Abstract
Objective
To evaluate the effectiveness of non-surgical treatment as an alternative in the management of central giant cell granuloma (CGCG).
Material and methods
A literature search was carried out in accordance with the PRISMA statement in order to answer the question “Are non-surgical treatments effective as an alternative in the treatment of CGCG?”. Two examiners independently assessed eligibility, risk of bias, and extracted data, which included therapeutic protocol, side effects, and need for surgical supplementation.
Results
Among 1712 studies, 15 were included, totaling 145 patients. Calcitonin, intralesional corticosteroids, and denosumab were the medications used. For calcitonin (n = 61), complete remission was found in 30 cases. For intralesional triamcinolone (n = 68), reduction in size was observed in most cases (n = 39). Four cases received subcutaneous denosumab and showed absence of active bone metabolism in the region, of which three presented ossification. Combination of drug therapies (n = 29) was reported in one study and included subcutaneous interferon and oral imatinib. More and less side effects were found for interferon and corticosteroids, respectively. Forty percent of patients required additional surgical treatment.
Conclusion
Despite the side effects presented and the need for additional surgery in some patients, in general, all non-surgical treatments could provide positive results as an alternative for the management of CGCG, especially with regard to reducing the size of the lesion.
Clinical relevance
CGCG is a benign bone lesion that mainly affects young individuals. Although the most common therapy is surgery, its contraindication in some patients, the large extension, and high recurrence rate of the aggressive variant have led the search for non-surgical therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Central giant cell granuloma (CGCG) is a benign bone lesion that preferentially affects young individuals (25.8 ± 15.3 years) of the female sex (1.56: 1), with the mandible being the most affected site [1,2,3]. The lesions are classified according to their clinical and radiographic behavior in non-aggressive and aggressive variants [4]. Non-aggressive lesions are often asymptomatic, grow slowly without perforating bone cortex or reabsorbing roots, and have lower recurrence rates compared to the aggressive variant. Aggressive lesions are more common in children and young patients, can be painful, increasing rapidly with cortical expansion and perforation, root resorption, tooth displacement, sizes larger than 5 cm and a high tendency to recurrence [2, 4,5,6,7,8], with rates ranging from 11 to 49% [2, 3].
Although the most common therapy is surgical (ranging from curettage to en bloc resection), the contraindication of surgical procedures in some patients and the high rate of recurrence of the aggressive variant and its tendency to affect children and young patients have led the search for non-surgical therapeutic options, aiming at the progressive reduction of these lesions and prevention of recurrences [9,10,11]. They are also alternatives for the management of extensive lesions, with the aim of reducing their size for further surgery. Intralesional corticosteroids, subcutaneous or nasal calcitonin, subcutaneous interferon alpha [12,13,14], imatinib [6], and denosumab [7, 13, 15,16,17] have been reported for this purpose.
Considering the challenge that is the management of CGCG, especially the aggressive variant, the difficult management of extensive lesions in young patients and children, which can lead to mutilations and aesthetic and functional defects, as well as the variety of non-surgical treatments available and the scarcity of evidence-based protocols, the objective of this systematic review was to evaluate the effectiveness of non-surgical treatments as an alternative in the management of CGCG.
Material and methods
A systematic review of the best evidence available in the literature was conducted in accordance with the PRISMA statement [18] to answer the following clinical question: “Are non-surgical treatments effective as an alternative in the management of CGCG?”. The PICOS question was as follows: participants, patients with CGCG; intervention, non-surgical therapies; comparisons, with surgical treatment, between non-surgical treatments or no comparison; outcome, disease-free survival; complete or partial lesion reduction after non-surgical treatment; and study design, intervention studies. Disease-free survival (primary outcome) was assessed by the absence of radiographic and clinical evidence of recurrence. The secondary outcomes were complete or partial lesion reduction after non-surgical treatment. The outcomes of interest were those that estimated clinical, histological or radiological efficacy in a defined way. The PROSPERO (International prospective register of systematic reviews) record is CRD42020152482.
Search strategy
Initially, the PubMed database (all years to March 2021—no time restriction) was electronically searched using keywords and their entry terms, without language restrictions. The terms were used separately, and then the results were merged using the Boolean term AND (Table 1). Further search was performed through Embase, Web of Science, Scopus, SciELO, Google Scholar and The Cochrane Library. Additionally, gray literature was consulted, through IBICT (Brazilian Institute of Information in Science and Technology). A manual search was also performed on the reference lists of all selected articles.
Eligibility and quality assessment
Two independent reviewers performed the overall article selection process according to the following eligibility criteria: original clinical studies on humans (randomized controlled trials, cohort studies, prospective, retrospective, case series, and case–control studies); patients with confirmed CGCG undergoing non-surgical treatment alone or prior to surgery; and minimum follow-up of 6 months and minimum of 3 patients. The exclusion criteria were patients with Cherubism or Brown Tumor of hyperparathyroidism; patients undergoing surgical treatment prior to non-surgical treatment; animal or in vitro studies; case reports, literature reviews, annals, and presentations at congresses. When there was no agreement, both examiners argued until a consensus was reached. If there was still no agreement, a third external examiner was consulted.
