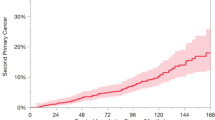
Abstract
Objectives
Second primary cancer is a common event in patients with head and neck squamous cell carcinoma. However, the incidence and relevant factors vary by studies. We conducted a systematic review and meta-analysis of observational studies to estimate the incidence and relevant risk factors.
Materials and methods
PubMed and Web of Science were searched for studies published between January 2000 and December 2020 that reported the incidence of SPC in HNSCC patients. Per 1000-person-year incidence and odds ratios were used to estimate the incidence and potential risk factors. Due to the high heterogeneity, random-effects models were used to estimate the incidence and 95% confidence interval.
Results
Seven thousand seven hundred thirteen articles were identified from the databases, in which 60 studies were included in this meta-analysis. The pooled incidence of the total, synchronous, and metachronous SPC in patients with HNSCC were 29.116 per 1000-person-year, 6.960 per 1000-person-year, and 26.025 per 1000-person-year, respectively. The head and neck region was the most common area where SPC occurred, followed by the lung (7.472 per 1000-person-year) and upper digestive tract (2.696 per 1000-person-year). Smoking, alcohol consumption, betel quid chewing, primary cancer of T1–2, and N0 were risk factors, while HPV infection (OR 0.47, 95% CI 0.30–0.72) was the protective factor.
Conclusions
SPC is frequently observed in HNSCC patients and had great impact on the prognosis. The findings could promote a more individualized follow-up strategy for SPC in HNSCC patients.
Clinical relevance
This systemic review and meta-analysis provide sufficient evidence for the establishment of the follow-up strategy for head and neck squamous cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer survivors are suffering from a great risk of developing and dying from a second primary cancer (SPC), with head and neck cancer (HNC) being one of the regions with highest risk among all kinds of first primary cancers [1]. As patients have a lifelong risk of developing an SPC, it has attributed to a quarter to one-third of all deaths in patients with head and neck squamous cell carcinoma (HNSCC) and has been identified as the leading cause of non-HNSCC death [2,3,4]. The mean incidence of SPC in patients with HNC was 13.2% with 5.3% for synchronous SPC (SSPC) and 9.4% for metachronous SPC (MSPC) reported in a conference report in EGPRN meeting [5]. However, the insufficient involvement of countries and regions leads to the inaccuracy of the results. What’s more, the duration of follow-up time was not taken into consideration in the research, which varies considerably among institutions, leading to the heterogeneity in incidence described by the percentage of the occurrence. Therefore, conducting an analysis of SPC incidence based on person-time denominators is essential.
Whether the risk factors of SPC are closely related to that of primary HNSCC remains controversial, where further investigation is required. The increasing risk of SPC is related to various reasons, including genetic predisposition, carcinogenic cancer treatments, and mostly, the environmental and lifestyle-related risk factors shared by the first and second cancers [6]. As one of the most representative risk factors for HNSCC, smoking could elevate the risk of SPC in the head and neck area and other smoking-related SPCs, such as lung cancer [1]. The carcinogenesis of radiotherapy could partially be reflected in SPC development at sites close to primary cancer [7].
SPC has a different choice of appropriate treatment and follow-up strategy comparing with other secondary events such as recurrence and metastasis, emphasizing the importance of its early detection and diagnosis. However, there are still no complete follow-up protocols concerning SPC after HNSCC and further investigation is urgently needed. American Cancer Society Head and Neck Cancer Survivorship Care Guideline and National Comprehensive Cancer Network only emphasize the screening and early detection of the three major sites which have the comparatively high risk of developing SPC, lacking the specific details of more subdivided subsites and the concrete recommendation of follow-up schedule and time interval [8]. It would be of benefit to the clinical work in the detection and diagnosis of SPC if the relatively accurate incidence of the subdivided subsites developing SPC was calculated and the follow-up time in SPC patients after HNSCC was summarized.
This systemic review and meta-analysis aimed to evaluate the SPC risk in HNSCC patients and further explore its predilection sites and risk factors. We tried to provide a more evidence-based approach for establishing an individualized SPC follow-up strategy according to the degree of risk in HNSCC patients and eventually promote earlier diagnosis and better prognosis.
Methods
This systematic review and meta-analysis have been registered on the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42020189273). Our study was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [9].
Search strategy
PubMed and Web of Science were searched for studies concerning SPC in HNSCC patients published between January 2000 and December 2020. The search strategy was as follows: (second primary malignancy OR second primary cancer OR second primary carcinoma) AND ((head and neck OR oral OR nasopharyngeal OR oropharyngeal OR hypopharyngeal OR laryngeal) AND (squamous cell carcinoma OR cancer OR carcinoma)). The initial search had no restriction on language or publication type.
Study selection
Three independent reviewers (XZ, DL, and HS) screened the titles and abstracts to exclude irrelevant studies. Full texts were assessed for eligibility when any information on the occurrence of SPC in HNSCC patients was mentioned in the abstract. The reference lists of included articles were also checked for additional relevant studies. Disagreements were resolved by consensus or involvement of a fourth reviewer as necessary.
