelB
Cellulase activity was evaluated by measuring the reducing sugars using CMC as the substrate. Purified enzyme (0.05 nmol) was added to a reaction mixture containing 5 mg mL−1 suspension of CMC in 0.05 M buffer. The reaction mixtures in 0.05 M Tris–HCl (pH8.8) buffer were incubated at a range of temperatures from 20 to 70°C for 30 min to investigate the effect of temperature on CelB activity. The amount of reducing sugar was determined by dinitrosalicylic acid (DNS) according to the standard method (Miller et al. 1960), and glucose was used as a standard. One unit of enzyme activity was defined as the amount of enzyme capable of releasing 1 μmol of reducing sugar per minute under the assay conditions.
The pH profile of CelB activity was determined at 35°C without NaCl or 55°C with 2.5 M NaCl, using four buffer systems containing 0.05 M Na2HPO4–citric acid (pH 4.0–6.0), 0.05 M sodium phosphate (pH 6.0–7.5), 0.05 M Tris–HCl (pH 7.5–8.8) and 0.05 M glycine–NaOH (pH 8.8–10.6), respectively. Every assay was performed in triplicate.
The influence of salt concentration on CelB activity was analyzed in 0.05 M Tris–HCl (pH 8.8) buffer with different concentrations of NaCl (0–4 M) and KCl (0–3.5 M), in which pH values were varied only slightly by different concentrations of salt.
The kinetic parameters of CelB in the absence of NaCl were determined at 35°C in 50 mM sodium phosphate buffer (pH 6.0), and reactions were conducted for 6 min with 0.25 nmol of CelB enzyme and CMC (6, 7, 8, 9, 10 mg mL−1). The kinetic parameters of CelB in the presence of 2.5 M NaCl were determined at 55°C. Reactions were conducted for 8 min with 0.05 nmol of CelB enzyme and CMC (1, 2.5, 5, 7.5, and 10 mg mL−1). The Michaelis–Menten parameters were calculated by double-reciprocal plot.
A viscometric assay was performed using Brookfield viscometer (Brookfield Engineering Laboratories, Inc., Middleboro, USA). The reaction mixture was composed of 50 mM sodium phosphate buffer (pH 6.0), 1% CMC, 2.5 M NaCl. The total volume was 50 mL. The decrease in viscosity in the mixture was measured at 55°C at timed intervals. Timed samples (0.5 mL) were withdrawn, and the reducing sugars released were measured by the DNS reagent to calculate the extent of CMC cleavage.
The thermal stability of CelB was measured at different temperatures. The CelB fractions without NaCl or in the presence of 2.5 M NaCl were pre-incubated for 30 or 15 min in the absence of substrates at 40, 50 and 60°C. Then, the cellulase activities were measured at 55°C for 30 min in sodium phosphate, 2.5 M NaCl, pH 5.0.
The pH stability of CelB also was measured. The CelB fractions were diluted in different buffers from pH 4–10 without NaCl or with 2.5 M NaCl, at 4°C for 16 h. The residual activity was measured at 55°C for 30 min in sodium phosphate, 2.5 M NaCl, pH 5.0.
Effects of various metal ions and chemicals on the activity of recombinant CelB were determined at the final concentrations of 5 mM or 0.5% (w/w) at 55°C with 2.5 M NaCl. The enzyme activity is presented as a percentage of the ratio of residual activity to complete enzyme activity in the control sample without addition of metal ions or chemical agents.
The substrate specificity of recombinant CelB also was assayed under optimal conditions (30 min incubation at 55°C, in sodium phosphate, 2.5 M NaCl, pH 5.0).
Nucleotide sequence accession number
The nucleotide sequence of cellulase gene celB, including its flanking regions from the plasmid pHBM803-BG, has been deposited at GenBank with the accession number of GU581315.
