Abstract
Objectives
To evaluate the effects of Nd:YAG laser irradiation on the microstructures of dentin surfaces and the long-term bond strength of dentin under simulated pulpal pressure.
Materials and methods
Under simulated pulp pressure, 30 freshly extracted caries-free third molars were cut into 2-mm-thick dentin samples and then divided into five groups: the control and laser groups (93.3 J/cm2; 124.4 J/cm2; 155.5 J/cm2; 186.6 J/cm2). Scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR), and Vickers hardness were used to analyze the surface morphology, composition, and mechanical properties of the dentin before and after laser irradiation. Another 80 caries-free third molars were removed and treated as described above, and the resin was bonded to the dentin surface with Single Bond Universal (SBU) adhesive in self-etch mode to make stick specimens. Microtensile bond strength (µTBS), confocal laser scanning microscopy (CLSM), and interfacial silver nanoleakage tests before and after 10,000 times thermocycling were then performed to analyze the bonding properties and interfacial durability of each group.
Results
SEM observations revealed that the surfaces of all laser group specimens were rough with open dentin tubules. Laser irradiation altered the surface composition of dentin while removing some collagen fibers but did not affect its surface hardness or crystallographic characteristics. Furthermore, laser irradiation with an energy density of 124.4 J/cm2 significantly promoted the immediate and aging bond strengths and reduced nanoleakage compared to those of the control group.
Conclusions
Under simulated pulp pressure, Nd:YAG laser pretreatment altered the chemical composition of dentin and improved the immediate and long-term bond strength.
Clinical relevance
This study investigated the optimal parameters for Nd:YAG laser pretreatment of dentin, which has potential as a clinical method to strengthen bonding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Modern bonding techniques have improved dramatically since the introduction of acid etching to enamel bonding by Buonocore [1]. Dentin, on the other hand, has a more complex bonding mechanism than enamel due to its structural complexity. Dentin is a tubular, liquid-rich mineralized tissue composed of inorganic matter, organic matter, and water, with organic matter accounting for as high as 30% of the total composition [2]. The dentin tubules contain dentin fluid and are connected to the pulp, and the diameters and water contents of the tubules vary with the layer of the dentin [3]. The surface of dentin has wettability and there is a smear layer after cutting, which prevents direct interaction between the adhesive and dentin [4]. Therefore, it is necessary to pretreat the dentin surface to improve the bonding effect.
The etch-and-rinse technique for dentin bonding relies on the adhesive penetrating both the dentin tubules and demineralized collagen fiber network and insitu polymerization, forming resin tags and a hybrid layer to generate the main retention force [5]. Due to the complexity of the structure of the collagen fiber network and progressive penetration of the adhesive monomers, it is difficult for existing adhesives to completely penetrate and encapsulate the demineralized collagen fibers, resulting in exposed collagen fibers at the bottom of the hybrid layer [6]. In addition, residual water molecules in this area form localized water-rich and resin-poor zones, which expand over time. Moreover, the activated endogenous collagen hydrolases in dentin gradually degrade the exposed collagen fibers, ultimately leading to the destruction of the bonding interface and the failure of adhesive restorations [7].
Lasers were introduced into the medical field in the 1960s, and since then, their applicability in various areas of the dentistry fields has expanded rapidly [8]. Thus far, the main types of lasers used include Er,Cr:YSGG lasers, Er:YAG lasers, Nd:YAG lasers and diode lasers [9,10,11]. Compared with traditional instruments, laser therapy is faster, safer and more effective because it does not generate vibration, noise or pain nor does it touch the tooth surface [12, 13]. Furthermore, laser irradiation preserves additional tooth tissue and reduces the invasive treatments [14]. Laser irradiation of dentin roughens the surface, removes the smear layer and opens the dentin tubules, thereby creating ideal conditions for bonding [15]. In addition, after laser irradiation, a microspace is formed in the dentin, which makes the dentin resistant to acid and less susceptible to corrosion, thus protecting the bonding interface [16]. However, some scholars have suggested that laser irradiation can degrade some collagen, and the remaining denatured collagen fibers fuse together, limiting the penetrability of the resin in dentin and preventing the improvement in the bonding strength of dentin [17]. This inconsistency may be related to the different types of lasers and parameters used in the experiments.
Nd:YAG lasers are infrared pulsed lasers with a wavelength of 1064 nm. This laser light source has a solid active medium consisting of neodymium-doped yttrium aluminium garnet crystals, which can be effectively absorbed by colored tissues [18]. Therefore, light is not absorbed by healthy dentin, and surrounding healthy tissues can be protected; in contrast, dark caries absorb a large portion of the energy [19]. Second, the energy of the Nd:YAG laser is less absorbed in dentin and water and instead disperses in or penetrates biological tissues [20]. This laser can raise the temperature of the tissue to the melting point. The surface of melted dentin rapidly cools and returns to a solid state, presenting the occurrence of a lava-like appearance [21]. In addition, due to its wavelength and pulsation characteristics, the Nd:YAG laser has a good bactericidal effect and can eliminate pathogenic bacteria residing inside the deep layers of dentin [22].
Dentin tubules communicate with the pulp chamber, and the dentin fluid will slowly flow from the tubules to the dentin surface by capillary movement under pulpal pressure [23]. The permeability and surface wetness of dentin affect the quality of the bonding interface [3]. Therefore, when testing the in vitro bond strength of adhesives to simulate in vivo conditions, simulating pulp pressure is an important and reliable strategy for evaluating resin-dentin sealing and durability. Multiple researchers have shown that dentin bond strength decreases to a relatively great extent in experimental groups employing pulp pressure, which closely resembles the degradation of the bonding interface in clinical practice [24]. In experiments that do not feature this technique, the decline in bond strength may be slowed and the effect of bonding materials can be exaggerated, which is not conducive to proper evaluation and selection of bonding materials [25].