Studies were included if they were original studies that reported the incidence of total SPC, SSPC, or MSPC after diagnosis of HNSCC. We included the studies that had investigated SSPC and MSPC according to the definitions reported by International Agency for Research on Cancer (IARC) or by Warren and Gates criteria or by Surveillance Epidemiology and End Results (SEER) program. According to IARC, an MSPC is defined as a SPC occurring more than 6 months after the diagnosis of the primary cancer. According to SEER program, an MSPC is defined as a SPC occurring 2 months after the diagnosis of the primary cancer and an SSPC is defined as that occurring within 2 months after the primary cancer [1, 10]. Where there appeared to be multiple studies concerning the same cohort, the most recent one was included if they contained exactly the same data on SPC. Studies were excluded if they (1) were reviews, meta-analysis, case reports, books, letters, or conference abstracts; (2) were published in non-English language; (3) did not make clear of the histological type of primary HNC; or (4) reported the incidence of SPC after HNC, including squamous cell cancer as a subtype of primary HNC, but we failed to obtain relevant data after contacting the authors. Total SPC, or tSPC, refers to the combination of SSPC and MSPC, the incidence for which can be obtained in two kinds of situations: firstly, data on SSPC and MSPC were both available and the incidence of tSPC was simply the addition of the two; secondly, SPC was taken as a whole without distinguishment of SSPC and MSPC.
Data extraction and risk of bias assessment
Data extraction was performed independently by three reviewers (XZ, DL, and HS) using a standardized data extraction form and disagreements were resolved by consensus. The following data were extracted from each study: study characteristics, demographic characteristics, primary HNSCC characteristics, and SPC characteristics (the overall and subgroup incidence of SPC, diagnosis criteria, inspection method, inspected sites, and time interval).
The risk of bias in individual studies was evaluated using the Newcastle–Ottawa scale (NOS)164. In brief, the list consists of nine items examining the quality of studies based on the selection of cases, factors controlling, and ascertainment of exposure. A full score is 9 points, and a score ≥ 7 indicates high quality, while a score < 6 for low quality. The NOS checklist was assessed independently by two reviewers. Discrepancies in the score were resolved through discussion by the reviewers. The risk of bias across studies was evaluated with funnel plots and the Egger test and considered significant when p < 0.05.
Data synthesis and statistical analysis
In each study, the numbers of patients with primary HNSCC, tSPC, SSPC, and MSPC were combined respectively to give a pooled incidence of SPC based on 1000-person-year denominators.
Subgroup analyses were conducted to compare the pooled incidence according to different primary sites and SPC sites and the source of heterogeneity. The risk of SPC was assessed according to proposed risk factors, using an OR, with a 95% confidence interval (95% CI). For the studies that concerned the same cohort with overlapping data on subgroup and risk factors analyses, only the most recent one was used.
Heterogeneity between studies was assessed using the I2 statistic, with I2 value > 50% indicating high heterogeneity. A p-value for I2 < 0.05 was considered statistically significant. Data were pooled using a random-effects model if I2 > 50%; otherwise, a fixed-effect model was used. R version 4.0.2 was used for all meta-analyses.
Results
The search strategy identified 7717 citations. After removing duplicates, the titles and abstracts of 4921 studies were reviewed, and 334 articles were further assessed in full text. Ultimately, we identified 60 articles that fulfilled the eligibility criteria [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] (Fig. 1), representing 60 separate study cohorts, containing 148,534 subjects in 17 countries. Detailed characteristics of all included studies were provided in eTable 1.
The pooled per 1000-person-year incidence of developing a SPC after primary HNSCC was 29.116 per 1000-person-year (95%CI 23.920–34.805, I2 = 97.64%) for tSPC (Fig. 2), 6.960 per 1000-person-year for SSPC (95% CI 4.461–10.009; I2 = 96.76%), and 26.025 per 1000-person-year for MSPC (95%CI 19.526–33.427; I2 = 98.31%).
Pooled incidence of SPC according to different primary cancer sites and SPC sites
We selected 27 studies that took the whole body as a screening area for SPC to estimate the pooled per 1000-person-year incidence according to subsites of primary HNSCC. Hypopharyngeal cancer had the highest incidence of tSPC (41.562 per 1000-person-year) while larynx cancer had the lowest (22.557 per 1000-person-year). The tSPC incidence of other primary HNSCC was 38.729 per 1000-person-year for the oral cavity, 36.811 per 1000-person-year for the oropharynx (Table 1 and eFig1).
We selected the studies that took primary HNSCC as a whole to investigate the site distribution of SPC. SPC sites were divided into five anatomical areas referring to the published studies, including head and neck region (oral cavity, oropharynx, nasopharynx, hypopharynx, larynx, and thyroid), lower respiratory system, upper digestive tract (esophagus and stomach), lower digestive tract, and genitourinary system. The head and neck region was the most common area where SPC occurred (13.127 per 1000-person-year, 95% CI 9.282–18.564, I2 = 97.57%). The SPC-developed incidence of the subsites of head and neck region are as follows: oropharynx (30.258 per 1000-person-year, 95% CI 24.357–37.588, I2 = 88.67%), (2.254 per 1000-person-year, 95% CI6. 1.013–5.011, I2 = 78.89%), and oral cavity (10.321 per 1000-person-year, 95% CI6.397–16.654, I2 = 95.02%). The lower respiratory system (7.472 per 1000-person-year, 95% CI 5.740–9.726, I2 = 92.43%) and upper digestive tract (2.696 per 1000-person-year, 95% CI 1.739–4.180, I2 = 86.85%) came the second and the third (Table 1). In order to make out the characteristic of HNSCC survivors for SPC systematical distribution and incidence, we compared the incidence of developing a new primary cancer in the top three anatomical areas as has just been noted (Fig. 3 and Table 1), in HNSCC group, the general population (data extracted from WHO CANCER TODAY in 2020), and all primary cancer survivors without site-limitation (data summarized from five released meta-analyses, see details in the figure legend). Interestingly, for the other two groups, SPC was most likely to occur in the lower respiratory system, while HNSCC survivors have the highest SPC incidence in the head and neck region. Such a distinct difference provided crucial insight into the more individualized follow-up strategy for SPC in HNSCC patients. The head and neck region should be paid prior attention to in HNSCC survivors for better prevention or early detection of SPC.