Results
Cloning of the cellulase-encoding gene (celB) from the BG-CS10 genomic library
The total DNA of alkaliphilic strain BG-CS10 was isolated, and a genomic library composed of about 20,000 transformants was constructed. This library was screened on the LAK medium and induced by IPTG. A single colony was found that had a blur zone on LAK medium (Fig. 1a), implying presence of active cellulase, mannanase or glucomannanase since konjac is crude and natural substrate mainly constituted of β-1,4-linked d-mannose and d-glucose in a ratio of 1.6:1 (Dey and Del Campillo 1984). Plasmid pHBM803-BG harboring a 4.0-kb insert was isolated from this colony. Sequence analysis of this 4.0 kb fragment revealed three possible ORFs, including ribonucleaseH rnaH, cellulase celB and esterase estA (Fig. 1b). A putative promoter of celB locating 25 bp before the initiation codon (ATG) of celB also was identified. The conserved sequences for −35 (5′-atgtct-3′) and −10 (5′-cgttaaact-3′) areas also were found. A potential Shine–Dalgarno-type ribosome binding site (5′-GGAGG-3′) was found to be 6 bp preceding the start codon ATG of celB.
The halos and map of plasmid pHBM803-BG. a shows the halos of the target colony with pHBM803-BG. b is the map of plasmid pHBM803-BG. Plac is the promoter of the lactose operon; rnaH is the ribonucleaseH encoding gene; celB is the cellulase-encoding gene in this study; estA is the esterase encoding gene
Bioinformatic analysis of the deduced protein (CelB) encoded by celB
CelB contains 569 amino acids, including a proposed 33-residue secretion signal peptide. Blast analysis showed that CelB belongs to the cellulase superfamily and DUF291 family (an unknown function domain). It has the highest identity (80%) and similarity (90%) with putative cellulase from Bacillus agaradhaerens and an uncultured bacterium [GenBank accession number: AJ537596 and AJ537597, (Ghauri et al. 2003)], followed by cellulase B from Bacillus halodurans C-125 [64%/77%, GenBank accession number: NC002570, (Takami et al. 2000)], endoglucanase from Paenibacillus sp. KSM-N546 [58%/75%, GenBank accession number: AB167733, (Ogawa et al. 2009)], cellulase from Geobacillus sp. Y412MC10 [55%/73%, GenBank accession number: ABRG01000004, not published], celB from P. lautus [54%/69%, GenBank accession number: P23550, (Hansen et al. 1992)], and Cel5B from Paenibacillus polymyxa [53%/69%, GenBank accession number: EF621409, (Cho et al. 2008)].
The predicted mature protein CelB has a calculated molecular weight of 62 kDa and pI of 4.37. Further alignment indicated that CelB contains one potential N-glycosylation site (507-NWT), but no putative disulfide linkage. The highly conserved catalytic residue Glu-180 is crucial for enzyme activity of the family 5 of glycoside hydrolases (Henrissat 1991). A large number of negative charge amino acids were found in the mature protein with percentages of 7.45 and 8.95 for Asp and Glu, respectively, whereas a small number of basic amino acids were found with a percentage of 2.73 for Arg and 2.42 for Lys. This result is similar to that of halophilic malate dehydrogenase (Cendrin et al. 1993; Mevarech et al. 2000) from halophilic archaea Haloarcula marismortui, where Asp and Glu constitute 20.5% of all residues, while the content of Lys along with Arg is only 7.6%. Significantly, no report has revealed the salt effects on enzyme activity of cellulase Cel5B from Paenibacillus polymyxa, which has the highest sequence identity with CelB, among all the cellulases with well-characterized enzymatic properties. The total composition of Asp and Glu in Cel5B was 9.92%, which is lower than that in CelB. Conversely, the Arg and Lys were 9.74%, which are higher than that of CelB. Paenibacillus polymyxa is not a halotolerant bacterium. This comparison indicates the CelB is more likely to be a halophilic or halostable protein in the composition of amino acids because of an abundance of acidic residues (Elcock and McCammon 1998).