Recently, some new adhesives that have been labeled as “universal” or “multi-mode”, have been widely used in clinical practice. Their most important feature is that they are not affected by the bonding mode and can be used in etch-and-rinse mode or self-etch mode, which provides a more convenient operation for the clinic. However, it has been shown that the use of phosphoric acid to dissolve intertubular dentin on the surface of laser-prepared teeth may lead to dentin demineralization at unpredictable depths, which may interfere with the diffusion of the bonding monomer [26]. In addition, compared to those in the self-etch mode, the amount of calcium ions in dentin decreased after phosphate acid etching, and the hydroxyapatite crystals were almost completely removed. This may affect the chemical interactions between the hydroxyapatite crystals and the MDP monomer, thus affecting the bonding efficiency [27].
This study aims to explore the effects of Nd:YAG laser pretreatment on the microstructures of dentin surfaces and bond durability under simulated pulp pressure. The null hypothesis posits that the application of laser irradiation on the dentin surface before bonding has no significant effect on the microstructure of dentin, bond strength, and interfacial nanoleakage characteristics.
Materials and methods
Sample preparation
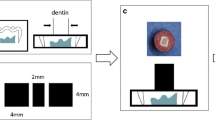
One hundred and ten freshly extracted, healthy third molars were collected after obtaining approval from the Ethics Committee for Human and Animal Studies of the Institute of Stomatology of Jilin University. There were no restorations, caries, fractures, or endodontic or periapical disease in the teeth prior to extraction. Organic debris and tissue residues were removed from the surface using scalpel blades and periodontal scrapers and stored for no more than 3 months in a 0.5% chloramine-T solution at 4 °C. The storage solution was replaced weekly. The enamel and dentin were cut from the crown with the laboratory low-speed cutter (Kejing, China) under running water. The root was subsequently removed 1 mm below the cemento enamel junction in order to expose the pulp cavity, and the pulp soft tissue was removed with forceps. Measurements were conducted with a thickness caliper, with 2 mm serving as the standard thickness at the highest pulp horn. To create a standard smear layer, the prepared dentin samples were sanded under running water in a circular motion on 600-grit SiC paper for 60 s.
Simulated pulpal pressure
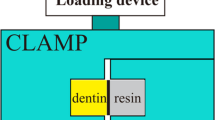
A perforation was made at the midpoint of the acrylic plate, followed by the insertion of a 16-gauge stainless steel needle. The opposite end of the needle was connected to a 50 mL syringe barrel. Dentin samples were fixed to an acrylic plate with cyanoacrylate adhesive and dental wax, and the syringe was filled with deionized water so that the height from the horizontal plane in the syringe to the dentin surface was 15 cm, thus simulating a pulpal pressure of 1.47 kPa. At 37 °C, this pressure was maintained for 24 h before proceeding with subsequent manipulation.
Experimental design
The Nd:YAG laser device utilized in this investigation was a LighterWalker (Fotona, Germany) operating at a wavelength of 1064 nm with a tip fiber diameter of 320 μm. The dentin samples were randomly divided into 5 groups according to the energy density parameters of the Nd:YAG laser; Group 1 received no treatment, while the energy density parameters of the remaining 4 groups were calculated based on the laser power, frequency, and fiber diameter, which were 93.3 J/cm2, 124.4 J/cm2, 155.5 J/cm2, and 186.6 J/cm2, respectively. The laser was irradiated in noncontact mode with a scanning time of 60 s. A fixation device was fabricated using self-curing acrylic resin during laser irradiation to maintain a stable distance of 2 mm at an angle of 90° from the surface of the dentin samples. The irradiations were moved in a sweeping fashion by hand, and a zigzag scan was performed on the whole dentin surface during the exposure period. The experimental grouping and design schematic are shown in Table 1; Fig. 1.
Microstructure of dentin
SEM-EDS analysis
One dentin sample was selected at random from each group and immersed in 2.5% glutaraldehyde solution (Solarbio, China) for 24 h. The sample was then dehydrated with progressively stronger ethanol solutions (25%, 50%, 75%, 95%, and 100%) for 20 min each and submerged in hexamethyldisilazane (HMDS; Sigma, USA) for 10 min. After drying and Au coating, SEM (S-4800; Hitachi, Japan) was used to examine the surface morphology of the dentin at an accelerating voltage of 15 kV. Moreover, EDS (EDAX Genesis 2000; Ammetek, USA) was used to detect and record changes in the compositions of the samples within the observation area. Four EDS measurements were taken for each sample, and the Ca and P contents (wt%) and Ca/P weight ratio (WR%) were recorded.
ATR-FTIR analysis
Two dentin samples were chosen at random from each group, and ATR-FTIR spectra (Nicolet; Madison, USA) were acquired. The average value was obtained by randomly selecting and testing five distinct places on the surface of the sample. The spectral data were expressed as absorbance values and were analyzed with OMNIC 8 software (Nicolet, Madison, USA). The PO4v3 band (900–1200 cm–1) corresponds to hydroxyapatite (HAP) crystals and the amide I band (1570–1790 cm–1) corresponds to dentin collagen fibers. The area ratio of the main phosphate to the amide I band represents the relative amount of the mineral to the organic matrix (PO4/amide I) when evaluating the effect of laser irradiation on the collagen fibers of dentin.
XRD analysis
According to the preparation requirements of the XRD samples, 1 molar was selected from each group, and 1-mm-thick slices of dentin were cut perpendicular to the long axis of the tooth. Each sample was first sanded with water sandpaper and then polished and ultrasonically cleaned. After laser treatment, the specimens were analyzed using an X-ray diffractometer (Rigaku Dmax, Japan) in continuous scanning mode with an accelerating voltage of 40 kV and a current of 40 mA. The scanning speed was 4°/min, and the scanning range was 20–60°.
Hardness measurements
Two dentin samples were chosen at random from each group, and the surface hardness values of the specimens were examined with a Vickers hardness tester (HMV-G21ST; Shimadz, Japan) with a load of 980.7 mN and a dwell time of 10 s after laser treatment. The values for each dentin sample were averaged after 4 measurements in a relatively flat and uniform location, with each indentation being 100 μm apart.
Bonding performance
Dentin bonding procedures
After the dentin samples were cemented to the pulp pressure model for 24 h, the dentin surfaces were first irradiated at a uniform rate with the Nd:YAG laser energy density parameter set for each group and then blown dry. The SBU adhesive (3M ESPE, USA) was applied on the dentin surface in a self-etching mode according to the manufacturer’s recommendations. After the adhesive procedures, two layers of resin composite (Filtek Z350, 3M ESPE, USA) with thickness of 2 mm each were applied and light cured separately for 20 s (light intensity 1000 mW/cm2). All of the samples were then stored in deionized water at 37 °C in the dark for 24 h to completely cure the resin.
