Abstract
Antipsychotic drugs raise seizure risk in adults, and antipsychotic drug use is increasing among pediatric psychiatric disorder patients. However, few studies have examined seizure risk in this younger patient population. To evaluate seizure risk in pediatric patients on antipsychotics, we conducted a nested case–control study using a nationwide database. Patient information was retrieved from the Korean Health Insurance Review and Assessment (HIRA) database from 2008–2018. Antipsychotic use among newly diagnosed psychiatric patients was gathered starting 1 year before the index date and categorized as recent, past, consistent, or none. Seizure cases among these patients were defined based on (1) prescription of antiepileptic drugs or (2) an electroencephalography (EEG) examination among patients with seizure diagnostic code. A conditional logistic regression model was constructed to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for seizure risk due to antipsychotic use. In total, 1523 seizure cases and 6092 seizure-free controls aged 8–19 years with newly diagnosed psychiatric disorders were included for analysis. Logistic regression revealed a significant association between antipsychotic use and seizure development (recent users OR = 4.03, 95% CI 3.4–4.79; consistent users: OR = 2.84, 95% CI 2.44–3.3). Seizure risk enhanced further with an increase in the number of antipsychotic drugs used. Risperidone, aripiprazole, quetiapine, olanzapine, paliperidone, and blonanserin were independently associated with greater seizure risk. Pediatric patients receiving antipsychotics, especially new or multiple antipsychotic users, should be carefully monitored for seizure development.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Antipsychotic prescriptions for children and adolescents have increased substantially in recent years [1,2,3,4,5,6,7,8]. While most studies documenting this rise have originated from the US and Europe [1, 2, 4, 5, 7, 8], a few studies from Asia have reported a similar increase in pediatric antipsychotic prescriptions [3, 6]. Despite this growing use, there is a lack of evidence demonstrating the long-term therapeutic efficacy of antipsychotics for children and adolescents [1, 9], and only a few such drugs have been approved for this age group, primarily for autism spectrum disorders, bipolar disorder, and schizophrenia. Thus, pediatric patients often receive multiple antipsychotic drugs for non-approved psychiatric conditions [9, 10], and there is great potential for serious adverse reactions, such as seizures.
Antipsychotics are well known to lower the seizure threshold and increase seizure risk in adults [11,12,13], but such an association has not been clearly demonstrated for children and adolescents even though the consequences may be more severe in the developing brain [14]. Indeed, pediatric seizures are associated with reductions in brain tissue volume, cognitive ability [15, 16], and quality of life [17]. Given the recent increase in antipsychotic use among pediatric patients and the general lack of information on potentially deleterious health effects, it is crucial to investigate the seizure risk in this population. To the best of our knowledge, only two studies have reported on the associations between antipsychotics and adverse events including seizure among pediatric patients in Western countries [18, 19], but neither examined the seizure risks associated with specific agents or the influences of drug dosage, exposure duration, and other relevant clinicodemographic factors. Further, these studies did not consider pre-existing health conditions or medication histories for seizure-associated neurologic conditions that could predispose to psychiatric disorders. To examine the risk of seizures in pediatric patients receiving antipsychotics, we utilized a nationwide population-based dataset containing extensive information on disease conditions and drug treatment history. We aimed to investigate the impact of the following factors on seizure development: (1) antipsychotic prescription status (i.e., recent user, past user, consistent user, or non-user), (2) antipsychotic polypharmacy, and (3) the specific type of antipsychotic agent.
Methods
Data source
We used claims data for pediatric patients with a diagnosed psychiatric disorder from the Health Insurance Review and Assessment (HIRA) database from 2010 to 2018. The Republic of Korea has a universal health care system that covers the entire population, the vast majority (97%) through the National Health Insurance Services (NHIS) and 3% by the Medical Aid Program. The HIRA database consists of claims records for all NHIS beneficiaries submitted by medical institutions for reimbursing healthcare providers. Thus, this database contains medical information for nearly all of the Korean population. From the claims data, we used age, sex, diagnosis, prescription records, and type of medical insurance to examine seizure incidence among the pediatric population receiving antipsychotic agents and associated risk factors. Prescription records provided information about active ingredients, issue dates, treatment duration, and route of administration. The study was approved by the Institutional Review Board of Kyungpook National University (IRB number: KNU 2018-0141). The need for informed patient/guardian consent was waved as the data were anonymized.