Expression and purification of cellulase CelB in E. coli
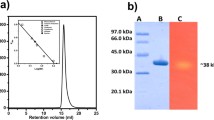
The plasmid pET28a-celB, containing the mature ORF region of CelB was constructed and expressed in E. coli Rosetta (DE3). The cellulase CelB was purified as described in the “Materials and methods” section. The summary of the methodology is shown in Table 1. The purity of CelB was analyzed by SDS-PAGE (Fig. 2). The purified protein showed a single band with molecular weight of 62 kDa, which is consistent with the predicted size of CelB.
SDS-PAGE analysis of CelB protein at each purification step. Lane 1 is the protein molecular weight marker (100, 75, 50, 35, 25 kDa); lane 2 is the cell-free extract of E. coli pET28a-celB; lane 3 is the CelB after purification step using Ni–NTA; lane 4 is the CelB after purification step using Superdex 200
Enzymatic characterization of CelB
The enzymatic activity of CelB was measured at different temperatures and pH values. Data analysis showed that the optimal reaction temperature for CelB was 35°C (Fig. 3) and the optimal pH was 5 (Fig. 4a). We found that at pH 7.5, the enzyme activity in sodium phosphate buffer was twice as high as that in the Tris–HCl buffer. Considering that CelB originates from a strain isolated from a salt lake, the effects of salts on CelB activity were further investigated. Figure 5 shows the enzymatic activities of CelB as a function of NaCl and KCl. Maximal activities of the enzyme were detected in 2.5 M NaCl or 3 M KCl, which shows a tenfold higher activity than that in the absence of salt. The CelB activity remained higher than 80% in 4 M NaCl or 3.5 M KCl. In the presence of 2.5 M NaCl, the optimal pH and temperature were measured as 5 (Fig. 4b) and 55°C (Fig. 3), respectively. The recombinant CelB displayed an optimal temperature of 45 and 55°C in the presence of 0.5 M and 1 M NaCl, respectively (Fig. 3). With 2.5 M NaCl, 50% residual activity remained between pH 4.5 and 9.2, suggesting its stability in a quite broad pH range.
Effects of pH on enzyme activity in the absence or presence of salt. CelB was incubated for 30 min at the indicated pH values in 50 mM buffers in the absence of NaCl (a at 35°C) or in the presence of 2.5 M NaCl (b at 55°C). The enzyme activity is presented as a percentage of the maximum activity in each group
The K m and V max values of CelB for CMC in the presence of 2.5 M NaCl were 3.18 mg mL−1 and 1.5 nM s−1, and the calculated k cat was 26 s−1. Without NaCl, the K m and V max values of CelB for CMC were 6.6 mg mL−1 and 0.53 nM s−1, and the calculated k cat was 2.1 s−1. These data imply that salt can change binding ability between enzyme and substrate.
CelB cannot hydrolyze pNP-β-1,4-glucopyranoside to produce yellow pNP. HPLC was performed to identify the products and results show that the CelB does not hydrolyze cellobiose to produce glucose. No cellotriose was detected based on glucose and cellobiose. These results suggest that CelB has no activity of exoglucanase, β-glucosidase and transglycosidase. CelB drastically reduces the viscosity of CMC solution. It decreases as much as 60% of the initial viscosity within 5 min, whereas the amount of reducing sugar increased by 30% (Fig. 6). These results also indicate that CelB has endo-cleaving activity for CMC (Liu et al. 2009). Thus, CelB is a true endoglucanase for soluble cellulose.
Ratio of viscosity reduction and reducing sugar increase. CelB was added to the reaction mixture, which was composed of 50 mM sodium phosphate buffer (pH 6.0), 1% CMC, 2.5 M NaCl and the enzyme in a total volume of 50 mL. The decrease in viscosity (filled square) in the mixture was measured at 55°C at timed intervals. Timed samples (0.5 mL) were withdrawn, and the released reducing sugars (filled diamond) were measured by the DNS reagent to calculate the extent of CMC cleavage
To characterize the thermal stability of CelB, enzymes in the presence of 2.5 M NaCl and in the absence of NaCl were pre-incubated at different temperatures for 30 or 15 min without addition of substrates. Then, activities were detected at 55°C for 30 min with 2.5 M NaCl. The results (Table 2) show that the CelB was stable after 30 min pre-incubation at 60°C with 2.5 M NaCl and 40°C without NaCl, while enzymatic activity was lost completely after 15 min pre-incubation at 50°C without NaCl. These results suggest that salt significantly improves the thermal stability of CelB.