µTBS and failure mode analysis
Fourteen samples of dentin-composite resin were chosen at random from each group, and half of them were embedded in epoxy resin and then cut into 0.9 × 0.9-mm sticks using a diamond saw (n = 35). The cross-sectional area of each stick should not exceed 1 mm2. The sticks were adhered to a microtensile mold using cyanoacrylate glue. The immediate µTBS was evaluated with universal testing equipment (AG-X plus; Shimadzu, Japan) at a loading rate of 1 mm/min until the sticks broke. Measure the exact cross-sectional area of each sample after fracture and the µTBS is determined by dividing the maximum load at which a specimen breaks by its cross-sectional area. The remaining samples underwent 10,000 thermal cycles, alternating between water baths at temperatures of 5 and 55 °C for 30 s each, and the above operations were subsequently repeated.
The fracture pattern of the specimen was observed under a stereomicroscope. The failure modes were recorded as adhesive failure (failure at the adhesive interface), cohesive failure (failure in the dentin or composite resin), or mixed failure (combined adhesive and cohesive failure).
SEM analysis
The laser group with the optimal parameter (124.4 J/cm2) was chosen for SEM analysis based on the µTBS results. Two samples of dentin-composite resin were selected from each group (control and laser), and half of them were embedded in epoxy resin and then cut into 1-mm-thick slabs along the long axis of the tooth. Selected slabs near the center of the tooth were first demineralized with 37% phosphoric acid gel for 2 min and then immersed in 1% NaClO for 10 min. After rinsing them in running water and fixing them in 2.5% glutaraldehyde solution for 24 h, the samples were dehydrated with increasing concentrations of ethanol solution and placed in HMDS for 10 min. They were then air-dried, coated with Au, and finally fixed on the SEM sample stage to observe the microstructure of the bonding interface. This process was repeated for the remaining half of the samples after 10,000 thermal cycles.
CLSM analysis
One dentin slab was selected from each of the control and laser groups, and rhodamine B (RB) was mixed into the SBU adhesive at 0.1 wt%. The bonding procedure was the same as the Dentin bonding procedures described above. After cutting the samples into 0.5-mm-thick resin-dentin slabs, SiC papers were used to grind and polish the samples step by step to 3000 mesh. To prevent tissue shrinkage caused by drying and dehydration, the samples were kept moist before observation and analysis were conducted. The prepared samples were placed on coverslips, and images were captured inside the bonding surface along the fixed axis with CLSM (LSM-800; Carl Zeiss, Germany).
Nanoleakage analysis
Two teeth in each group were selected to prepare dentin slabs for nanoleakage assessment according to the SEM analysis procedures described above. The slabs were first coated with nail varnish, excluding the area within 1 mm of the bonding interface, and then immersed in 50 wt% ammoniated silver nitrate solution in the darkness for 24 h. Subsequently, the samples were rinsed under deionized water for 5 min to thoroughly remove the soaking solution and then placed in a photodeveloping solution under fluorescent light for 8 h. The samples were polished step by step under running water with SiC papers and cleaned by ultrasonic vibration. Finally, the samples were coated with Au and analyzed using FE-SEM in the backscattered electron mode. This process was repeated for the remaining half of the samples after 10,000 thermal cycles.
Statistical analysis
The EDS, ATR-FTIR, microhardness and µTBS results exhibited normal distributions and homogeneous variances. Therefore, the differences in EDS, ATR-FTIR and microhardness values between the various treatment groups were analyzed with one-way ANOVA and Tukey’s post hoc tests. The µTBS values before and after aging at different energy densities were analyzed by two-way ANOVA, followed by Tukey’s post hoc multiple comparison tests. The significance level was set at P < 0.05.
Results
SEM-EDS analysis
According to the morphological observations, the surface of the dentin in the control group was relatively flat, with the smear layer blocking the dentin tubules. The dentin surface pretreated with the Nd:YAG laser was irregularly cratered due to recrystallization of the molten dentin, which resembled lava. The smear layer on the dentin surface disappeared, and the dentin tubules were open (Fig. 2).
The EDS plot showed changes in the chemical compositions of the dentin surfaces before and after laser irradiation at different energy densities (Fig. 3A). The Ca and Ca/P ratios on the dentin surface increased following Nd:YAG laser irradiation, but the variations in the values did not differ among the four groups with different energy densities. Furthermore, the differences in P contents between the groups before and after laser irradiation were not statistically significant (Table 2).
(A) Changes in the chemical composition of dentin surface before and after laser irradiation. (B) The ATR-FTIR spectrums of control dentin and laser-treated dentin. (C) Column graph of the apatite/collagen ratio of laser-irradiated and control dentin surfaces. (D) XRD spectra of dentin samples of control dentin surface and laser-irradiated dentin surface
ATR-FTIR analysis
The ATR-FTIR spectra and PO4/amide I ratios for the control and each experimental group are shown in Fig. 3B, C. The infrared spectra of the laser-irradiated dentin showed no additional peak formation. In comparison to the control group (6.14 ± 0.23), the peak corresponding to the amide I band (1570–1790 cm–1) was significantly weaker in all the laser groups and the ratios of PO4/amide I (93.3 J/cm2: 8.63 ± 0.71; 124.4 J/cm2: 8.18 ± 0.74; 155.5 J/cm2: 8.62 ± 0.22; and 186.6 J/cm2: 7.70 ± 0.62) were significantly greater.
XRD analysis
The dentin of both the control and experimental groups were characterized with an X-ray diffractometer, and the corresponding spectra are displayed in Fig. 3D. All of the diffraction peaks corresponded well to the main characteristic diffraction peaks in the standard JCPD card of hydroxyapatite, and the peak shapes of each group before and after laser irradiation were similar.
Hardness measurements
Table 3 displays the hardnesses of the dentin before and after laser irradiation. The microhardness of the dentin surface was slightly lower after laser pretreatment than in the control group, but the difference was not statistically significant.
