Abstract
Purpose
The diagnosis of a second primary cancer (SPC) is a major concern in the follow-up of survivors of a primary head and neck cancer (HNC), but the anatomic subsites in the head and neck area are close, making it difficult to distinguish a SPC of a recurrence and therefore register it correctly.
Methods
We performed a retrospective cohort study using data from two population-based cancer registries in Catalonia, Spain: the Tarragona Cancer Registry and the Girona Cancer Registry. All patients diagnosed with HNC during the period 1994–2013 were registered and followed-up to collect cases of SPC. We analysed the standardized incidence ratio (SIR) and the excess absolute risk (EAR) to determine the risk of second malignancies following a prior HNC.
Results
923 SPC were found in a cohort of 5646 patients diagnosed of a first head and neck cancer. Men had an increased risk of a SPC with a SIR of 2.22 and an EAR of 216.76. Women also had an increased risk with a SIR of 2.02 and an EAR of 95.70. We show the risk for different tumour sites and discuss the difficulties of the analysis.
Conclusion
The risks of a SPC following a prior HNC in Tarragona and Girona are similar to those previously found in other similar cohorts. It would appear to be advisable to make a revision of the international rules of classification of multiple tumours, grouping the sites of head and neck area with new aetiological criteria to better determine and interpret the risks of SPC obtained in these studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A second primary cancer (SPC) is defined as an invasive cancer diagnosed after a first primary cancer which is not an extension or a recurrence of that. The diagnosis of a SPC has long been a major concern in the follow-up of survivors of a first head and neck cancer (HNC) as it represents a frequent cause of long-term mortality in those patients [1]. The most commonly diagnosed SPC after a first HNC are those arising from lung, oesophagus or another head and neck site.
As regards the causes of SPC, they can be categorized into three groups: those due to shared aetiologic exposure to carcinogens, those related to the treatment for the first cancer and those related to an increased hereditary or familial risk [2].
The increased risk of head and neck SPC arising after the first HNC reinforces the concept of “field cancerization,” initially proposed by Slaughter in 1953. Based on “field cancerization”, environmental carcinogens such as tobacco and alcohol may induce multifocal development process of cancer within a field of mucosa with anaplastic tendency involving many cells at once [3, 4]. The relationships between these neoplasms at a molecular level have also been defined [5]. At the same time, Human Papilloma Virus (HPV)-related oropharyngeal cancer patients have a significant lower risk of SPC development than non-HPV-related oropharyngeal cancer patients particularly in those sites related to tobacco use or alcohol consumption [6]. The analysis of SPC also provides useful information on common aetiologies and epidemiological trends [7, 8].
The different anatomical sites in which a HNC can arise are located in a small space, sometimes very close to one another. In addition, the predominant morphology in almost all of them is squamous carcinoma. Therefore, distinguishing between an SPC and a recurrence is not easy and cancer registries have also difficulties to register and codify them correctly.
The objective of this study was to analyse the risk of suffering from an SPC in two population-based cohorts of patients diagnosed with an initial HNC, using data from two cancer registries that use the same case recording methodology.
Materials and methods
Patients
For the analysis, we used data from two population-based cancer registries in Catalonia, Spain: the Tarragona Cancer Registry (TCR) and the Girona Cancer Registry (GCR) which have covered the population of the provinces of Tarragona since 1980 and Girona since 1994, respectively.
Both registries have always registered all new (first and successive) tumours diagnosed in different three-digit sites of the successive International Classification of Diseases for Oncology (ICD-O) editions. They have also registered those tumours of the same patient diagnosed in the same three-digit sites with different morphology according to the table “Groups of malignant neoplasms considered to be histologically different for the purpose of defining multiple tumours” first following the criteria adapted from Berg in 1982 [9] and later following the criteria of the 2004 international standards from the International Association of Cancer Registries—International Agency for Research on Cancer—European Network of Cancer Registries (2004 IACR-IARC-ENCR) [10].