The pH stability of CelB in the presence of 2.5 M NaCl and in the absence of NaCl also was measured (Fig. 7). The results in the presence or absence of NaCl show that the CelB was very stable from pH 5 to 10. The activity was lower only when the pH was lower than 5, suggesting that salt does not affect the pH stability.
The effects of metal ions and some chemicals on the activity of CelB also were investigated (Tables 3, 4). In the presence of 2.5 M NaCl, Li+, Rb+, NH4 + increased its activity by 6–15%, and other metal ions reproducibly inhibited its activity by 47–81%. Hg2+ was found to significantly inhibit the activity of most glycoside hydrolase, since Hg2+ can oxidize indole rings (Hu et al. 2008) of tryptophan residues of the enzyme. However, our data show that 45% residual activity of CelB remained in the presence of 2.5 M NaCl along with 5 mM Hg2+, although there were 15 tryptophan residues in CelB sequence. DMSO, triton X-405 and isopropanol (5%) did not show obvious inhibition to CelB activity. Glycerol, methanol (5%), 5 mM EDTA and 0.025% SDS could partially inhibit the activity of CelB. This suggests that CelB can act in a high-salt and high osmotic pressure environment.
The substrate specificity and specific activity of CelB were measured under the optimal condition (pH 5, 2.5 M NaCl and 55°C) as described above. It was observed that CelB could efficiently hydrolyze CMC (33 U mg−1), β-d-glucan from barley (83.5 U mg−1), methyl cellulose (7 U mg−1) and konjac glucomannan (30 U mg−1). Additionally, it did not show any activity on insoluble cellulose, such as cellulose microcrystalline, cellulose CM-52, cellulose powder and dextran, nor with other polysaccharides, like β-1,3-glucan, laminarin, mannan (Guar Gum and locust bean gum), xylan, curdlan, pectin and soluble starch. Thus, the enzyme has beta-1,4-linkages bond specificity on soluble cellulose, and we speculate that CelB does not contain cellulose-binding domain (CBD). In order to identify the activity of CelB to glucosidic bonds between glucose and mannose, the substrates konjac glucomannan and CMC with concentration of 5 mg mL−1 were hydrolyzed with excessive CelB during an overnight reaction. Then, the reducing sugars were measured, respectively. The results show that CelB acts on konjac glucomannan with tenfold relative activity compared to CMC. These results suggest that CelB can act on glucosidic bonds between glucose and mannose, which is consistent with the recent report by Vanfossen et al. (2011).
Discussion
Among the 20,000 transformants of the genomic library of alkaphilic strain BG-CS10, no positive target clone with cellulase or mannanase activity was detected by regular screening methods, when the transformants were spread on konjac-containing plates. We speculate that the native promoter of celB does not work in E. coli. A positive clone was obtained once the 0.1 mM IPTG was added in the konjac-containing plates to act on the strong promoter Plac and induce gene expression (Fig. 1a). This promoter crosses gene rnaH and reads through (Fig. 1b) so that the gene celB can be expressed weakly. A blurring halo was observed after incubation at 37°C overnight followed by 4°C for another 2 days (Fig. 1a); thus an efficient method was applied to screen a useful biocatalyst from extremophiles or extreme environmental samples.