µTBS and failure mode analysis
The mean and standard deviations of the immediate and aging µTBS are shown in Fig. 4A. The results of the µTBS were analyzed with two-way ANOVA (variables: surface treatment and 10,000 thermal cycles) (Table 4). The main effects of the surface treatment (F = 21.420, p < 0.001) and aging (F = 63.520, p < 0.001) were significant, while the interaction effect was not significant.
(A) Immediate and aged microtensile bond strength test results. Different upper case letters represent statistically significant differences between different energy density groups (p < 0.05). Different lower case letters indicate statistically significant differences between the groups before and after thermocycling (p < 0.05). (B) Prevalence (in percent) of the failure mode in each group
The immediate bond strengths in all of the laser groups, except for the group treated with the 186.6 J/cm2 energy density, were significantly higher than that of the control group. In particular, the group irradiated by a laser with an energy density of 124.4 J/cm2 exhibited the highest bond strength, and the difference in µTBS among the remaining experimental groups was not significant. After 10,000 thermal cycles, the 124.4 J/cm2 group still exhibited greater bond strength than the aging control group. The µTBS did not significantly differ among the other groups, except for that of the 124.4 J/cm2 group. Moreover, the bond strengths of both the control and laser groups were significantly lower after aging.
Figure 4B displays the results of both the immediate and aging fracture patterns. The fracture pattern of each group was primarily dominated by adhesive fracture, followed by mixed fracture, and the percentage of adhesive fracture increased after aging. The proportions of adhesive fractures in the experimental group were lower both immediately and after aging compared to the control group. The percentage of adhesive fractures in the laser group tended to first decrease and then increase with progressive increases in laser energy density.
SEM analysis
Figure 5 displays SEM images of the bonding interface of each group. Few short resin tags, which were cylindrical in shape with smooth surfaces, were formed in the dentin tubules of the immediate control group. There were more and thicker resin tags deeper into the dentin tubules for the laser groups than for the control group. The surfaces of the resin tags were relatively rough, with a few short lateral branching protrusions. At the bonding interface after aging, the morphologies of the tubules and the number of resin tags were roughly similar to those under immediate conditions, respectively.
CLSM analysis
The results of the CLSM observation of the bonding interface are shown in Fig. 6A. Three-dimensional reconstruction displayed the overall morphology of the hybrid layer resin tags at a certain depth without limiting the layer sweep depth. The resin tags that formed at the bonding interface in the control group were short and sparse. In contrast, the density and penetration depth values of the resin tags in the laser group significantly increased.
The evaluation influence of Nd:YAG laser on the durability of resin–dentin bonding interface. (A) CLSM image of bonding interface of different groups. Lower case letters are 3D reconstructed images of upper case letters. (B) Representative images (1000x) of interfacial nanoleakage expression of different groups
Nanoleakage
Figure 6B displays the FE-SEM images of each group of nanoleakage both before and after aging, and the highlighted area at the bottom of the hybrid layer arose due to silver ion deposition. Under immediate conditions, the degree of silver staining at the interface was much lower in the Nd:YAG laser pretreatment group than in the control group, and the bonding interface was spotted or discontinuous with sparse silver staining lines.
Significantly greater levels of nanoleakage were observed after aging than before. In the aging control group, a dense and continuous layer of silver particles was observed at the bonding interface. In contrast, in the aging laser-pretreated groups, the silver particles exhibited a continuous and sparse distribution.
Discussion
The existing bonding process mainly utilizes two different techniques: etch-and-rinse approach (E&R) and self-etch approach (SE). The E&R technique removed the smear layer, resulting in the formation of a demineralized dentin layer on the dentin surface. However, because the depth of penetration of the adhesive monomer cannot reach the depth of acid etching, the exposed collagen fibers were hydrolyzed in the wet environment provided by residual moisture, which led to microleakage [28, 29]. SE adhesives reduce the difference between the depth of demineralization and penetration because they occur simultaneously [30]. However, the SE adhesives did not eliminate the smear layer but rather modified it, which may impact dentin bonding [31].
Since they are applied in the oral cavity, lasers have demonstrated unique advantages when combined with or used as an alternative to traditional methods. The Nd:YAG laser is also increasingly used to treat clinical oral diseases, such as root canal disinfection, tooth bleaching, peri-implantitis, dentin hypersensitivity, and surgical procedures [32,33,34,35]. Some scholars have also studied the effects of laser irradiation on dental hard tissues. Laser irradiation of tooth tissues not only reduces microleakage but also has a bactericidal effect [36]. However, there has been contradictory reporting of the effects of laser irradiation in the literature. The Nd:YAG laser primarily affects dentin through thermal action, which generates high temperatures even within a short action time and induces apatite crystals to melt, recrystallize and form a crater [37]. This study was designed to assess the effects of laser irradiation on the surface characteristics and adhesive qualities of dentin. These goals were achieved by simulating the pressure of the pulp in vitro and by setting parameters, such as power, frequency, and fiber diameter, based on previous experiments to determine the most suitable laser parameters. The results showed that the laser treatment altered the dentin surface morphology and performed better in terms of µTBS and interfacial nanoleakage. Therefore the null hypothesis was rejected.
The SEM results indicated that pretreating the dentin surface with Nd:YAG laser increased its roughness and opened the dentin tubules, which was consistent with the observations of Özdoğan et al. [38]. The open dentin tubules facilitated the penetration of subsequent adhesives and the formation of a hybrid layer. Moreover, an increase in roughness increased the bonding area of the dentin surface, which was conducive to the combination of resin and the dentin surface to form a mechanical inlay. The statistical results of the EDS test data showed that the content of Ca was significantly greater after laser irradiation, suggesting a decrease in the solubility of the dentin surface, which may improve the acid resistance to a certain extent and prevent the occurrence of secondary caries [19]. According to Yoshida et al., SBU adhesives contain the 10-MDP monomer, which can stably bind to Ca [39]. The chemical structure of –OP(OH)2 can form a 4 nm thick hydrophobic nanolayer structure with calcium ions; these ions are tightly adsorbed on the surface of HAP and can provide additional chemical binding sites for the adhesive, thus enhancing the durability of dentin bonding [40, 41].