Cohort population
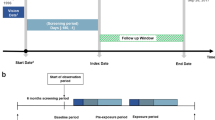
For this nested case–control study, we created an eligible cohort consisting of pediatric patients newly diagnosed with a psychiatric disorder and without seizures at baseline. The eligible cohort population excluded youth ≤ 7 years of age as such patients are rarely prescribed antipsychotics [3] and seizures are more likely related to other risk factors such as fever compared to older pediatric patients [20,21,22]. From the pediatric population aged 8–19 years, we first selected 29,069 individuals who were newly diagnosed with a psychiatric disorder and without a history of any psychiatric disorder for at least 2 years prior to the initial 2010–2018 HIRA claims date. The psychiatric disorder was identified by the F code for mental, behavioral, and neurodevelopmental disorders of the International Classification of Diseases (ICD)-10. We then reviewed all medical records for the 2 years before the first date of psychiatric diagnosis to rule out pre-existing seizures, neuropathology underlying seizures, and other neurological diseases related to seizures because most seizure cases due to neurologic conditions emerge within 2 years of diagnosis [23,24,25]. Patients with a diagnosis of seizure, prescription of an antiepileptic drug (AED), or neurologic disorders within 2 years before the first date of psychiatric diagnosis were excluded from our cohort. Supplemental Tables 1 and 2 summarize the ICD-10 codes of neurologic disorders and psychiatric drugs, respectively, for the final cohort included in the analysis.
Selection of cases and controls
The cases and controls were selected from this eligible cohort population. Seizure cases were defined as individuals receiving an AED prescription or an electroencephalographic (EEG) examination within 90 days after seizure diagnosis. Controls were selected from the subjects without seizure development in a 4:1 ratio by matching to cases according to age, sex, and the first date of psychiatric diagnosis. The index date of cases was defined as the first date of seizure diagnosis. For controls, the index date was assigned as the same index date of the matched cases.
Assessment of antipsychotic exposure
The classification of antipsychotic exposure was based on prescription records gathered starting the year before the index date. We included both typical and atypical antipsychotics in Anatomical Therapeutic Chemical (ATC) class N05A (except lithium; Supplemental Table 3). Antipsychotic use was categorized into the following four groups to account for duration and regency: (1) past user, with an antipsychotic prescription > 90 days before the index date; (2) recent user, with an antipsychotic prescription within 90 days before the index date; (3) consistent user, with antipsychotic prescriptions throughout the observation period; (4) non-user, without records of antipsychotic prescriptions during the observation period. The average number of antipsychotic agents per day was used as a metric of antipsychotic polypharmacy. The value was calculated by dividing the sum of total prescription days per each antipsychotic drug by the observation period. We also assessed the individual seizure risks for the 10 most frequently prescribed antipsychotic agents. Patients who received an antipsychotic drug for ≥ 30% of days during the year before the index date were defined as users of that agent.
Concomitant prescription of other psychiatric medications and mental health
In previous studies, the use of other psychiatric medications and the specific psychiatric condition were included as potential factors influencing seizure risk [26,27,28,29]. Therefore, we identified prescription records for other psychiatric drugs during the year prior to the index date using the following ATC codes: N04A (anticholinergic drugs), N05B (anti-anxiety drugs), N06A (antidepressant drugs), N06B (attention-deficit/hyperactivity disorder drugs, ADHD drugs), and lithium (Supplemental Table 2). Only patients receiving these drugs for ≥ 30% of days during the observation period were considered users. Psychiatric diagnoses were identified during the same period using the ICD-10 codes indicated in Supplemental Table 4.