Protein sequence comparisons between CelB and other cellulases revealed that CelB belongs to the cellulase superfamily and DUF291 family. There are over 20 cellulases with 2 domains cellulase superfamily and DUF291 family, sharing 30–80% similarity with CelB. Up to date only three cellulases from Paenibacillus sp. KSM-N546, P. polymyxa and P. lautus were characterized and published; however, no report about the relationship of enzyme and salt is available. In addition, Paenibacillus sp. KSM-N546, P. polymyxa and P. lautus are not halotolerant or halophilic strains. CelB and other 17 cellulases with characterization reports from Genbank were selected to construct the phylogenetic tree (data not shown). Cellulases from Paenibacillus sp. KSM-N546, P. polymyxa and P. lautus (Hansen et al. 1992) with cellulase superfamily and DUF291 family showed high identity (59, 53 and 54%) with CelB. Another 14 cellulases without DUF291 domain showed lower similarity with CelB.
The function of the DUF291 domain is unknown, but it may be involved in mediating interactions with carbohydrates (Cho et al. 2008). The structure of this module is known, but it consists of an Ig-like fold (Mosbah et al. 2000). In the present study, we found that the DUF291 domain is not a cellulose-binding domain, since CelB cannot hydrolyze insoluble cellulose, such as cellulose microcrystalline, cellulose CM-52 and cellulose powder. The same result was observed with endoglucanase from Paenibacillus sp. KSM-N546, while CelA from P. lautus can degrade microcrystalline, Avicel cellulose and acid-swollen cellulose. Cel5B from P. polymyxa had no related data. The 40 amino acids in the C-terminal of CelB were found to be critical for cellulase activity. No cellulase activity of recombinant protein was detected when the 40 amino acids were deleted. Endoglucanase from Paenibacillus sp. KSM-N546 also was truncated and no activity was detected (Ogawa et al. 2009). The study of the function of DUF291 domain will be continued in our future work.
To our knowledge, the effect of salt on activity has been investigated in only five cellulases. The halostable cellulase from Salinivibrio sp. strain NTU-05 has the highest activity in 5% NaCl (Wang et al. 2009). The halo-alkaliphilic, thermo-stable endoglucanase from moderate halophilic Bacillus sp. C14 isolated from Van Soda Lake, has the highest activity in 20% NaCl, which is 1.3 times higher than that with no salt (Aygan and Arikan 2008). The endoglucanase from Bacillus agaradhaerens JAM-KU023 is a salt-activated endoglucanase. The enzyme activity increased about fourfold by adding 0.2–2.0 M NaCl (Hirasawa et al. 2006). The endoglucanase was purified from the culture broth, the molecular mass was about 38 kDa and the N-terminal 19 amino acids were identical to Cel5A from Bacillus agaradhaerens DSM8721T, which is different from that of CelB. A halotolerant cellulase-encoding gene from metagenomes of uncultured microorganisms was expressed in E. coli and the recombinant enzyme retained 86–87% activity after incubation in 3 M NaCl, 3 M RbCl or 4 M KCl for 20 h (Voget et al. 2006). The Egl from Bacillus subtilis AU-1 was activated by 1.37 times in the presence of 0.1 M NaCl at pH 6 (Au and Chan 1987). CelB has the highest activity in 2.5 M (14.6%) NaCl. Its enzyme activity increased approximately tenfold with 2.5 M NaCl compared to that without NaCl (Table 5). Among the six halostable or halophilic cellulases, only two from uncultured microorganisms and B. agaradhaerens have protein sequence data. These two sequences were also aligned with CelB. Beyond this, our alignments showed that three salt-related cellulases belong to different branches of the phylogenetic tree, and no obvious sequence homology was found.
The optimal pH is 5 for CelB, which originated from a strain with optimal growth at pH 10. We speculate that the function of CelB of BG-CS10 has degenerated, since BG-CS10 may possess other cellulases. Considering evolutionary associations, CelB may be derived from other neutral microorganisms.