Laser irradiation instantaneously increases the surface temperature of the dentin. It has been shown that when the surface temperature of dentin reached 60–80 ℃, collagen fibers began to degenerate, coagulate or necrose [42]. Based on the ATR-FTIR spectral analysis, the relative collagen content on the dentin surface was dramatically reduced following laser irradiation compared to the control group, suggesting that the collagen was removed from the dentin bonding interface. Benetti et al. [43] used ATR-FTIR to verify the changes in bone tissue induced by laser irradiation and found that the concentrations of all organic components and water were significantly reduced. Some studies have similarly shown the ablation of collagen fibers by scanning electron microscopy [44]. These changes may affect the diffusion of the resin in the collagen fibers and the formation of hybrid layer, which is not conducive to obtaining good bond strength. In contrast, Gan et al. [45] discovered that laser treatment enhanced the longevity of dentin bonding by removing collagen fibers and water, thereby forming a dry bonding interface and preventing dentin collagen degradation.
Some scholars believe that the thermal effect produced by a laser leads to melting and recrystallization of the dentin surface, thereby changing the inorganic phase structure and composition of the hard tissue [46]. For dentin bonding, a change in the inorganic crystal phase changes the ionic distribution on the dentin surface, which affects the chemical reaction pattern between the adhesive and dentin and subsequently changes the bonding effect [47]. The XRD patterns generated before and after laser irradiation were similar, indicating that the inorganic phase of the dentin was unchanged and that the main component was HAP crystals. These results were consistent with the ATR-FTIR spectra. The laser energy density parameters employed in this work did not increase the surface temperature of the dentin enough to change the inorganic phase structure.
Al-Omari [48] and Lee et al. [49] concluded that Nd:YAG is a high-energy-density laser, and the irradiated dentin surface exhibited lesser hardness compared to the normal dentin surface. On the contrary, some scholars have found that the application of Nd:YAG laser resulted in significantly greater hardness of the dentin [42]. The change in hardness is still controversial, and further research is needed. The results of this study showed no significant effect of the laser treatment on dentin surface hardness.
Low-energy laser irradiation can seal most dentin tubules, preventing the adhesive from penetrating into the tubules and ultimately affecting the bonding effect. A higher energy density may cause the formation of microcracks after tooth tissue bursts, while the high temperatures generated may harm the dental pulp [50]. Therefore, it is particularly important to screen lasers with the correct energy density. This study examined the impact of laser irradiation on the bonding strength of dentin through the utilization of the microtensile testing method, which is currently the universal standard bond strength testing method [51]. The effect of the Nd:YAG laser on pulp safety is one of the necessary factors to be considered because it lacks a cooling system. Some scholars have found that when the temperature increases to 5.5℃, irreversible damage to the pulp occurs, so the laser power should be controlled within 1.5 W [52]. In addition, the pulpal temperature is related not only to the power of the laser but also to the thickness of the dentin. Santis et al. [23] demonstrated that when the thickness of remaining dentin tissue is below 1 mm, an increase in temperature during laser irradiation may cause damage to the pulp tissue. Therefore, we standardized the remaining dentin at the highest pulp horn, which can ensure the safety of the pulp and simulate the experimental effect of pulp pressure.
There is still a lack of consensus among different scholars on the experimental results of resin-dentin bond strength after pretreatment of dentin with Nd:YAG laser, which may be related to the type and brand of laser, treatment method and time, and energy density parameters [53]. The bond durability between the adhesive system and tooth structure is crucial for long-term success of the restoration. Thermal cycles can simulate the intraoral environment to a certain extent. Blumer et al. [54] reported that 10,000 thermal cycles were equivalent to 1 year of function in the oral environment. In this study, both immediately and after thermal cycling, the laser irradiation group has significantly greater µTBS values compared to the control group, especially when an Nd:YAG laser with an energy density of 124.4 J/cm2 was used. It is shown that laser irradiation not only improved the short-term bond strength of the dentin under simulated pulpal pressure but also reduced the detrimental effects of aging on the bond strength to some extent, thus improving the longevity of the bonding interface. This difference may have arisen from the stable interaction between 10-MDP-Ca salts and dentin collagen fibers at the molecular level, which protects the exposed collagen fibers from degradation by matrix metalloproteinases (MMPs) and maintains the integrity of the bonding interface [55, 56]. In addition, In addition, there exist variations in bond strength among the different energy density parameter groups. As the energy density increased sequentially from Group 2 to Group 5, the µTBS values tended to first increase and then decrease slightly, both immediately and after aging. The elevated bond strength was attributed to the increased roughness and surface area of the dentin and penetration of the adhesives through the dentin tubules. The subsequent decrease in bond strength may have occurred due to the high energy density of the laser, which caused microfractures on the dentin surface, affecting the stability and integrity of the bonding interface [38]. In addition, high-energy lasers may not be able to control the degree of demineralization, resulting in suboptimal resin penetration of deep demineralized dentin and insufficient formation of the hybrid layer.
The observations of fracture patterns indicated that the distributions of the results were similar both immediately and after aging, with adhesive fracture predominating and increasing after thermal cycling; this was consistent with the results obtained by Özdoğan [38] and Ribeiro et al. [57]. The weakness of the bonding was mainly concentrated at the bonding interface between the adhesive and dentin or composite resin, affecting the durability of the bonding. Moreover, the proportion of adhesive fractures tended to first decrease and then slightly increase with increasing energy density, indicating that laser irradiation with suitable parameters can reduce the proportion of adhesive fractures to a certain extent.
Observation of the overall morphologies of the resin tags at the bonding interface and the penetration of the adhesive into the dentin by SEM and CLSM can help to more visually analyze the reasons for contributing to the enhancement of dentin bond strength through laser irradiation. The densities and lengths of the resin tags formed in the laser groups were markedly greater than those in the control group. However, in the laser groups, a few short protrusions appeared on the relatively rough surface of the resin tags. These results all indicated that laser irradiation is beneficial for enhancing dentin bonding under conditions that simulate pulp pressure.