Data analysis
The characteristics of cases and controls are presented as numbers of patients and proportions. A conditional logistic regression model was constructed to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between various covariates and seizure development. We also constructed two multiple logistic regression equations to estimate the influences of antipsychotic use and antipsychotic polypharmacy on seizure risk after adjusting for covariates. Model 1 included antipsychotic use, history of inpatient stay, and insurance type as variables. Model 2 added each additional psychiatric drug used as a covariate because other psychiatric drugs have also been identified as seizure risk factors [26]. Separate conditional logistic models were constructed to estimate the effects of individual antipsychotic agents on seizure risk. We also performed an additional adjustment for psychiatric diagnosis 1 year before the index date as supplementary information [26,27,28,29]. All statistical analyses were performed using SAS Enterprise Guide 6.1 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 1523 cases and 6092 controls were selected from an eligible population of 18,579 (Fig. 1). Table 1 summarizes the demographic and clinical variables of cases and controls as well as the univariate associations between these variables and seizure incidence. Among cases, 55.4% (844/1523) received a prescription for antipsychotics compared to only 30.8% (1879/6092) of controls. Although the primary psychiatric disease was not used as a matching variable, the distribution of disorders was similar between control and case groups. Depression was the most common primary diagnosis in both groups (case 26.2%, control 25.48%), followed by a reaction to severe stress and adjustment disorder.
About half of the case-patients used antipsychotics consistently (27.9%) or recently (22.9%). The average daily number of antipsychotic medications used was also higher in cases (mean 0.36, standard deviation [SD] 0.50) than controls (mean 0.22, SD 0.46). According to univariate logistic analysis, recent antipsychotic users were at greatest risk of seizure development compared to non-users (OR 4.03, 95% CI 3.40, 4.79), followed by consistent users (OR 2.84, 95% CI 2.44, 3.30). The risk of seizure was also higher in patients prescribed other psychiatric medications within 1 year before the index date.
Table 2 presents the seizure risk from antipsychotic use with and without adjustment for other psychiatric medications (e.g., antianxiety and antidepressant drugs). In both models, recent and consistent users were at significantly higher risk of seizure development than non-users (Model 1: OR = 4.07, 95% CI 3.42, 4.83; Model 2: OR = 3.97, 95% CI 3.32, 4.74) while past use of antipsychotics was not significantly associated with seizure incidence (Model 1: OR = 1.19, 95% CI 0.90, 1.58; Model 2, OR = 1.16, 95% CI 0.88, 1.54). Moreover, multivariable logistic regression identified significant associations between seizure incidence and both antianxiety drug and MAO inhibitor use within 1 year before the index date.
The relationships between the average daily number of antipsychotic agents and seizure risk are shown in Table 3. There was a positive association between seizure incidence and the average number of antipsychotics used both without and with adjustment for other psychiatric medications (model 1, OR = 1.73, 95% CI 1.55, 1.93; model 2, OR 1.47, 95% CI 1.27, 1.70). Antianxiety drugs, SSRIs/SNRIs, MAO inhibitors, and lithium were all associated with increased risk of seizures in the multivariable-adjusted model.
In addition, we confirmed the same tendency of antipsychotic seizure risk after adjustment for the specific psychiatric disease instead of other psychiatric drugs. Supplementary Table 6 presents the associations between seizure incidence and antipsychotic use after adjustment for specific psychiatric disease. In Supplementary Table 7, we present the associations between seizure incidence and antipsychotic polypharmacy after adjustment for psychiatric disease.
Finally, Table 4 presents the individual associations between the ten most frequently prescribed antipsychotic agents and seizure incidence after adjustment for insurance type and hospitalization (inpatient history). The most frequently prescribed antipsychotic was risperidone, followed by aripiprazole. Risperidone, aripiprazole, quetiapine, olanzapine, paliperidone, and blonanserin were significantly associated with greater seizure incidence.
Discussion
Several reports have demonstrated an association between seizure onset and the use of antipsychotics in adults [30], but there were limited reports in pediatrics. Using data from general practice and hospital records in the UK between 1999 and 2015, Brophy et al. reported that the seizure rate increased 1.4-fold in children after antipsychotic use [19]. A study by Jerrell et al. based on Medicaid data from 1996 to 2015 in the US also found elevated seizure risk in pediatric patients prescribed risperidone (OR = 1.62, 95% CI 1.13, 2.31) or multiple antipsychotics (OR = 3.41, 95% CI 2.34, 4.91) compared to age-matched patients receiving no antipsychotic medication [18]. In the current study, we confirm this association in the Korean pediatric population using a large nationally representative dataset.