Site distribution of tSPC occurring after the four primary cancers. For each figure, the SPC site was listed above the horizontal line. Primary cancer sites were represented by dots of different colors and listed below the line in order of incidence from highest to lowest. 95% confidence interval was shown within the brackets. (A) SPC sites were divided into five anatomic areas: head and neck region, lower respiratory system, upper digestive tract, lower digestive tract, and genitourinary system; (B) SPCs in the head and neck region were further divided into those occurred in the oral cavity, oropharynx, hypopharynx, larynx, and thyroid; (C) incidence of developing SPC from not-limited primary cancer sites was summarized from study Sung, H. et al., 2020, Feller, Anita et al., 2020, Jégu, Jérémie et al., 2014, Donin, Nicholas M et al., 2016, Donin, Nicholas et al., 2019; c: Pooled incidence of developing SPC in three sites above among patients with HNSCC, according to our meta-analysis; (D) the crude rate of estimated new cancer cases among general population was extracted from WHO CANCER TODAY in 2020; abbreviations: SPC, second primary cancer
We further explored the predilection for where SPC occurred according to different primary HNSCC site (Fig. 3). The most common SPC site for patients with oral cancer was the head and neck region (27.685 per 1000-person-year). SPC was also more common for patients with oropharyngeal cancer in the head and neck region (10.959 per 1000-person-year) and lower respiratory system (10.153 per 1000-person-year). For patients with laryngeal cancer, it was the upper digestive tract (6.485 per 1000-person-year).
Pooled incidence of SPC according to potential risk factors
We selected ten potential risk factors frequently discussed in published studies, and 17 studies were included in this analysis (Table 2). In terms of demographic characteristics, those aged ≥ 50 years were at a higher risk of SPC (OR 1.69, 95% CI 1.06–2.69). Gender was proved to be an irrelevant factor (OR 1.72, 95% CI 1.63–1.83).
Unhealthy lifestyles, such as smoking, alcohol consumption, and betel quid chewing, have already been identified as risk factors for HNSCC and were proved to be related to SPC after that as well. The OR in smokers compared with non-smokers (OR 1.79, 95% CI 0.62–5.11; I2 = 92.0%), alcohol drinkers compared with non-drinkers (OR 1.68, 95% CI 1.32–2.12; I2 = 92.0%), and betel quid chewers compared with non-chewers (OR 5.62, 95% CI 0.61–51.73; I2 = 85.0%) indicated these three as risk factors for SPC after HNSCC. HPV infection, however, appeared to be a protective factor (OR 0.47, 95% CI 0.30–0.72).
We were also able to confirm that primary HNSCC with specific oncologic characteristics were more likely to develop an SPC (Fig. 4). Primary cancers of T1–2 (OR 1.57, 95% CI 1.23–2.02) and N0 (OR 1.58, 95% CI 1.15–2.18) were demonstrated to be at a higher risk of SPC compared with those of T3–T4 and N1–N3.
Forest plots of OR for SPC according to potential risk factors. (A) OR for tSPC, SSPC, and MSPC in patients with HNSCC of early stage versus advanced stage; (B) OR for SPC in patients with HNSCC of T1–2 versus T3–4; (C) OR for SPC in patients with HNSCC of N0 versus N1–3. Abbreviations: OR, odds ratio; CI, confidence interval; SPC, second primary cancer; tSPC, total second primary caner; SSPC, synchronous second primary caner; MSPC, metachronous second primary cancer
Risk of bias assessment and heterogeneity analysis
The details of risk of bias in individual studies using the Newcastle–Ottawa scale (NOS) are available in eTable 3. The number of high-quality studies is 9, which indicates the studies with comprehensive information provided. The details of risk of bias across studies are available in eFig 2. There was no evidence of publication bias with the Egger test (p = 0.12).
As we observed significant heterogeneity among included studies, geographical region, sampling method, sample size, and year of primary cancer diagnosis were selected as the potential source of heterogeneity for subgroup analysis (eTable 3). However, we only observed a few faint changes in pooled incidence and a minor decline in I2. Thus, we failed to find the source of heterogeneity in the baseline by subgroup analysis.
Discussion
In this study, we summarized the incidence of SPC in patients with HNSCC and proved it to be a common event, with the pooled per 1000-person-year incidence of 29.116 for tSPC, 6.960 for SSPC, and 26.025 for MSPC. The most common SPC sites were the head and neck region, lung, and upper digestive tract. Different primary cancers had their predilection for where SPC occurred. Age over 40 years, smoking, alcohol consumption, betel quid chewing, family cancer history, primary cancer of T1–T2, N0, or well/moderate differentiation were found to be risk factors for SPC after HNSCC, while HPV infection and radiotherapy were protective factors. To our knowledge, this is the most extensive systemic review of SPC in patients with HNSCC, the first meta-analysis to assess the risk factors, and the first to calculate the incidence of developing SPC after the HNSCC based on the person-year analysis [71,72,73].