Endpoints
A retrospective cohort design was used. The study cohort included all patients of the TCR and GCR databases whose first incident of an invasive HNC diagnosis occurred between 1994 and 2013. This cohort was followed until 31 December 2013 to find all new invasive cancers. For the analysis and presentation of results, HNCs were classified according to the International Classification of Diseases 10th revision (ICD–10) [11]: lip (C00), oral cavity (C02–C06, except C05.1 and C05.2), salivary glands (C07, C08), oropharynx (C01, C05.1, C05.2, C09–C10), nasopharynx (C11), hypopharynx (C12–C13), nasal cavity and sinus (C30.0, C31) and larynx (C32, C10.1).
This grouping is similar to that used by the Surveillance, Epidemiology and End Results (SEER) Program in the monograph “New malignancies among Cancer survivors: SEER Cancer Registries, 1973–2000” [12]. The only exception is the lip that, in this study, has been analysed separately from mouth or oropharyngeal tumours. The reason for not having used the 2004 IACR-IARC-ENCR criteria for the definition of multiple primaries is based on the fact that its aetiological factors are somehow different from the rest of the sites, especially in cases related to HPV [13,14,15]. Tumours belonging to the same morphological group were not included if they were bilateral tumours and tumours of the same subsite.
To ensure the quality of data, we especially checked the diagnosis of all pairs of cancers in which both involved oral cavity and/or pharynx reviewing their clinical and pathological data. SPC registered exclusively from a death certificate and non-melanoma skin cancers were excluded from the analyses. Because non-melanoma skin cancer has traditionally been underdiagnosed and therefore under-recorded, it has not been included in the final analysis, as is usual in other epidemiological studies. Third and subsequent primary cancers were not considered to be SPC.
Statistical analysis
The standardized incidence ratio (SIR) was calculated as the ratio between the observed number of SPC and the number that would be expected if patients in the cohort experienced the same cancer rates as the general reference population. The observed number of cases included all SPC diagnosed in the cohort. The expected number of cases was computed by multiplying the cumulated person-years observed by the incidence rates by cancer site, 5-year age group and calendar-year of the general population.
In each patient, person-years at risk (PYO) was defined as the period comprised from the first HNC diagnosis to the date of second cancer diagnosis, the date of death or the date of end of follow-up, whichever date came first.
The SIR was calculated by sex and time between the first and second cancer for all tumours as a whole and for all the combinations of the 32 studied tumour types, including HNC: lip (C00), oral cavity (C02–C06 except C05.1 and C05.2), oropharynx (C01, C05.1, C05.2, C09,C10), salivary glands (C07, C08), nasopharynx (C11), hypopharynx (C12, C13), oesophagus (C15), stomach (C16), colorectal (C18–C21), liver (C22), gallbladder and biliary tree (C23–C24), pancreas (C25), nasal cavity and paranasal sinus (C30, C31), larynx (C32), lung and bronchus (C33–C34), bone (C40–41), skin melanoma (C43), soft tissue (C47–C49), breast (C50), cervix uteri (C53), corpus uteri (C54), ovary (C56), prostate (C61), testis (C62–C63), kidney (C64), urinary tract (C65–C67), central nervous system (C70–C72), thyroid (C80), Hodgkin’s lymphoma (C81), non-Hodgkin lymphoma (C81–C85, C96), myeloma (C90) and leukaemia (C91–C95).
We included all SPC even those considered synchronous (those diagnosed up to 60 days after the first HNC diagnosis).
The Excess Absolute Risk (EAR) was calculated by subtracting the expected number of SPC from the observed number of SPC and dividing the difference by PYO, expressing the number of cases in excess or deficit by 10,000 PYO.
To observe if there were differences of risk of having an SPC over time, we have calculated the SIR of developing an SPC at 5 years of follow-up in two different periods (1994–2000 and 2001–2007).