Nanoleakage is often regarded as a crucial metric for assessing the sealing ability and bonding performance of adhesive systems or interventions [58]. In this study, the degrees of nanoleakage at the bonding interface of the laser-irradiated samples in both the immediate and aging groups were significantly reduced compared to those in the control group, which is consistent with the µTBS and SEM results. This difference may be due to the water-insoluble MDP-Ca salt protecting the bonding interface and collagen fibers from degradation [59]. de Oliveira et al. [60] found under TEM that there were no gaps or debonding areas between the irradiated dentin and the adhesive layers, and there was less silver deposition at the bonding interface. This indicated that the laser-irradiated hybrid layer had good sealing ability and integrity, which can effectively prevent water ingress and facilitate bonding durability.
The limitation of this study is that only the self-etch mode of SBU and one type of dentin substrate were used. Whether the use of the etch-and-rinse mode or other dentin substrates, such as caries-affected dentin can achieve the same effect remains to be further explored in future experiments.
Conclusions
To summarize, the following conclusions could be drawn:
-
1.
Under conditions that simulate pulp pressure, Nd:YAG laser irradiation changed the surface morphology and composition of dentin but did not affect its mechanical properties or crystallographic characteristics.
-
2.
Nd:YAG laser irradiation improved the immediate bond strength of dentin, facilitated a reduction in nanoleakage at the bond interface, and reduced to some extent the effect of thermocycling on the long-term bond strength. The energy density of 124.4 J/cm2 was determined to be optimal in this study.
Data availability
Data will be made available on request.
References
Buonocore MG (1955) A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res 34:849–853
Perdigão J (2020) Current perspectives on dental adhesion: (1) dentin adhesion - not there yet. Jpn Dent Sci Rev 56:190–207. https://doi.org/10.1016/j.jdsr.2020.08.004
Silva TM, Gonçalves LL, Fonseca BM, Esteves SR, Barcellos DC, Damião AJ et al (2016) Influence of nd:YAG laser on intrapulpal temperature and bond strength of human dentin under simulated pulpal pressure. Lasers Med Sci 31:49–56. https://doi.org/10.1007/s10103-015-1827-1
Saikaew P, Sattabanasuk V, Harnirattisai C, Chowdhury A, Carvalho R, Sano H (2022) Role of the smear layer in adhesive dentistry and the clinical applications to improve bonding performance. Jpn Dent Sci Rev 58:59–66. https://doi.org/10.1016/j.jdsr.2021.12.001
Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S et al (2013) Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent Mater 29:999–1011. https://doi.org/10.1016/j.dental.2013.07.016
Tjäderhane L (2015) Dentin bonding: can we make it last? Oper Dent 40:4–18. https://doi.org/10.2341/14-095-bl
Fialho MPN, Hass V, Nogueira RP, França FMG, Turssi CP, Basting RT et al (2019) Effect of epigallocatechin-3- gallate solutions on bond durability at the adhesive interface in caries-affected dentin. J Mech Behav Biomed Mater 91:398–405. https://doi.org/10.1016/j.jmbbm.2018.11.022
Yavuz T, Özyılmaz ÖY, Dilber E, Tobi ES, Kiliç H (2017) Effect of different surface treatments on Porcelain-Resin Bond Strength. J Prosthodont 26:446–454. https://doi.org/10.1111/jopr.12387
Reddy N, Golob Deeb J, Kitten T, Carrico CK, Grzech-Leśniak K (2022) The In Vitro Effect of Laser Irradiation (Er:YAG and CO(2)) and Chemical reagents (hydrogen peroxide, Sodium Hypochlorite, Chlorhexidine, or Sodium Fluoride) alone or in combination on reducing Root caries Bacteria. Int J Mol Sci 23. https://doi.org/10.3390/ijms232415732
Alkhudhairy F, Neiva GF (2023) Effect of Er, Cr: YSGG, nd: YAG, and diode laser against different photosensitizers on tensile and shear bond strength of bonded composite to caries affected dentin. Eur Rev Med Pharmacol Sci 27:8350–8359. https://doi.org/10.26355/eurrev_202309_33757
Bürklein S, Abdi I, Schäfer E, Appel C, Donnermeyer D (2023) Influence of pulse energy, tip design and insertion depth during Er:YAG-activated irrigation on cleaning efficacy in simulated severely curved complex root canal systems in vitro. Int Endod J. https://doi.org/10.1111/iej.13992
Najeeb S, Khurshid Z, Zafar MS, Ajlal S (2016) Applications of light amplification by Stimulated Emission of Radiation (Lasers) for restorative Dentistry. Med Princ Pract 25:201–211. https://doi.org/10.1159/000443144
Shamsudeen SM, Thavarajah R, Joshua E, Rao UDK, Kannan R (2019) Evaluating and comparing the morphological and histopathological changes induced by erbium:yttrium-aluminum-garnet laser and diamond bur on enamel, dentin and pulp tissue. J Investig Clin Dent 10:e12475. https://doi.org/10.1111/jicd.12475
Trevelin LT, da Silva BTF, de Freitas PM, Matos AB (2019) Influence of Er:YAG laser pulse duration on the long-term stability of organic matrix and resin-dentin interface. Lasers Med Sci 34:1391–1399. https://doi.org/10.1007/s10103-019-02739-y
Karadas M, Çağlar İ (2017) The effect of Er:YAG laser irradiation on the bond stability of self-etch adhesives at different dentin depths. Lasers Med Sci 32:967–974. https://doi.org/10.1007/s10103-017-2194-x
Maeda FA, Fukushima KA, Tedesco TK, Correa Aranha AC, Raggio DP, Miranda Junior WG et al (2016) Effect of erosive challenge and nd:YAG laser irradiation on bond strength of adhesive systems to dentin. Int J Adhes Adhes 64:60–64. https://doi.org/10.1016/j.ijadhadh.2015.09.008