Site distribution of SPC after primary HNSCC was frequently discussed in previous studies, sharing generally consistent results with slight differences. The most common SPC site for patients with primary SCC in the oral cavity and oropharynx was the head and neck; for patients with hypopharyngeal cancer, it was the lung and esophagus; for those with laryngeal cancer, it was the lung [69, 71, 73,74,75,76,77]. These findings are not surprising as the SCC of the lung, head and neck, and esophagus shares similar risk factors and morphologic features as well as mechanisms of tumorigenesis. Genetic predisposition could also play a role. The HNSCC has been confirmed to be associated with molecular-level changes such as the epidermal growth factor receptor (EGFR) and the genetic polymorphism of p21 [78, 79], which may explain the reason why second primary cancer of HNSCC is most likely to occur in the head and neck. Studies have confirmed that the fibroblast growth factor receptor 1 (FGFR1) gene located on chromosome 8 in HNSCC is highly variable, while frequent amplification of FGFR1, a cell surface receptor of fibroblast growth factor involved in the regulation of cell proliferation and differentiation, can also be detected in primary lung squamous cell carcinoma [80]. This may provide guidance for HNSCC treatment and prognostic testing programs to reduce the incidence of secondary primary cancer.
The evaluation of risk factors for SPC is of great importance during the follow-up of cancer survivors, contributing to earlier diagnosis and treatment of SPC, and eventually improves the prognosis of HNSCC. Lifestyle factors such as smoking, alcohol consumption, and betel quid chewing have already been proved to be risk factors for sure, and those who continued smoking after a cancer diagnosis seem to have a higher risk of SPC [81, 82]. The phenomenon could be partly explained by the classic theory of field cancerization [82]. This theory hypothesizes the tissue in a certain site is exposed to carcinogenic factors such as tobacco, alcohol for a long time, and those genes in the tissue cells are abnormally changed, which leads to an increased risk of malignant lesions in the epithelial layer of the entire area, and multiple malignant lesions may occur simultaneously or successively. That is to say, risk factors for primary cancer affect other sites at the same time (for example, continuous exposure to tobacco harms the esophagus and airway), which may increase their incidence of SPC. This reminds us of the importance of encouraging healthy lifestyles and routine comprehensive examination in cancer survivors.
HNSCC of advanced stage or poor differentiation is more likely to undergo recurrence and metastasis within the 5 years after treatment. On the contrary, those of T1–T2 and N0 stage or well/moderate differentiation tend to suffer from SPC. Part of the reason may be that they have a longer survival time to develop a second cancer and the incidence of SPC rises with the extension of follow-up time.
The impact of radiotherapy on the occurrence of SPC in HNSCC patients still remains controversial [6]. So far, the most consensus view is that the risk of SPC in patients receiving radiotherapy decreases during the first 5–10 years after HNSCC diagnosis, but then increases beyond the risk in those without radiotherapy [6, 83]. A possible theory is that radiation may sterilize occult synchronous malignancies and premalignant tissues, thus reducing the incidence of SPC in or near the irradiated fields [84, 85]. While in terms of its carcinogenic effect, it often takes years of latency to be revealed. So it may result in underestimating actual incidence due to limited follow-up time [65, 86]. As the mean follow-up time of all included studies was mainly within 5 years, it is not surprising that radiotherapy was a protective factor in the present study.
Some problems in follow-up strategy of SPC still exist nowadays. For example, the need of an individually tailored approach that considers the specific performance status and the impact on quality of life of the diagnosis of SPC in asymptomatic patients [87]. The incidence based on person-year analysis, which takes the follow-up time into considerations as a part of the denominator, provides relatively more accurate data. The three major sites with high risk in developing the SPC after HNSCC are head and neck region, lung, and upper digestive tract, which is almost the same as the result of the sites developing SPC after HNC [8]. The subsites with highest incidence in head and neck region are as follows: oropharynx, oral cavity, and hypopharynx, suggesting that more targeted inspections are needed in screening and diagnosis of head and neck region and upper digestive tract. The data of the ranking of most common SPC sites in different primary HNSCC sites can provide the detailed evidence in personalized follow-up strategy in clinical work. For example, the patients with larynx primary cancer should pay more attention to lung SPC than head and neck region according to the data.
Some limitations of the present study should be interpreted. Firstly, there was significant heterogeneity in most of our analyses and we failed to explain it through subgroup analysis. We assumed that the heterogeneity might be partly attributed to the differences in the diagnostic criteria for SPC, which were diversely modified according to the Warren and Gates criteria and strikingly varied among the included studies. Even though we tried to categorize the studies according to different criteria throughout the analysis process, similar results were obtained as those shown in the present study. Secondly, ORs were calculated using raw data extracted from studies, not adjusting for potential underlying differences between individuals. The connections between the first and second primary cancer were mediated by complex interactions between them, including genetic predispositions, aging, treatment exposures, lifestyle factors, socioeconomic status, and access to health care [88], which could not be independently analyzed. However, the direction of the effect, protective or harmful, could still be identified in the present study.
Conclusions
SPC is frequently observed after the diagnosis of HNSCC and the incidence varies with different sites of primary cancer and SPC. Unhealthy lifestyles, anatomical subsites, initial treatments, and other oncological factors of primary HNSCC should all be taken into consideration when assessing the risk of SPC. Thus, a more individualized screening and follow-up strategy should be applied according to different degrees of risk, with the reasonable arrangement of various examinations at the proper time of points.
Data availability
The original contributions presented in the study are included in the supplementary material. Further inquiries can be directed to the corresponding authors.