CelB is unable to hydrolyze mannan (Guar Gum and locust bean gum), but it acts on konjac glucomannan with tenfold relative activity, compared to CMC. Moreover, Vanfossen et al. (2011) reported that the backbone of glucomannan was mainly composed of β-1,4-glucosidic bonds between glucose and mannose. Also, Dey and Del Campillo (1984) reported that it is mannose that constitutes the majority of glucomannan. These results suggest that CelB can hydrolyze glucosidic bonds between two glucose units and between glucose and mannose. Thus, CelB is a cellulase with glucomannanase activity. So far, only cellulase Cel45A from Trichoderma reesei (Karlsson et al. 2002), endoglucanase from Paenibacillus sp. KSM-N546 (Ogawa et al. 2009) and CelB from Caldicellulosiruptor saccharolyticus have been reported as glucomannanase and endoglucanase.
The salt can enhance the enzyme activity and thermostability of CelB. In order to explore why the activity of the enzyme is stimulated by adding salt, we used a fluorescence spectrophotometer and circular dichroism spectrum (CD) to analyze CelB at different salt concentrations, but no obvious change was observed (online supplemental material SM-Fig. 1 and SM-Fig. 2). One-dimensional 1H NMR spectra of CelB in the presence of 0.5 M NaCl and no NaCl also were investigated (online supplemental material SM-Fig. 3), but no significant alteration was found. It is likely that the overall structural change is too minimal to be detected. In order to explore the underlying mechanism of CelB activity in salt, we are currently working on solving the X-ray structure of CelB proteins.
In summary, our results present efficient gene cloning, expression and enzymatic properties about a different halophilic cellulase CelB with glucomannanase activity. The activity of CelB is tenfold higher in the presence of 2.5 M NaCl, and salt can improve thermostability, but CelB showed lower sequence similarity in comparison to other salt-related cellulases. The thermo-stable, salt and pH-tolerant cellulase is a promising candidate for cellulose degradation under high-salt conditions, and also provides a new model to study salt effects on the structure of protein.
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402
Au KS, Chan KY (1987) Purification and properties of the endo-1,4-β-glucanase from Bacillus subtilis. J Gen Microbiol 133:2155–2162
Aygan A, Arikan B (2008) A new halo-alkaliphilic, thermostable endoglucanase from moderately halophilic Bacillus sp. C14 isolated from Van soda lake. Int J Agric Biol 10:369–374
Cendrin F, Chroboczek J, Zaccai G, Eisenberg H, Mevarech M (1993) Cloning, sequencing, and expression in Escherichia coli of the gene coding for malate dehydrogenase of the extremely halophilic archaebacterium Haloarcula marismortui. Biochemistry 32(16):4308–4313
Cho KM, Hong SJ, Math RK, Islam SM, Kim JO, Lee YH, Kim H, Yun HD (2008) Cloning of two cellulase genes from endophytic Paenibacillus polymyxa GS01 and comparison with cel 44C-man 26A. J Basic Microbiol 48(6):464–472
Dey PM, Del Campillo E (1984) Biochemistry of the multiple forms of glycosidases in plants. Adv Enzymol Relat Areas Mol Biol 56:141–249
Elcock AH, McCammon JA (1998) Electrostatic contributions to the stability of halophilic proteins. J Mol Biol 280(4):731–748
Ghauri MA, Khalid AM, Grant S, Heaphy S, Grant WD (2003) Phylogenetic analysis of different isolates of Sulfobacillus spp isolated from uranium-rich environments and recovery of genes using integron-specific primers. Extremophiles 7(5):341–345
Hansen CK, Diderichsen B, Jorgensen PL (1992) CelA from Bacillus lautus PL236 encodes a novel cellulose-binding endo-β-1,4-glucanase. J Bacteriol 174(11):3522–3531
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280(Pt 2):309–316