Finally, to observe if there were global differences of risk of having an SPC between the two Catalan provinces over time, we have calculated the SIR of an SPC in two different periods (1994–2003 and 2004–2013) in each area.
The assumption that the observed number of SPC followed a Poisson distribution was used to calculate 95% confidence intervals (CI). Results are considered statistically significant if 95% CI does not include 1. All the analyses were computed using R software.
Results
Table 1 shows the characteristics of the cohort by province, sex, age at diagnosis, site of cancer, period of diagnosis, follow-up time and interval between the first and the second cancer.
The cohort of patients diagnosed with a first HNC in Girona between 1994 and 2013 was 2612 (2209 men and 403 women), of which 391 had an SPC, 364 men and 27 women. In Tarragona, 3034 patients (2594 men and 440 women) were diagnosed with a first HNC with 532 SPC (485 men and 47 women). The total cohort of both cancer registries was 5646 patients out of which 923 SPC were found. The median age at diagnosis was 63 years old. The mean follow-up time was 4.5 years. The median time from the diagnosis of the first cancer to diagnosis of SPC was 3.19 years.
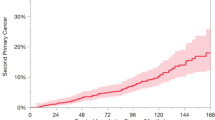
The diagnosis of SPC was relatively constant over time, as reflected in the free survival of SPC curve (Fig. 1). In this figure, any SPC count as an event and any death as a withdrawal of follow-up. At 15 years of follow-up, survival free of SPC was 59.5%.
The results of the associations between pairs of tumours based on sex are summarized in Table 2, showing the results of both populations as a whole. It only displays the statistically significant combinations. In Supplementary Table 1 are shown the results of each cancer registry separately.
In the 2 provinces as a whole, the cohort was diagnosed with 923 SPC, 849 in men and 74 in women. Men had an increased risk of an SPC with a SIR of 2.22 (95% CI: 2.07–2.37) and an EAR of 216.76 (95% CI 190–63-244.27). Women also had an increased risk with an SIR of 2.02 (95% CI 1.59–2.54) and an EAR of 95.70 (95% CI: 55.02–144.14) (Table 2).
In Girona, the cohort of men with a prior HNC had an increased risk of an SPC with an SIR of 2.09 and an EAR of 197.38 per 10,000 person-years. In women, the increased risk of a SPC was not statistically significant (SIR of 1.48; EAR of 45.90). Conversely, in Tarragona, both men (SIR of 2.31; EAR of 232.53) and women (SIR of 2.56); EAR of 143.43) had a significant increased risk of an SPC (Supplementary Table 1).
In oral cavity cancer, men had an increased risk of an SPC of oral cavity, oropharynx, hypopharynx, larynx, all head and neck sites as a whole, oesophagus, lung and all sites as a whole in both cancer registries. We also found an increased risk of a SPC in the lip in Tarragona and in urinary tract in Girona (Table 2 and Supplementary Table 1).
Men with oropharynx cancer had an increased risk of suffering from an SPC of oral cavity, hypopharynx, larynx, all head and neck sites, oesophagus, lung, thyroid and all sites.
Men diagnosed with the first cancer of hypopharynx had an increased risk of SPC of oral cavity, oropharynx, larynx, all head and neck sites, oesophagus, stomach, lung and all sites.
When the first diagnosed cancer in men occurred in the larynx, an increased risk of suffering from an oral cavity, oropharynx, hypopharynx, all head and neck sites, oesophagus, lung, urinary tract, thyroid, Hodgkin lymphoma or all sites was observed. SPC of oral cavity and urinary tract only had a significant increase in Tarragona (supplementary Table 1).
No increased risk of an SPC was found in men diagnosed with a prior cancer of the salivary gland or nasopharynx in either of the two population-based registries.