References
Sung H, Hyun N, Leach CR et al (2020) Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. JAMA 324(24):2521–2535. https://doi.org/10.1001/jama.2020.23130
Baxi SS, Pinheiro LC, Patil SM et al (2014) Causes of death in long-term survivors of head and neck cancer. Cancer 120(10):1507–1513. https://doi.org/10.1002/cncr.28588
Simpson MC, Massa ST, Boakye EA et al (2018) Primary cancer vs competing causes of death in survivors of head and neck cancer. JAMA Oncol 4(2):257–259. https://doi.org/10.1001/jamaoncol.2017.4478
van der Schroeff MP, van de Schans SA, Piccirillo JF et al (2010) Conditional relative survival in head and neck squamous cell carcinoma: permanent excess mortality risk for long-term survivors. Head Neck 32(12):1613–1618. https://doi.org/10.1002/hed.21369
Collins C, Frese T, Merode TV et al (2020) General practice and the community: research on health service, quality improvements and training. Selected abstracts from the EGPRN Meeting in Vigo, Spain, 17–20 October 2019. Eur J Gen Pract 26(1):42–50. https://doi.org/10.1080/13814788.2020.1719994
Travis LB, Demark Wahnefried W, Allan JM et al (2013) Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol 10(5):289–301. https://doi.org/10.1038/nrclinonc.2013.41
Berrington de Gonzalez A, Kutsenko A, Rajaraman P (2012) Sarcoma risk after radiation exposure. Clin Sarcoma Res 2(1):18. https://doi.org/10.1186/2045-3329-2-18
Cohen EEW, LaMonte SJ, Erb NL et al (2016) American Cancer Society head and neck cancer survivorship care guideline. CA: Cancer J Clin 66(3):203–239. https://doi.org/10.3322/caac.21343
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372:n71. https://doi.org/10.1136/bmj.n71
Supramaniam R (2008) New malignancies among cancer survivors: SEER cancer registries, 1973–2000. J Epidemiol Community Health 62:375–376. https://doi.org/10.1136/jech.2007.063560
Cloos J, Leemans CR, van der Sterre ML et al (2000) Mutagen sensitivity as a biomarker for second primary tumors after head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 9(7):713–717
Nikolaou AC, Markou CD, Petridis DG et al (2000) Second primary neoplasms in patients with laryngeal carcinoma. Laryngoscope 110(1):58–64. https://doi.org/10.1097/00005537-200001000-00012
Wang CC, Chen ML, Hsu KH et al (2000) Second malignant tumors in patients with nasopharyngeal carcinoma and their association with Epstein-Barr virus. Int J Cancer 87(2):228–231. https://doi.org/10.1002/1097-0215(20000715)87:2%3c228::aid-ijc12%3e3.0.co;2-t
Homann N, Nees M, Conradt C et al (2001) Overexpression of p53 in tumor-distant epithelia of head and neck cancer patients is associated with an increased incidence of second primary carcinoma. Clin Cancer Res 7(2):290–296
Jacob R, Welkoborsky HJ, Bittinger F et al (2001) Histological grading, growth fraction and DNA-ploidy as criteria for the treatment of pharyngeal and supraglottic squamous cell carcinomas: a preliminary, prospective study. ORL J Otorhinolaryngol Relat Spec 63(5):314–320. https://doi.org/10.1159/000055765
Khuri FR, Kim ES, Lee JJ et al (2001) The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev 10(8):823–829
Mayne ST, Cartmel B, Baum M et al (2001) Randomized trial of supplemental beta-carotene to prevent second head and neck cancer. Cancer Res 61(4):1457–1463
Munker R, Purmale L, Aydemir U et al (2001) Advanced head and neck cancer: long-term results of chemo-radiotherapy, complications and induction of second malignancies. Onkologie 24(6):553–558. https://doi.org/10.1159/000055143
Do KA, Johnson MM, Lee JJ et al (2004) Longitudinal study of smoking patterns in relation to the development of smoking-related secondary primary tumors in patients with upper aerodigestive tract malignancies. Cancer 101(12):2837–2842. https://doi.org/10.1002/cncr.20714
Do KA, Johnson MM, Doherty DA et al (2003) Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States). Cancer Causes Control 14(2):131–138. https://doi.org/10.1023/a:1023060315781
Hanna E, Alexiou M, Morgan J et al (2004) Intensive chemoradiotherapy as a primary treatment for organ preservation in patients with advanced cancer of the head and neck: efficacy, toxic effects, and limitations. Arch Otolaryngol Head Neck Surg 130(7):861–867. https://doi.org/10.1001/archotol.130.7.861
Brouwer J, Senft A, de Bree R et al (2006) Screening for distant metastases in patients with head and neck cancer: is there a role for (18)FDG-PET? Oral Oncol 42(3):275–280. https://doi.org/10.1016/j.oraloncology.2005.07.009
Licitra L, Perrone F, Bossi P et al (2006) High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 24(36):5630–5636. https://doi.org/10.1200/jco.2005.04.6136
Yao M, Nguyen T, Buatti JM et al (2006) Changing failure patterns in oropharyngeal squamous cell carcinoma treated with intensity modulated radiotherapy and implications for future research. Am J Clin Oncol 29(6):606–612. https://doi.org/10.1097/01.coc.0000242294.89536.d6