Hirasawa K, Uchimura K, Kashiwa M, Grant WD, Ito S, Kobayashi T, Horikoshi K (2006) Salt-activated endoglucanase of a strain of alkaliphilic Bacillus agaradhaerens. Antonie van Leeuwenhoek 89(2):211–219
Horikoshi K (1971) Production of alkaline enzymes by alkalophilic microorganisms. Part I. Alkaline protease produced by Bacillus no. 221. Agric Biol Chem 36:1407–1414
Hu Y, Zhang GM, Li AY, Chen J, Ma LX (2008) Cloning and enzymatic characterization of a xylanase gene from a soil-derived metagenomic library with an efficient approach. Appl Microbiol Biotechnol 80(5):823–830
Karlsson J, Siika-aho M, Tenkanen M, Tjerneld F (2002) Enzymatic properties of the low molecular mass endoglucanases Cel12A (EG III) and Cel45A (EG V) of Trichoderma reesei. J Biotechnol 99(1):63–78
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66(1):10–26
Liu L, Feng Y, Duan CJ, Pang H, Tang JL, Feng JX (2009) Isolation of a gene encoding endoglucanase activity from uncultured microorganisms in buffalo rumen. World J Microbiol Biotechnol 25:1035–1042
Ma Y, Xue Y, Grant WD, Collins NC, Duckworth AW, Van Steenbergen RP, Jones BE (2004) Alkalimonas amylolytica gen nov., sp. nov., and Alkalimonas delamerensis gen. nov., sp. nov., novel alkaliphilic bacteria from soda lakes in China and East Africa. Extremophiles 8(3):193–200
Mevarech M, Frolow F, Gloss LM (2000) Halophilic enzymes: proteins with a grain of salt. Biophys Chem 86(2–3):155–164
Miller GL, Blum R, Glennon WE, Burton AL (1960) Measurement of carboxymethyl cellulase activity. Anal Biochem 2:127–132
Mosbah A, Belaich A, Bornet O, Belaich JP, Henrissat B, Darbon H (2000) Solution structure of the module X2 1 of unknown function of the cellulosomal scaffolding protein CipC of Clostridium cellulolyticum. J Mol Biol 304(2):201–217
Ogawa A, Sumitomo N, Hakamada Y, Saeki K, Ozaki K, Kobayashi T (2009) Nucleotide sequence of a Paenibacillus endoglucanase gene and characterization of the recombinant enzyme. J Appl Glycosci 56:253–259
Takami H, Nakasone K, Takaki Y, Maeno G, Sasaki R, Masui N, Fuji F, Hirama C, Nakamura Y, Ogasawara N, Kuhara S, Horikoshi K (2000) Complete genome sequence of the alkaliphilic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis. Nucleic Acids Res 28(21):4317–4331
Voget S, Steele HL, Streit WR (2006) Characterization of a metagenome-derived halotolerant cellulase. J Biotechnol 126:26–36
Vanfossen AL, Ozdemir I, Zelin SL, Kelly RM (2011) Glycoside hydrolase inventory drives plant polysaccharide deconstruction by the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Biotechnol Bioeng 108(7):1559–1569
Wang CY, Hsieh YR, Ng CC, Chan H, Lin HT, Tzeng WS, Shyu YT (2009) Purification and characterization of a novel halostable cellulase from Salinivibrio sp. strain NTU-05. Enzym Microb Technol 44:373–379
Zhang GM, Hu Y, Zhuang YH, Ma LX, Zhang XE (2006) Molecular cloning and expression in Pichia pastoris of a xylanase gene from Bacillus pumilus HBP8. Biocatal Biotransformation 24(5):371–379
Acknowledgments
We thank Dr. Guo Reyting for his critical review of this manuscript and helpful discussions. This research project was supported by the National Natural Science Foundation of China (31170068), the Ministry of Sciences and Technology of China (973 programs 2007CB707801 and 2012CB721000, 863 programs 2006AA020201 and 2007AA021306).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. da Costa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, G., Li, S., Xue, Y. et al. Effects of salts on activity of halophilic cellulase with glucomannanase activity isolated from alkaliphilic and halophilic Bacillus sp. BG-CS10. Extremophiles 16, 35–43 (2012). https://doi.org/10.1007/s00792-011-0403-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-011-0403-2