Women diagnosed with a first HNC were at a slightly lower risk of being affected by an SPC than men. Some of the associations between tumours acquired significance only when grouping both populations, similar to what happened with a SPC of the breast after a prior cancer of the nasopharynx or salivary gland. In GCR, we did not find significant associations in contrast to TCR, in which we found an increased risk for women to suffer from a SPC for the combinations of oral cavity, oropharynx, all head and neck and all sites after oral cavity cancer and lung and all sites after laryngeal cancer.
In relation to the Excess Absolute Risk (EAR), the sites with the highest values were oropharynx (534), hypopharynx (452), oral cavity (319) and larynx (224), coincident with the sites with the highest values of SIR.
Using data from both registries as a whole, we also made a comparison between the cases registered in two periods of time: 1994–2000 and 2001–2007. As shown in Table 3, the second period contains more relationships than the first one between tumours with a statistically significant SIR.
Furthermore, to analyse differences by time between cancer registries, Table 4 shows the risk of developing an SPC after 5 years of diagnosis for each registry in two 10-year calendar periods only focusing on the results of all head and neck sites as a whole and all sites. In men, the population of Tarragona shows slightly higher global risks than those of GCR and the second period shows higher risks than the first one. In women, only Tarragona shows significant higher risks and those of the second period are higher than those of the first one.
Discussion
As expected, our cohort of HNC has an increased risk of suffering from an SPC in the other head and neck and tobacco-related sites, such as lung, oesophagus and urinary tract, confirming the extensively described strong association between those cancers with the same aetiological cause [7, 8, 16,17,18]. At the same time, as in other previous studies, the risk and distribution of SPC differ significantly according to the subsite of the first cancer.
Our findings not only show the above mentioned relationships, but we also found associations unrelated to either the same carcinogenic exposure or treatment, such as Hodgkin lymphoma after lip cancer. In any case, a small increase in the number of cases in a cohort where they are less expected can dramatically change the results. It should be noted that since we have examined a large number of combinations of cancers, some of these positive results were likely due to chance.
In both cancer registries, we found an association between laryngeal cancer and thyroid cancer, as previously published [16]. This result could have also been influenced by the follow-up techniques, detecting cancers in the subclinical phase or even over-diagnosed cancers, instead of being related to the use of radiotherapy.
The risk of SPC after HNC is mainly explained by the use of tobacco. Men diagnosed with a non-tobacco-related HNC, such as cancers of nasopharynx or salivary glands, were not at an increased risk of an SPC.
The results in women in both registries were less consistent due to the lower incidence of HNC and, therefore, to have a smaller cohort. The women in the cohort were also at an increased risk of SPCs, but with less significant associations between cancers. Globally, they also had an SIR greater than 2 of developing an SPC of the head and neck area. Nevertheless, the higher risk was only significant in the TCR.
The increased risk of breast cancer after cancer of salivary glands or nasopharynx is noticeable in women, but must be taken into account that the cohort was small and the number of expected cases was very low. Therefore, a very small number of observed cases produce a significant result.
The concordance of our results, performed in two cancer registries with a similar population and the same method of registration and analysis, confirms that SPC following a prior HNC appear early in the follow-up time.
One of the biggest difficulties in the analysis of neoplasm in the head and neck area is that different ICD-O-3 sites are very close and most of those cancers are squamous carcinomas. Therefore, it can be difficult to clarify whether an SPC is actually a recurrence or a new one. In both TCR and GCR, all SPCs have been reviewed and those with classification discordance are encoded with clinical criteria provided by physicians. This difficulty to unify the clinical criteria with the IARC rules may be one of the factors for explaining that there are more cases in TCR than in GCR.