Several mechanisms have been proposed to explain elevated seizure risk from antipsychotic treatment. Although antipsychotics mainly target dopamine D2 receptors, they may also block several other receptors in the dopamine receptor family (D1, D3, D4, and D5) as well as histamine H1 receptors and α1-adrenoreceptors, which can lower seizure threshold [31, 32] and disturb the neurosteroid system involved in seizure development [26, 33]. Further, repeated stimulation by antipsychotics may facilitate seizure occurrence by a ‘kindling effect’ [32, 34].
In our study population, an association between antipsychotic use and seizures was observed only in patients who recently (within 90 days before the index date) or consistently received antipsychotics, but not in patients who received antipsychotics in the past (> 90 days before the index date) even after adjusting for covariates. These results suggest that the effect of antipsychotics on seizure risk may disappear after discontinuation. Consistent with this notion, Thabet et al. reported that a healthy 3-year-old girl completely recovered 3 days from the date of incidental aripiprazole ingestion (30 mg tablets) [35]. In our study, the point estimates for ORs in recent users were higher than in continuous users, and the confidence intervals for estimated ORs did not overlap between the groups in model 1. Therefore, new users in particular should be carefully monitored for the emergence of seizures.
Our results also support previous studies showing an even higher seizure risk under antipsychotic polypharmacy. Jerrell et al. [18] reported a 3.41-fold greater seizure risk among pediatric patients prescribed multiple antipsychotics compared to pediatric patients not receiving any psychiatric medication. Similarly, multiple studies have reported that seizures are a dose-dependent side effect of antipsychotics [36,37,38,39]. Further, co-prescription of antipsychotics tends to increase the total dose [40, 41], and may further enhance seizure risk through the drug-drug interaction. Most antipsychotics are metabolized by cytochromes P450 (CYP) enzymes in the liver, such as CYP1A2, CYP3A4, and CYP2D6 [42]. Thus, antipsychotic polypharmacy could alter CYP enzyme activity, resulting in additional adverse drug–drug interactions.
Among the most frequently prescribed antipsychotics, we found that the atypical antipsychotics (i.e., risperidone, aripiprazole, quetiapine, and olanzapine) independently associated with greater seizure incidence. Lertxundi et al. suggested that atypical antipsychotics may have a higher seizure risk than typical antipsychotics [43]. However, a review by Hedges et al. concluded that both typical and atypical antipsychotics can increase the seizure risk [30]. In our study, the number of patients treated with typical antipsychotics was too small (less than 2% in both cases and controls) to confirm an association with seizures, so this issue requires additional investigation.
Moreover, our findings suggest differences in seizure risk for individual agents between adults and children. Aripiprazole treatment reportedly carries a lower seizure risk than other agents in adult patients [30, 43]. Indeed, a WHO report of adverse drug reactions reported the lowest seizure rate (2.59%) among patients taking aripiprazole [44]. A cohort study of adults in Taiwan by Wu et al. [12] also reported a 0.41-fold lower risk for seizures while taking aripiprazole compared to risperidone (although the difference did not reach statistical significance). In contrast to previous studies in adults [11,12,13, 39], the seizure risk of aripiprazole treatment was not lower than that of other agents in our study. Also inconsistent with previous studies in adults [11,12,13, 39], we did not observe a significant relationship between clozapine and seizure risk. These discrepancies underscore the importance of evaluating individual agent risk separately in children. For instance, antipsychotic-drug pharmacokinetics and pharmacodynamics may differ between children and adults [45]. Indeed, an analysis of the FDA Adverse Event Reporting System database revealed a significant difference in the safety profile of antipsychotics across age groups [46].
In addition to antipsychotics, we found associations between seizure risk and other psychiatric drugs, including anti-anxiety drugs, SSRIs, MAO inhibitors, and lithium, after adjusting for the use of antipsychotics. All of these drugs have been shown to increase seizure risk and are frequently co-prescribed with antipsychotic drugs in psychiatric patients [12]. Thus, future studies should explore the effects of co-prescribed psychiatric medications on seizure risk.