The results were combined, and, after duplicate removal (EndNote web software, Clarivate Analytics, Philadelphia, Pennsylvania, USA), the reviewers screened the yielded titles and abstracts. After the evaluation of the full text, the selected articles were submitted to the quality assessment and final review.
The quality assessment was performed according to the revised Cochrane risk-of bias-tool for randomized trials – rob [19, 20] for randomized controlled trials; NOS scale (Newcastle–Ottawa Scale) [21] for prospective and retrospective cohort studies and non-randomized case–control studies; and the checklist of the Institution Joana Briggs (JBI Critical Appraisal Checklist for Case Series) [22] for case series. Studies with a high risk of bias were excluded from the analysis.
Data extraction
The following data were extracted: number of patients, sex, age, size and location of the lesion (maxilla or mandible), classification [2] (aggressive and non-aggressive), non-surgical treatment applied (protocol), side effects, follow-up, and outcome.
Results
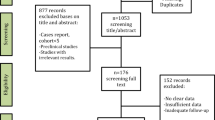
The results are described in Fig. 1. The database and manual search yielded 1712 relevant references. After duplicate removal, 1073 studies were screened by title and abstract resulting in 51 potential articles for full-text review. Thirty-six were excluded, as they did not completely meet the eligibility criteria. The remaining 15 articles were included in the qualitative synthesis: 13 case series [15, 23,24,25,26,27,28,29,30,31,32,33,34] (Table 2), one retrospective cohort study [6] (Table 3), and one randomized controlled double-blind clinical trial [12] (Fig. 2). Authors, study design, number of patients, sex, average age, site and average size of the lesion, classification [2], treatment protocol, outcome, follow-up, and conclusion of the studies are summarized in Tables 4, 5, and 6.
The sample ranged from three [23] to 29 patients [6], totaling 145 patients, 75 (51.72%) women and 70 (48.28%) men, with a mean age of 19.91 years. The maxilla was affected in 45 (31.04%) cases, the mandible in 97 (66.89%) and in 3 (2.07%) cases both jaws were affected. Sixty-three lesions (64.95%) were aggressive and 34 (35.05%) non-aggressive. In six studies [24,25,26,27,28,29], this classification was not carried out (n = 48).
Of the selected studies, seven used only calcitonin as the main treatment [12, 24,25,26, 30,31,32], six used intralesional corticosteroids [15, 23, 27, 28, 33, 34], one used subcutaneous denosumab [29], and one combined drug therapies [6], which included oral imatinib and interferons.
Overall, of the 145 patients who underwent non-surgical treatment, 40% (n = 58) needed to undergo additional surgical treatment, with 44 curettages on the remainder of the lesion, 12 aesthetic osteoplasty, and 2 resections.
Meta-analysis could not be performed due to the lack of data and the heterogeneity of the studies.
Calcitonin
The studies that administered calcitonin included a total of 61 patients. Thirty-one (50.8%) presented the aggressive lesion and 17 (27.8%) the non-aggressive (Table 4). In three studies the classification was not specified [24,25,26]. The mean age was 20.36 years, with the mandible being most affected (42 patients). The average size of the lesions was 4.12 cm, but it was not mentioned in three studies [25, 26, 32]. The protocol was similar in the studies, varying the time of treatment and the form of administration of calcitonin. The nasal spray of salmon calcitonin was used in 34 patients (55.8%) and subcutaneous human calcitonin in 22 (36%), and in 5 patients (8.2%), there was an association of both types.
In all studies, the results were evaluated by panoramic radiographs [6, 24,25,26, 30,31,32] or computed tomography [12, 25]. Only De Lange et al. [12] reported the differences found between aggressive and non-aggressive variants. They found borderline differences in reducing the size of the lesion. The lesions reduced their volume by 22.5% in patients with the non-aggressive variant (n = 10) after 6 months of follow-up. In two patients with the aggressive variant, the lesion increased at the end of the treatment period. Complete remission was not observed in any patient [12]. In the other studies (n = 47) [6, 24,25,26, 30, 32], in 63.82% of cases, there was complete resolution of the lesion (n = 30), in 17.02% reduction in size (n = 8), in 14.89% complete ossification (n = 7), 2.12% growth limitation (n = 1), and 2.12% definition of limits (n = 1).
Regarding side effects, two studies did not mention [24, 31], in one there was no side effects [30], and in five studies nausea, headache, flushing, diarrhea, and epistaxis were reported [6, 12, 25, 26, 32]. The average follow-up was 19.79 months, ranging from six [12] to 34 months [6, 30].
Surgical supplementation was not necessary in two studies (n = 9) [25, 30]. Curettage of the remainder of the lesion was performed in 16 patients [6, 24, 26, 31, 32] and in one patient only aesthetic osteoplasty was performed [24]. The need or performance of additional surgery was not mentioned in one study [12]. Recurrences were found in two patients after 13.2 (n = 7) [6] and 26 months (n = 10) [31] of the end of treatment. Only one patient discontinued treatment [32].