Tsou YA, Hua CH, Tseng HC et al (2007) Survival study and treatment strategy for second primary malignancies in patients with head and neck squamous cell carcinoma and nasopharyngeal carcinoma. Acta Otolaryngol 127(6):651–657. https://doi.org/10.1080/00016480600951517
Hsu YB, Chang SY, Lan MC et al (2008) Second primary malignancies in squamous cell carcinomas of the tongue and larynx: an analysis of incidence, pattern, and outcome. J Chin Med Assoc 71(2):86–91. https://doi.org/10.1016/s1726-4901(08)70080-7
Sackett MK, Bairati I, Meyer F et al (2008) Prognostic significance of cyclooxygenase-2 overexpression in glottic cancer. Clin Cancer Res 14(1):67–73. https://doi.org/10.1158/1078-0432.Ccr-07-2028
Farhadieh RD, Smee R, Rees CG et al (2009) Mutant p53 and cyclin A1 protein expression in primary laryngeal squamous cell carcinomas do not correlate to second primary tumours of the head and neck. ANZ J Surg 79(1–2):48–54. https://doi.org/10.1111/j.1445-2197.2008.04799.x
Peck BW et al (2013) Low risk of second primary malignancies among never smokers with human papillomavirus-associated index oropharyngeal cancers. Head Neck 35(6):794–9. https://doi.org/10.1002/hed.23033
Lee DH et al (2013) Second cancer incidence, risk factor, and specific mortality in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg 149(4):579–86. https://doi.org/10.1177/0194599813496373
Mochizuki Y et al (2015) Clinical characteristics of multiple primary carcinomas of the oral cavity. Oral Oncol 51(2):182–9. https://doi.org/10.1016/j.oraloncology.2014.11.013
Li F, Sturgis EM, Zafereo ME et al (2009) p73 G4C14-to-A4T14 polymorphism and risk of second primary malignancy after index squamous cell carcinoma of head and neck. Int J Cancer 125(11):2660–2665. https://doi.org/10.1002/ijc.24570
van der Haring IS, Schaapveld MS, Roodenburg JL et al (2009) Second primary tumours after a squamous cell carcinoma of the oral cavity or oropharynx using the cumulative incidence method. Int J Oral Maxillofac Surg 38(4):332–338. https://doi.org/10.1016/j.ijom.2008.12.015
Cohen EE, Haraf DJ, Kunnavakkam R et al (2010) Epidermal growth factor receptor inhibitor gefitinib added to chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 28(20):3336–3343. https://doi.org/10.1200/jco.2009.27.0397
Eisbruch A, Harris J, Garden AS et al (2010) Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00–22). Int J Radiat Oncol Biol Phys 76(5):1333–1338. https://doi.org/10.1016/j.ijrobp.2009.04.011
Priante AV, Carvalho AL, Kowalski LP (2010) Second primary tumor in patients with upper aerodigestive tract cancer. Braz J Otorhinolaryngol 76(2):251–256
Ramroth H, Schoeps A, Rudolph E et al (2011) Factors predicting survival after diagnosis of laryngeal cancer. Oral Oncol 47(12):1154–1158. https://doi.org/10.1016/j.oraloncology.2011.08.003
Sasaki M, Aoki T, Karakida K et al (2011) Postoperative follow-up strategy in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg 69(6):e105–e111. https://doi.org/10.1016/j.joms.2010.11.039
Azad AK, Bairati I, Samson E et al (2012) Genetic sequence variants and the development of secondary primary cancers in patients with head and neck cancers. Cancer 118(6):1554–1565. https://doi.org/10.1002/cncr.26446
Chu PY, Tsai TL, Tai SK et al (2012) Effectiveness of narrow band imaging in patients with oral squamous cell carcinoma after treatment. Head Neck 34(2):155–161. https://doi.org/10.1002/hed.21704
Huang SF, Li HF, Liao CT et al (2012) Association of HPV infections with second primary tumors in early-staged oral cavity cancer. Oral Dis 18(8):809–815. https://doi.org/10.1111/j.1601-0825.2012.01950.x
Nakahara R, Kodaira T, Furutani K et al (2012) Treatment outcomes of definitive chemoradiotherapy for patients with hypopharyngeal cancer. J Radiat Res 53(6):906–915. https://doi.org/10.1093/jrr/rrs052
Hakeem AH, Pradhan SA, Tubachi J et al (2013) Outcome of per oral wide excision of T1–2 N0 localized squamous cell cancer of the buccal mucosaanalysis of 156 cases. Laryngoscope 123(1):177–180. https://doi.org/10.1002/lary.23707
Ho MW, Field EA, Field JK et al (2013) Outcomes of oral squamous cell carcinoma arising from oral epithelial dysplasia: rationale for monitoring premalignant oral lesions in a multidisciplinary clinic. Br J Oral Maxillofac Surg 51(7):594–599. https://doi.org/10.1016/j.bjoms.2013.03.014
Hsu SH, Wong YK, Wang CP et al (2013) Survival analysis of patients with oral squamous cell carcinoma with simultaneous second primary tumors. Head Neck 35(12):1801–1807. https://doi.org/10.1002/hed.23242
Kämmerer PW, Koch FP, Schiegnitz E et al (2013) Associations between single-nucleotide polymorphisms of the VEGF gene and long-term prognosis of oral squamous cell carcinoma. J Oral Pathol Med 42(5):374–381. https://doi.org/10.1111/jop.12026
Koo K, Barrowman R, McCullough M et al (2013) Non-smoking non-drinking elderly females: a clinically distinct subgroup of oral squamous cell carcinoma patients. Int J Oral Maxillofac Surg 42(8):929–933. https://doi.org/10.1016/j.ijom.2013.04.010