There are several limitations to this study. First, associations were based on prescription data from claims records, which do not necessarily reflect the actual dose taken as the database contains no information on adherence. In fact, adherence in patients with psychiatric disorders may be substantially lower than among patients with physical disorders [47]. Second, seizure incidence was based on insurance claims data rather than actually seizure records and so does not reflect the patient’s clinical condition (i.e., seizure frequency and severity). Nonetheless, all patients were either receiving AEDs or were confirmed by EEG examinations. Third, antipsychotic users may have been at a more severe disease stage than non-users. To minimize the effect of disease severity on seizure risk, we used the first date of psychiatric diagnosis as a matching covariate when selecting the controls and adjusted for recent psychiatric diagnosis 1 year before the index date. However, we cannot completely rule out a residual effect due to the association between psychiatric disease severity and seizure development, particularly in cases of ADHD or autism [28, 29]. In addition, this study could not assess the impact of antipsychotic dose [13] due to a lack of information in the database, which remains further study to know the dose-dependent effect of them in pediatric patients.
Using real-world data, we demonstrate enhanced seizure risk in pediatric psychiatric patients taking antipsychotics, especially among new users. In addition, this risk is further elevated by the administration of multiple antipsychotic agents (polypharmacy). Our findings indicate the need for careful monitoring of children and adolescents receiving antipsychotic treatment and for appropriate management of treatment regimens to reduce seizure risk.
References
Bachmann CJ et al (2014) Antipsychotic prescription in children and adolescents: an analysis of data from a German statutory health insurance company from 2005 to 2012. Dtsch Arztebl Int 111(3):25–34
Cooper WO et al (2006) Trends in prescribing of antipsychotic medications for US children. Ambul Pediatr 6(2):79–83
Lee H et al (2018) Nationwide Epidemiologic Study of atypical antipsychotic use among pediatric population with mental illness in Korea. J Child Adolesc Psychopharmacol 28(3):205–215
Olfson M et al (2012) National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Arch Gen Psychiatry 69(12):1247–1256
Patel NC et al (2005) Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry 44(6):548–556
Song QY, Guo LT (2013) Trends in the prescribing of psychotropic medications for inpatient children and adolescents, 2000–2010: a study from China. Int Clin Psychopharmacol 28(4):193–199
Verdoux H et al (2015) Antipsychotic prescribing in youths: a French community-based study from 2006 to 2013. Eur Child Adolesc Psychiatry 24(10):1181–1191
Waszak PM et al (2018) Antipsychotic medication prescribing trends in a pediatric population in Northern Poland 2008–2012. J Child Adolesc Psychopharmacol 28(9):631–636
Zito JM et al (2008) Off-label psychopharmacologic prescribing for children: History supports close clinical monitoring. Child Adolesc Psychiatry Mental Health 2(1):24
Harrison JN, Cluxton-Keller F, Gross D (2012) Antipsychotic medication prescribing trends in children and adolescents. J Pediatric Health Care 26(2):139–145
Bloechliger M et al (2015) Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs 29(7):591–603
Wu CS et al (2016) Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry 77(5):e573–e579
Centorrino F et al (2002) EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry 159(1):109–115
Smith ML (2010) Neuropsychology in epilepsy: children are not small adults. Epilepsia 51(Suppl 1):68–69
Harrison RM, Taylor DC (1976) Childhood seizures: a 25-year follow-up: social and medical prognosis. The Lancet 307(7966):948–951
Hermann B et al (2002) The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia 43(9):1062–1071
Sabaz M et al (2001) The health-related quality of life of children with refractory epilepsy: a comparison of those with and without intellectual disability. Epilepsia 42(5):621–628
Jerrell JM, Hwang T-L, Livingston TS (2008) Neurological adverse events associated with antipsychotic treatment in children and adolescents. J Child Neurol 23(12):1392–1399