Nahas P, Zeinoun T, Namour M, Ayach T, Nammour S (2018) Effect of Er:YAG laser energy densities on thermally affected dentin layer: morphological study. Laser Ther 27:91–97. https://doi.org/10.5978/islsm.18-OR-07
Dortaj D, Bassir SH, Hakimiha N, Hong H, Aslroosta H, Fekrazad R et al (2022) Efficacy of nd:YAG laser-assisted periodontal therapy for the management of periodontitis: a double-blind split-mouth randomized controlled clinical trial. J Periodontol 93:662–672. https://doi.org/10.1002/jper.21-0242
Kaviani A, Khansari Nejad N (2021) Effect of nd:YAG and Er:YAG laser tooth conditioning on the microleakage of self-adhesive resin cement. Biomater Investig Dent 8:152–159. https://doi.org/10.1080/26415275.2021.1990063
Huang Q, Li Z, Lyu P, Zhou X, Fan Y (2023) Current applications and future directions of lasers in endodontics: a narrative review. Bioeng (Basel) 10. https://doi.org/10.3390/bioengineering10030296
João-Souza SH, Scaramucci T, Hara AT, Aranha AC (2015) Effect of nd:YAG laser irradiation and fluoride application in the progression of dentin erosion in vitro. Lasers Med Sci 30:2273–2279. https://doi.org/10.1007/s10103-015-1802-x
Jurič IB, Anić I (2014) The Use of lasers in Disinfection and Cleanliness of Root canals: a review. Acta Stomatol Croat 48:6–15. https://doi.org/10.15644/asc48/1/1
Santis LR, Silva TM, Haddad BA, Gonçalves LL, Gonçalves SE (2017) Influence of dentin thickness on intrapulpal temperature under simulated pulpal pressure during nd:YAG laser irradiation. Lasers Med Sci 32:161–167. https://doi.org/10.1007/s10103-016-2098-1
Pucci CR, Gu LS, Zeng C, Gou YP, Tay FR, Niu LN (2017) Susceptibility of contemporary single-bottle self-etch dentine adhesives to intrinsic water permeation. J Dent 66:52–61. https://doi.org/10.1016/j.jdent.2017.08.010
Kim RJ, Choi NS, Ferracane J, Lee IB (2014) Acoustic emission analysis of the effect of simulated pulpal pressure and cavity type on the tooth-composite interfacial de-bonding. Dent Mater 30:876–883. https://doi.org/10.1016/j.dental.2014.05.027
Silva AC, Melo P, Ferreira JC, Oliveira T, Gutknecht N (2019) Adhesion in dentin prepared with Er,Cr:YSGG Laser: systematic review. Contemp Clin Dent 10:129–134. https://doi.org/10.4103/ccd.ccd_302_18
Muñoz MA, Luque-Martinez I, Malaquias P, Hass V, Reis A, Campanha NH et al (2015) In vitro longevity of bonding properties of universal adhesives to dentin. Oper Dent 40:282–292. https://doi.org/10.2341/14-055-l
Breschi L, Maravic T, Cunha SR, Comba A, Cadenaro M, Tjäderhane L et al (2018) Dentin bonding systems: from dentin collagen structure to bond preservation and clinical applications. Dent Mater 34:78–96. https://doi.org/10.1016/j.dental.2017.11.005
Kaczor K, Gerula-Szymańska A, Smektała T, Safranow K, Lewusz K, Nowicka A (2018) Effects of different etching modes on the nanoleakage of universal adhesives: a systematic review and meta-analysis. J Esthet Restor Dent 30:287–298. https://doi.org/10.1111/jerd.12375
Gonçalves LL, Da Silva TM, Prakki A, Barcellos DC, Caneppele TMF, De Oliveira HPM et al (2022) Universal adhesive: the effect of different simulated pulpal pressure fluids and bonding modes to dentin. Odontology 110:62–69. https://doi.org/10.1007/s10266-021-00633-0
Saikaew P, Senawongse P, Chowdhury AA, Sano H, Harnirattisai C (2018) Effect of smear layer and surface roughness on resin-dentin bond strength of self-etching adhesives. Dent Mater J 37:973–980. https://doi.org/10.4012/dmj.2017-349
Wang X, Cheng X, Liu X, Wang Z, Wang J, Guo C et al (2018) Bactericidal effect of various laser Irradiation systems on Enterococcus faecalis Biofilms in Dentinal tubules: a confocal laser scanning Microscopy Study. Photomed Laser Surg 36:472–479. https://doi.org/10.1089/pho.2017.4430
Gao Y, Zhao Z, Li L, Zhang K, Liu Q (2020) In vitro evaluation of the effectiveness of bleaching agents activated by KTP and Nd:YAG laser. Photodiagnosis Photodyn Ther 31:101900. https://doi.org/10.1016/j.pdpdt.2020.101900
Fragkioudakis I, Kallis A, Kesidou E, Damianidou O, Sakellari D, Vouros I (2023) Surgical Treatment of Peri-implantitis using a combined nd: YAG and Er: YAG Laser Approach: investigation of clinical and bone loss biomarkers. Dent J (Basel) 11. https://doi.org/10.3390/dj11030061
Cattoni F, Ferrante L, Mandile S, Tetè G, Polizzi EM, Gastaldi G (2023) Comparison of lasers and desensitizing agents in Dentinal Hypersensitivity Therapy. Dent J (Basel) 11. https://doi.org/10.3390/dj11030063
Ramirez I, Bertolini GR, Candemil AP, Sousa-Neto MD, Souza-Gabriel AE (2023) Chemical and morphological analysis of dentin irradiated by different high-power lasers: a systematic review. Lasers Med Sci 38:255. https://doi.org/10.1007/s10103-023-03912-0
Lin CP, Lee BS, Lin FH, Kok SH, Lan WH (2001) Phase, compositional, and morphological changes of human dentin after nd:YAG laser treatment. J Endod 27:389–393. https://doi.org/10.1097/00004770-200106000-00004
Özdoğan MS, Karaokutan I, Yıldırım M, Aydemir KA, Karatay A, Aykent F (2021) Shear bond strength of a self-adhesive resin cement to dentin surface treated with nd:YAG and femtosecond lasers. Lasers Med Sci 36:219–226. https://doi.org/10.1007/s10103-020-03138-4
Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H et al (2004) Comparative study on adhesive performance of functional monomers. J Dent Res 83:454–458. https://doi.org/10.1177/154405910408300604
Yoshihara K, Nagaoka N, Yoshida Y, Van Meerbeek B, Hayakawa S (2019) Atomic level observation and structural analysis of phosphoric-acid ester interaction at dentin. Acta Biomater 97:544–556. https://doi.org/10.1016/j.actbio.2019.08.029