Lai WM, Chen CC, Lee JH et al (2013) Second primary tumors and myeloperoxidase expression in buccal mucosal squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 116(4):464–473. https://doi.org/10.1016/j.oooo.2013.06.018
Liu CH, Chen HJ, Wang PC et al (2013) Patterns of recurrence and second primary tumors in oral squamous cell carcinoma treated with surgery alone. Kaohsiung J Med Sci 29(10):554–559. https://doi.org/10.1016/j.kjms.2013.03.001
Mourad WF, Hu KS, Shasha D et al (2013) Long-term outcome of seropositive HIV patients with head and neck squamous cell carcinoma treated with radiation therapy and chemotherapy. Anticancer Res 33(12):5511–5516
Kwon M, Roh JL, Song J et al (2014) Noncancer health events as a leading cause of competing mortality in advanced head and neck cancer. Ann Oncol 25(6):1208–1214. https://doi.org/10.1093/annonc/mdu128
Priante AV, Gross JL, Sztokfisz CZ et al (2014) Diagnosis of second primary tumor and long-term survival after single initial triple endoscopy in patients with head and neck cancer. Eur Arch Otorhinolaryngol 271(8):2285–2292. https://doi.org/10.1007/s00405-013-2768-6
Tiwana MS, Hay J, Wu J et al (2014) Incidence of second metachronous head and neck cancers: population-based outcomes over 25 years. Laryngoscope 124(10):2287–2291. https://doi.org/10.1002/lary.24719
Gokavarapu S, Parvataneni N, Charan CR et al (2015) Multi centricity of oral verrucous carcinoma: a case series of 22 cases. Indian J Otolaryngol Head Neck Surg 67(2):138–142. https://doi.org/10.1007/s12070-015-0835-6
Koo K, Harris R, Wiesenfeld D et al (2015) A role for panendoscopy? Second primary tumour in early stage squamous cell carcinoma of the oral tongue. J Laryngol Otol 129:S27–S31. https://doi.org/10.1017/s0022215114002989
Kwon M, Roh J-L, Song J et al (2015) Effect of metformin on progression of head and neck cancers, occurrence of second primary cancers, and cause-specific survival. Oncologist 20(5):546–553. https://doi.org/10.1634/theoncologist.2014-0426
Lim H, Kim DH, Jung HY et al (2015) Clinical significance of early detection of esophageal cancer in patients with head and neck cancer. Gut Liver 9(2):159–165. https://doi.org/10.5009/gnl13401
Sato K, Kubota A, Furukawa M et al (2015) Definitive radiotherapy for early-stage hypopharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 272(8):2001–2006. https://doi.org/10.1007/s00405-014-3132-1
van Monsjou HS, Schaapveld M, van den Brekel MWM et al (2015) The epidemiology of head and neck squamous cell carcinoma in The Netherlands during the era of HPV-related oropharyngeal squamous cell carcinoma. Is there really evidence for a change? Oral Oncol 51(10):901–907. https://doi.org/10.1016/j.oraloncology.2015.06.011
Martel M, Alemany L, Taberna M et al (2017) The role of HPV on the risk of second primary neoplasia in patients with oropharyngeal carcinoma. Oral Oncol 64:37–43. https://doi.org/10.1016/j.oraloncology.2016.11.011
Boakye EA, Buchanan P, Hinyard L et al (2018) Incidence and risk of second primary malignant neoplasm after a first head and neck squamous cell carcinoma. Jama Otolaryngol-Head Neck Surg 144(8):727–737. https://doi.org/10.1001/jamaoto.2018.0993
Leoncini E, Vukovic V, Cadoni G et al (2018) Tumour stage and gender predict recurrence and second primary malignancies in head and neck cancer: a multicentre study within the INHANCE consortium. Eur J Epidemiol 33(12):1205–1218. https://doi.org/10.1007/s10654-018-0409-5
Sato K, Yabuki K, Sano D et al (2018) Analysis of prognostic factors, including the incidence of second primary cancer, in patients with early stage laryngeal squamous cell carcinoma treated by radiation-based therapy. Transl Cancer Res 7(4):890–900. https://doi.org/10.21037/tcr.2018.06.15
Tang D, Tao L, Zhou L et al (2018) Retrospective analysis of 659 laryngeal squamous cell carcinoma patients treated with open laryngeal function-preserving operations. Acta Otolaryngol 138(11):1043–1050. https://doi.org/10.1080/00016489.2018.1500711
Chow JCH, Au KH, Mang OWK et al (2019) Risk, pattern and survival impact of second primary tumors in patients with nasopharyngeal carcinoma following definitive intensity-modulated radiotherapy. Asia Pac J Clin Oncol 15(1):48–55. https://doi.org/10.1111/ajco.12994
Elicin O, Sermaxhaj B, Bojaxhiu B et al (2019) Incidence of second primary cancers after radiotherapy combined with platinum and/or cetuximab in head and neck cancer patients. Strahlenther Onkol 195(6):468–474. https://doi.org/10.1007/s00066-018-1400-5
Haremza C, Baert M, Pascual C et al (2019) Head and neck squamous cell carcinoma and metachronous second primaries. Eur Ann Otorhinolaryngol-Head Neck Dis 136(5):367–372. https://doi.org/10.1016/j.anorl.2019.05.006
Iwatsubo T, Ishihara R, Morishima T et al (2019) Impact of age at diagnosis of head and neck cancer on incidence of metachronous cancer. Bmc Cancer 19. https://doi.org/10.1186/s12885-018-5231-7
Ozdemir Y, Topkan E (2019) Second primary malignancies in laryngeal carcinoma patients treated with definitive radiotherapy. Indian J Cancer 56(1):29–34. https://doi.org/10.4103/ijc.IJC_273_18