We analyse lip site separate from oral cavity because of its different aetiology due to the influence of solar exposure. Regarding oropharynx, we analysed this site separately from hypopharynx and including the base of tongue. This has an evident aetiological explanation as HPV is a well-known aetiological factor in oropharyngeal cancer and, in recent years, the epidemiology of this tumour in United States of America (USA) has changed with an increase of HPV-related HNC while those tobacco-related decreased [19]. At the same time the patterns at risk of SPCs have been modified; since 1991, the risk of SPC has decreased significantly among patients with oropharyngeal cancer [20]. So far, there is no information on the evolution of HPV-related and non-HPV-related SPCs in Europe. In our cohort as a whole, the risk of developing an SPC after oral cavity, hypopharyngeal or laryngeal cancer does not vary too much between the two studied periods. However, contrary to what was observed in the USA, in oropharyngeal cancer, the risk for the period 2001–2007 is 2.27 times that for the period 1994–2000.
An increased risk of HPV-related SPC, such as cancers of cervix, vagina, vulva, anal canal and penis had been described [21, 22], but our study did not find any associations with these cancer sites. This may reflect that the number of HPV-related tumours in our area is not large enough and/or that the elapsed time is not long enough to observe an increased risk. However, there is little population-based information on the incidence of HPV-related tumours in our area. Castellsagué et al. [13] published a set of hospital series where the HPV positivity in oropharyngeal cancer in Southern Europe was below 10%, much lower than other areas such as Central-eastern and Northern Europe. This low incidence undoubtedly influences the lack of significant risks of SPC among HPV-related cancers in this study.
The analysis of risk can also be done excluding SPCs diagnosed within 60 days of the first cancer. This approach is done to minimize the risk of encoding an extension or metastasis of the first cancer during the diagnostic process as an SPC. As we carefully reviewed all cases, we finally decided to include synchronic cancers and also give importance to those second subclinical tumours diagnosed at the same time as the first one. In supplementary Table 2, we show the risk of developing a SPC after a first head and neck cancer in Tarragona and Girona excluding cases diagnosed up to 60 days after the first cancer.
We also made comparisons between two different periods, not using the cases of the most recent years of the cohort to not diminish the possibility of an SPC diagnosis. The results show a greater risk and wider range of combinations of significant tumours in the most recent period. This may be due to changes in the diagnostic completeness of SPC or the improvement of registration methods, but it may also reflect an epidemiological reality.
The results of our study show a lower risk of SPC after a first HNC than that observed in France, where the increased risk of having an SPC following a prior HNC was defined with an SIR of 3.89 and an EAR of 380.0 in men and an SIR of 3.43 and an EAR of 194.8 in women. In France, the risk of a head and neck SPC is also higher than that in our study. The authors cited high tobacco and alcohol consumption as the most probable hypothesis of the high risk of SPC in France.[23].
Although the patterns of association between tumours are well established in the literature, the differences between cancer registries in grouping the head and neck sites in addition to the changes in the rules on multiplicity over the years do not make it easy to compare our data with those of other studies. Two of the most comprehensive analyses of multiple cancer in the 2000s are that published by Curtis et al. [24] based on data from 9 cancer registries of the SEER Program from 1973 to 2000 and that performed by the AIRTUM (“Associazione Italiana Registri Tumori”) Working Group [25] with data from 1976 to 2010. Due to the differences in grouping sites between these two studies and ours, it is difficult to compare our results on the risk of suffering from an SPC. Nonetheless, to give an example unaffected by differences in groupings, in men, the SIR and EAR of presenting a lung SPC after a previous laryngeal cancer (excluding those diagnosed in the first two months) was 3.36 and 91.1 in SEER, 3.08 and 76.9 in AIRTUM and 5.00 and 109.2 in our study (Table Supplementary 2). Although the values of our study are based on a smaller sample size, in France (SIR = 6.93 and EAR = 133.3), these values are even higher [23]. To facilitate the comparison with the results obtained by other studies, Table S1 shows the results of the cancers with statistically significant values of SIRs excluding those SPCs diagnosed up to 60 days after SPC diagnosis.
Differences in the use of the rules for coding neoplasm can also make it difficult to establish comparisons between registries [26] and find out if this risk is increasing or not [27]. IARC-IACR-ENCR coding rules for multiple cancers, in which SPC that occur at the same site are considered to be one unless it has a different histology, are more restrictive than those used in the SEER program. This makes it difficult to establish the risk of SPC in the same site, such as the oral cavity, where these associations are clearly justified by the theory of “field cancerization”.