Landmayer K, da Silva JCV, Anhesini BH, Iatarola BO, Aranha ACC, Francisconi-Dos-Rios LF (2022) Effect of nd:YAG laser irradiation, used as a desensitizing strategy, on bond strength to simulated hypersensitive dentin. Clin Oral Investig 26:4109–4116. https://doi.org/10.1007/s00784-022-04380-6
Trevelin LT, Silva BTF, Arana-Chavez VE, Matos ABJLDS (2018) Impact of Er:YAG laser pulse duration on ultra-structure of dentin collagen fibrils
Benetti C, Santos MO, Rabelo JS, Ana PA, Correa PR, Zezell DM (2011) Detection of chemical changes in bone after irradiation with Er,Cr:YSGG laser. Photonic Ther Diagnostics Vii 7883. https://doi.org/10.1117/12.876533
Khalid A, Bashir S, Akram M, Ahmed QS (2017) The variation in surface morphology and hardness of human deciduous teeth samples after laser irradiation. Laser Phys 27. https://doi.org/10.1088/1555-6611/aa8cd5
Gan J, Liu S, Zhou L, Wang Y, Guo J, Huang C (2017) Effect of nd:YAG laser irradiation pretreatment on the long-term bond strength of etch-and-rinse adhesive to dentin. Oper Dent 42:62–72. https://doi.org/10.2341/15-268-l
Lin S, Pan D, Lin Q, Yin S, Chen D, Liu Q et al (2011) Evaluation of phase, microstructure and composition of human dentine after Er,Cr:YSGG laser irradiation. J Nanosci Nanotechnol 11:2421–2426. https://doi.org/10.1166/jnn.2011.3526
Nurrohman H, Nikaido T, Takagaki T, Sadr A, Ichinose S, Tagami J (2012) Apatite crystal protection against acid-attack beneath resin-dentin interface with four adhesives: TEM and crystallography evidence. Dent Mater 28:e89–98. https://doi.org/10.1016/j.dental.2012.04.025
Al-Omari WM, Palamara JE (2013) The effect of nd:YAG and Er,Cr:YSGG lasers on the microhardness of human dentin. Lasers Med Sci 28:151–156. https://doi.org/10.1007/s10103-012-1094-3
Lee BS, Lin CP, Lin FH, Li UM, Lan WH (2003) Effect of nd:YAG laser irradiation on the hardness and elastic modulus of human dentin. J Clin Laser Med Surg 21:41–46. https://doi.org/10.1089/10445470360516734
Liu M, Xu X, Liu Q, Zhang K, Xin P (2023) Effect of various Er:YAG laser conditioning energies on dentin surface: micromorphological investigation and dentin-resin shear bond strength test. Lasers Med Sci 38:242. https://doi.org/10.1007/s10103-023-03915-x
Sano H, Chowdhury AMA, Saikaew P, Matsumoto M, Hoshika S, Yamauti M (2020) The microtensile bond strength test: its historical background and application to bond testing. Jpn Dent Sci Rev 56:24–31. https://doi.org/10.1016/j.jdsr.2019.10.001
Lopes AO, Correa Aranha AC (2013) Comparative evaluation of the effects of Nd:YAG Laser and a Desensitizer Agent on the treatment of dentin hypersensitivity: a clinical study. Photomed Laser Surg 31:132–138. https://doi.org/10.1089/pho.2012.3386
Heredia A, da Silva DFF, Carracho HG, Borges GA, Spohr AM (2015) Influence of nd:YAG laser on the durability of resin-dentin bonds. J Laser Appl 27. https://doi.org/10.2351/1.4927608
Blumer L, Schmidli F, Weiger R, Fischer J (2015) A systematic approach to standardize artificial aging of resin composite cements. Dent Mater 31:855–863. https://doi.org/10.1016/j.dental.2015.04.015
Perdigão J, Reis A, Loguercio AD (2013) Dentin adhesion and MMPs: a comprehensive review. J Esthet Restor Dent 25:219–241. https://doi.org/10.1111/jerd.12016
Dressano D, Salvador MV, Oliveira MT, Marchi GM, Fronza BM, Hadis M et al (2020) Chemistry of novel and contemporary resin-based dental adhesives. J Mech Behav Biomed Mater 110:103875. https://doi.org/10.1016/j.jmbbm.2020.103875
Ribeiro CF, Gonçalves SE, Yui KC, Borges AB, Barcellos DC, Brayner R (2013) Dentin bond strength: influence of Er:YAG and nd:YAG lasers. Int J Periodontics Restor Dent 33:373–377. https://doi.org/10.11607/prd.1096
Yang H, Guo J, Guo J, Chen H, Somar M, Yue J et al (2015) Nanoleakage evaluation at adhesive-dentin interfaces by different observation methods. Dent Mater J 34:654–662. https://doi.org/10.4012/dmj.2015-051
Hurtado A, Fuentes V, Cura M, Tamayo A, Ceballos L (2023) Long-term in Vitro Adhesive properties of two universal adhesives to dentin. Mater (Basel) 16. https://doi.org/10.3390/ma16093458
de Oliveira MT, Reis AF, Arrais CAG, Cavalcanti AN, Aranha ACC, Eduardo CD et al (2013) Analysis of the interfacial micromorphology and bond strength of adhesive systems to Er:YAG laser-irradiated dentin. Lasers Med Sci 28:1069–1076. https://doi.org/10.1007/s10103-012-1157-5
Funding
This work was supported by National Natural Science Foundation Of China (82071163).
Author information
Authors and Affiliations
Contributions
GS: Conceptualization, Software, Methodology, Data curation, Investigation, Writing–original draft. HC: Formal analysis, Methodology, Data curation. HW: Software, Investigation, Data curation. XC: Investigation, Data curation. FW: Investigation, Data curation. TB: Methodology, Investigation. SZ: Conceptualization, Supervision, Funding acquisition, Writing–review and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The consent of the participants was written, as indicated and authorized by the Ethics Committee for Human and Animal Studies of the Institute of Stomatology of Jilin University (Number: JDKQ2023105).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, G., Chen, H., Wang, H. et al. Effects of Nd:YAG laser irradiation at different energy densities on dentin bond durability under simulated pulpal pressure. Clin Oral Invest 28, 202 (2024). https://doi.org/10.1007/s00784-024-05600-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05600-x