Brophy S et al (2018) Characteristics of children prescribed antipsychotics: analysis of routinely collected data. J Child Adolesc Psychopharmacol 28(3):180–191
Cross JH (2012) Fever and fever-related epilepsies. Epilepsia 53(Suppl 4):3–8
Seizures F (1980) Long-term management of children with fever-associated seizures. Pediatrics 66(6):1009
Graves RC, Oehler K, Tingle LE (2012) Febrile seizures: risks, evaluation, and prognosis. Am Fam Phys 85(2):149–153
Zafeiriou DI, Kontopoulos EE, Tsikoulas I (1999) Characteristics and prognosis of epilepsy in children with cerebral palsy. J Child Neurol 14(5):289–294
Gururaj AK et al (2003) Epilepsy in children with cerebral palsy. Seizure Eur J Epilepsy 12(2):110–114
Misra UK, Kumar M, Kalita J (2018) Seizures in tuberculous meningitis. Epilepsy Res 148:90–95
Pisani F et al (2002) Effects of psychotropic drugs on seizure threshold. Drug Saf 25(2):91–110
Martin RC et al (2014) Psychiatric and neurologic risk factors for incident cases of new-onset epilepsy in older adults: data from US Medicare beneficiaries. Epilepsia 55(7):1120–1127
Williams AE et al (2016) Epilepsy and attention-deficit hyperactivity disorder: links, risks, and challenges. Neuropsychiatr Dis Treat 12:287–296
Besag FM (2018) Epilepsy in patients with autism: links, risks and treatment challenges. Neuropsychiatr Dis Treat 14:1–10
Hedges D, Jeppson K, Whitehead P (2003) Antipsychotic medication and seizures: a review. Drugs Today (Barc) 39(7):551–557
Williams AM, Park SH (2015) Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs 29(2):101–111
Torta R, Monaco F (2002) Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia 43(Suppl 2):8–13
Maurice T et al (1999) Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol 81(2):125–155
Haller E, Binder RL (1990) Clozapine and seizures. Am J Psychiatry 147(8):1069–1071
Thabet FI, Sweis RT, Joseph SA (2013) Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol 36(1):29–30
Devinsky O, Honigfeld G, Patin J (1991) Clozapine-related seizures. Neurology 41(3):369–369
Günther W et al (1993) EEG alterations and seizures during treatment with clozapine. Pharmacopsychiatry 26(03):69–74
Logothetis J (1967) Spontaneous epileptic seizures and electroencephalographic changes in the course of phenothiazine therapy. Neurology 17(9):869–869
Pacia SV, Devinsky O (1994) Clozapine-related seizures: experience with 5629 patients. Neurology 44(12):2247–2249
Harrington M et al (2002) The results of a multi-centre audit of the prescribing of antipsychotic drugs for in-patients in the UK. Psychiatr Bull 26(11):414–418
Roh D et al (2014) Antipsychotic polypharmacy and high-dose prescription in schizophrenia: a 5-year comparison. Aust N Z J Psychiatry 48(1):52–60
Khoury R, Ghossoub E (2019) Antipsychotics and seizures: what are the risks? Curr Psychiatry 18:21–33
Lertxundi U et al (2013) Antipsychotics and seizures: higher risk with atypicals? Seizure 22(2):141–143
Kumlien E, Lundberg PO (2010) Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure 19(2):69–73
Kearns GL et al (2003) Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med 349(12):1157–1167
Sagreiya H et al (2017) Differences in antipsychotic-related adverse events in adult, pediatric, and geriatric populations. Cureus 9(2):e1059
Cramer JA, Rosenheck R (1998) Compliance with medication regimens for mental and physical disorders. Psychiatr Serv 49(2):196–201
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1B07043416).
Author information
Authors and Affiliations
Contributions
Soo Min Jeon, Susan Park, and Jin-Won Kwon conceived the study, and Soo Min Jeon, Susan Park, Dohoon Kim, and Jin-Won Kwon executed the analysis and reviewed the results. Soo Min Jeon and Susan Park wrote the first draft of the manuscript. Jin-Won Kwon coordinated the study, and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Ethical approval for the study was obtained from the Institutional Review Board of Kyungpook National University (IRB number: KNU 2018–0141). The requirement for informed consent from the study population was waived by the board because the data was anonymous.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jeon, S.M., Park, S., Kim, D. et al. Risk of seizures associated with antipsychotic treatment in pediatrics with psychiatric disorders: a nested case–control study in Korea. Eur Child Adolesc Psychiatry 30, 391–399 (2021). https://doi.org/10.1007/s00787-020-01525-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-020-01525-4