It is noteworthy that 33.5% of the SPC were diagnosed after 5 years of follow-up from the first. Although it is difficult to know whether an early diagnosis in the absence of symptoms can clearly influence the survival of patients with a SPC, these data should be taken into account by clinicians in the design of follow-up guidelines for patients with a first HNC.
In conclusion, the risks of a SPC following a prior HNC in Tarragona and Girona are similar to those previously found in other similar cohorts, supporting that these patients should be followed closely to detect if they develop a second neoplasm. The registration of SPC in the head and neck area should be carefully reviewed and analysed with aetiological criteria. Finally, it would appear to be advisable to make a revision of the international rules of classification of multiples tumours, grouping the sites of head and neck area with new aetiological criteria to better determine and interpret the risks of SPC obtained in this type of studies.
References
Schou G, Storm HH, Jensen OM. Second cancer following cancers of the buccal cavity and pharynx in Denmark, 1943–80. Natl Cancer Inst Monogr. 1985;68:253–76.
Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30(30):3734–45.
Slaughter M, Danely P, Harry W, et al. Field cancerization in oral stratified squamous epithelium clinical implications of multicentric origin. Cancer. 1953;6:963–8.
Mohan M, Jagannathan N. Oral field cancerization: an update on current concepts. Oncology Rev. 2014;8:244–50.
Ha PK, Califano JA. The molecular biology of mucosal field cancerization of the head and neck. Crit Rev Oral Biol Med. 2003;14:363–9.
Martel M, Alemany L, Taberna M, Mena M, Tous S, Bagué S, et al. The role of HPV on the risk of second primary neoplasia in patients with oropharyngeal carcinoma. Oral Oncol. 2017;64:37–433.
Engeland A, Bjorge T, Haldorsen T, Tretli S. Use of multiple primary cancers to indicate associations between smoking and cancer incidence: an analysis of 500000 cancer cases diagnosed in Norway during 1953–93. Int J Cancer. 1997;70:401–4017.
Shiels MS, Gibson T, Sampson J, Albanes D, Andreotti G, Freeman LB, Berrington de Gonzalez A, Caporaso N, Curtis RE, Elena J, Freedman ND, Robien K, Black A, Morton LM. Cigarette smoking prior to first cancer and risk of second smoking-associated cancers among survivors of bladder, kidney, head and neck, and stage I lung cancers. J Clin Oncol. 2014;32(35):3989–95.
Berg JW. Morphologic classification of human cancer. In: Schottenfeld D & Fraumeni JF. Jr. Cancer epidemiology and prevention, 1982, Chapter 5, pp 74–89. Saunders Company
(2005) Working Group of the International Association of Cancer Registries. International rules for multiple primary cancers (ICD-0 third edition). Eur J Cancer Prev. 14(4):307–308.
(1992) International statistical classification of diseases and related health problems, tenth revision. World Health Organization, Geneva.
Brown LM, McCarron P, Freedman M. Chapter 3: New malignancies following cancer of the buccal cavity and pharynx. In: Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF Jr. (eds). New malignancies among Cancer survivors: SEER Cancer Registries, 1973–2000. National Cancer Institute, NIH Publ. No. 05–5302. Bethesda, MD, 2006
Castellsagué X, Alemany L, Quer M, Halec G, Quirós B, Tous S, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403.
Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–20.
Lubin JH, Purdue M, Kelsey K, Zhang ZF, Winn D, Wei Q, et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2009;170(8):937–47.
Morris LG, Sikora AG, Hayes RB, Patel SG, Ganly I. Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Causes Control. 2011;22(5):671–9.