Silen S, Haapaniemi A, Dickinson A et al (2019) Presentation of second primary cancers in young laryngeal carcinoma patients. Acta Otolaryngol 139(1):85–89. https://doi.org/10.1080/00016489.2018.1527037
Hoxhaj I, Hysaj O, Vukovic V et al (2020) Occurrence of metachronous second primary cancer in head and neck cancer survivors: a systematic review and meta-analysis of the literature. Eur J Cancer Care (Engl) e13255. https://doi.org/10.1111/ecc.13255
Coca-Pelaz A, Rodrigo JP, Suarez C et al (2020) The risk of second primary tumors in head and neck cancer: a systematic review. Head Neck-J Sci Spec Head Neck 42(3):456–466. https://doi.org/10.1002/hed.26016
Haughey BH, Gates GA, Arfken CL et al (1992) Meta-analysis of second malignant tumors in head and neck cancer: the case for an endoscopic screening protocol. Ann Otol Rhinol Laryngol 101(2 Pt 1):105–112. https://doi.org/10.1177/000348949210100201
Morris LGT, Sikora AG, Patel SG et al (2011) Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol 29(6):739–746. https://doi.org/10.1200/jco.2010.31.8311
Sweet Ping N, Pollard C III, Kamal M et al (2019) Risk of second primary malignancies in head and neck cancer patients treated with definitive radiotherapy. Npj Precis Oncol 3. https://doi.org/10.1038/s41698-019-0097-y
Birkeland AC, Rosko AJ, Chinn SB et al (2016) Prevalence and outcomes of head and neck versus non-head and neck second primary malignancies in head and neck squamous cell carcinoma: an analysis of the surveillance, epidemiology, and end results Database. ORL J Otorhinolaryngol Relat Spec 78(2):61–69. https://doi.org/10.1159/000443768
Bertolini F, Trudu L, Banchelli F et al (2020) Second primary tumors in head and neck cancer patients: The importance of a “tailored” surveillance. Oral Dis. https://doi.org/10.1111/odi.13681
Li G, Liu Z, Sturgis EM et al (2005) Genetic polymorphisms of p21 are associated with risk of squamous cell carcinoma of the head and neck. Carcinogenesis 26(9):1596–1602. https://doi.org/10.1093/carcin/bgi105
Michmerhuizen NL, Birkeland AC, Bradford CR et al (2016) Genetic determinants in head and neck squamous cell carcinoma and their influence on global personalized medicine. Genes Cancer 7(5–6):182–200. https://doi.org/10.18632/genesandcancer.110
von Mässenhausen A, Franzen A, Heasley L et al (2013) FGFR1 as a novel prognostic and predictive biomarker in squamous cell cancers of the lung and the head and neck area. Ann Transl Med 1(3):23. https://doi.org/10.3978/j.issn.2305-5839.2013.06.08
Mohan M, Jagannathan N (2014) Oral field cancerization: an update on current concepts. Oncol Rev 8(1):244. https://doi.org/10.4081/oncol.2014.244
Slaughter DP, Southwick HW, Smejkal W (1953) Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6(5):963–968. https://doi.org/10.1002/1097-0142(195309)6:5%3c963::aid-cncr2820060515%3e3.0.co;2-q
Rennemo E, Zätterström U, Evensen J et al (2009) Reduced risk of head and neck second primary tumors after radiotherapy. Radiother Oncol 93(3):559–562. https://doi.org/10.1016/j.radonc.2009.08.005
Wang C, Kishan AU, Yu JB et al (2019) Association between long-term second malignancy risk and radiation: a comprehensive analysis of the entire surveillance, epidemiology, and end results database (1973–2014). Adv Radiat Oncol 4(4):738–747. https://doi.org/10.1016/j.adro.2019.05.003
Farhadieh RD, Otahal P, Rees CG et al (2009) Radiotherapy is not associated with an increased rate of Second Primary Tumours in Oral Squamous Carcinoma: a study of 370 patients. Oral Oncol 45(11):941–945. https://doi.org/10.1016/j.oraloncology.2009.05.634
Mroueh R, Bsc AN, Haapaniemi A et al (2020) Risk of second primary cancer in oral squamous cell carcinoma. Head Neck-J Sci Spec Head Neck. https://doi.org/10.1002/hed.26107
Denaro N, Merlano MC, Russi EG (2016) Follow-up in head and neck cancer: do more does it mean do better? A systematic review and our proposal based on our experience. Clin Exp Otorhinolaryngol 9(4):287–297. https://doi.org/10.21053/ceo.2015.00976
Perez DG, Loprinzi C, Ruddy KJ (2020) Lifestyle factors can lead to multiple cancers over a lifetime-here we go again. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2020.7360
Author information
Authors and Affiliations
Contributions
GL, YL, and LY contributed to conception and design of the study. XZ, DL, and HX contributed to data extraction. XZ organized the data. DL, BZ, and JG performed the statistical analysis. XZ, DL, HX, and JF wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Consent for publication is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dongheng Lu and Xinyu Zhou contributed equally to this work and share the first authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, D., Zhou, X., Sun, H. et al. Risk of second primary cancer in patients with head and neck squamous cell carcinoma: a systemic review and meta-analysis. Clin Oral Invest 27, 4897–4910 (2023). https://doi.org/10.1007/s00784-023-05066-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05066-3