Bosetti C, Scelo G, Chuang SC, Tonita JM, Tamaro S, Jonasson JG, Kliewer EV, Hemminki K, Weiderpass E, Pukkala E, Tracey E, Olsen JH, Pompe-Kirn V, Brewster DH, Martos C, Chia KS, Brennan P, Hashibe M, Levi F, La Vecchia C, Boffetta P. High constant incidence rates of second primary cancers of the head and neck: a pooled analysis of 13 cancer registries. Int J Cancer. 2011;129(1):173–9.
Coca-Palez A, Rodrigo JP, Suárez C, Nixon IJ, Mäkitie A, Sanabria A, et al. The risk of second primary tumours in head and neck cancer: a systematic review. Head Neck. 2020;42(3):456–66.
Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301.
Morris LG, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2010;29:739–46.
Neumann F, Jégu J, Mougin C, Prétet JL, Guizard AV, Lapôtre-Ledoux B, Bara S, Bouvier V, Colonna M, Troussard X, Trétarre B, Grosclaude P, Velten M, Woronoff AS. Risk of second primary cancer after a first potentially-human papillomavirus-related cancer: a population-based study. Prev Med. 2016;90:52–8.
Suk R, Mahale P, Sonawane K, Sikora AG, Chhatwal J, Schmeler KM, Sigel K, Cantor SB, Chiao EY, Deshmukh A. Trends in risks for second primary cancers associated with index human papillomavirus-associated cancers. JAMA Network Open. 2018;1(5):e181999.
Jégu J, Colonna M, Daubisse-Marliac L, Trétarre B, Ganry O, Guizard AV, Bara S, Troussard X, Bouvier V, Woronoff AS, Velten M. The effect of patient characteristics on second primary cancer risk in France. BMC Cancer. 2014;14:94–107.
Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF. New malignancies among cancer survivors: SEER Cancer Registries, 1973–2000. National Cancer Institute, NIH Publ. No 05–5302. Bethesda, MD, 2006
Buzzoni C, Crocetti E. AIRTUM working group. italian cancer figures report 2013: multiple tumours. Epidemiol Prev. 2013;37((4–5) suppl 1):1–152.
Weir HK, Johnson CJ, Ward KC, Coleman MP. The effect of multiple primary rules on cancer incidence rates and trends. Cancer Causes Control. 2016;3:377–90.
Ye Y, Neil AL, Wills KE, Venn AJ. Temporal trends in the risk of developing multiple primary cancers: a systematic review. BMC Cancer. 2016;16(1):849–66.
Acknowledgements
We thank Katie Linder for reviewing the English grammar of the manuscript.
Funding
This research did not receive any specific grants from public, commercial, or not-for-profit funding agencies.
Author information
Authors and Affiliations
Contributions
JRC, JGa, JM, AI, JB, MC, RMG and JGu contributed to study design and to the analysis and interpretation of the study results. JRC and JGa wrote the manuscript. MP was responsible for the databases in Girona Cancer Registry (GCR). LL were responsible for the databases in Tarragona Cancer Registry (TCR). AA performed the statistical analysis. JGa and MC are the coordinators of the TCR. RMG and AI are the coordinators of the GCR. All authors corrected and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study uses anonymous data and complies with all the laws and rules of the National Health System that regulates the activity of population-based cancer registries.
Informed consent
For this type of study formal consent is not required.
Availability of data and material
This study was carried out using anonymized data from the Girona Cancer Registry (GCR) and Tarragona Cancer Registry (TCR) which comply with the legal regulations for data protection and management of clinical data in force in Spain. Both registries belong to and comply with the regulations of the International Association of Cancer Registries (IACR) and the International Agency for Research in Cancer (IARC). No intervention has been performed on human or animal subjects. All procedures have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rubió-Casadevall, J., Galceran, J., Ameijide, A. et al. Population-based risk assessment of second primary cancers following a first head and neck cancer: patterns of association and difficulties of its analysis. Clin Transl Oncol 23, 788–798 (2021). https://doi.org/10.1007/s12094-020-02470-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02470-z