Corticosteroids
Of the 68 patients who underwent intralesional corticosteroids injections, 21 (30.89%) had the aggressive variant and 14 (20.58%) the non-aggressive, and three studies (n = 33; 48.53%) did not specified this classification [27, 28, 34] (Table 5). The mean age of the patients was 21.18 years. The mandible was more affected (n = 46; 67.65%). Only two studies mentioned the average size of the lesions, which ranged from 3.85 cm [28] to 5.5 cm [23].
In all studies, triamcinolone was used, with one [23, 28, 34] or two weekly injections [15, 27, 33], for 6 weeks, and the results were evaluated using panoramic radiographs [15, 23, 27, 28, 33, 34]. In 57.35% of the cases, there was a decrease in the lesion (n = 39), bone formation in 25% (n = 17), total resolution in 10.29% (n = 7) and no response to treatment in 7.35% (n = 5).
Four studies reported that there were no side effects [15, 23, 28, 33] and two did not mention it [27, 34]. The average follow-up time was 35.16 months, ranging from 21 [23] to 64 months [15]. In one study [27] this information was missing.
Surgical supplementation was performed in some case in all studies, totaling 34 patients (50%). In 20 patients, curettage of the remaining lesion was necessary [15, 23, 27, 28, 33, 34], aesthetic osteoplasty in 12 patients [15, 33], curettage associated with osteoplasty in two [23], and total resection in two [15]. Only one recurrence was reported [27]. In the study by Dolanmaz et al. [28], one patient discontinued treatment.
Denosumab
Of the five patients undergoing treatment with denosumab [29], four were included in this review, as one patient underwent surgical treatment during drug therapy. The authors did not specify the classification and the size of the lesions. The mean age was 15.5 years. The mandible was more affected (n = 3) (Table 6).
All patients received 1–2 doses of intralesional corticosteroids at the beginning of treatment. The dose of denosumab varied from 70 to 120 mg, applied subcutaneously 3 times in 2 weeks and then once a month for 1 year.
The results were analyzed using cone beam CT and PET-CT. Of the four patients, three (75%) exhibited good ossification, and, at the end of treatment, all showed no remaining metabolic activity in the region. The average follow-up was 37.25 months.
Side effects were observed in one patient who presented deficient wound healing and pain. In one patient, debulking with intralesional corticosteroid was necessary due to sudden pain after finishing treatment with denosumab. Recurrence was reported in one patient 1 year after the end of treatment.
Combined non-surgical therapies
Schreuder et al. [6] carried out a retrospective cohort study of 33 patients. Of these, four underwent only surgical treatment and were excluded from this review. Of the remaining 29 patients, 21 (72.41%) presented the aggressive variant and 8 (27.59%) the non-aggressive, with 15 cases affecting the mandible and 14 the maxilla. The mean age was 19.4 years. The average size of the lesions was 2.58 × 2.47 × 2.78 cm (length/height/depth).
Regarding the drug therapy, only one patient received a single treatment (salmon calcitonin nasal spray) and had complete remodeling. Thirteen patients who were treated initially with calcitonin nasal spray needed an additional pharmacological treatment, which included intralesional corticosteroid (n = 1), subcutaneous interferon alpha, beta and-or PEGylate (n = 4), human subcutaneous calcitonin with interferon alpha (n = 2), or isolated (n = 6). In 15 cases, pharmacological treatment with salmon calcitonin nasal spray was supplemented with conservative enucleation, with 5 patients still receiving other drugs before surgery oral imatinib (n = 2), PEG interferon (n = 4), interferon alpha (n = 2), or intralesional corticoid (n = 3) (Table 4).
The results were evaluated by helical CT scans and the mean follow-up was 38 months. Of the 14 patients that could be managed without additional surgery, one (7.1%) showed progression during follow-up. The overall long-term response in this group varied from complete remodeling or ossification (n = 9; 64.3%) to non-progressive residual lesions (n = 4; 28.6%). In these cases, further close follow-up was chosen instead of surgical intervention.
In the other 15 lesions who underwent additional surgery, with the exception of one case (6.6%), there was regression in response to drug therapy (delineation by a bone capsule with or without intralesional ossification and/or decrease in size). Surgery was performed for the following reasons: substantial volume of residual radiolucency (n = 4); lack of additional spontaneous regression after the interruption of drug therapy (n = 7); intolerance to the side effects (n = 1); aesthetic correction (n = 1); and a persistent bone cavity in the mandible (n = 1).
Side effects were encountered during all pharmaceutical interventions, except after corticosteroid injections. Treatment with interferon has been associated with most side effects, which included hypothyroidism, depression, neutropenia, loss of appetite, myalgia, fatigue, mild hair loss, fever diarrhea, fever, arthralgia, and malaise. Recurrence was reported in one patient 1.1 years after the end of treatment.
Discussion
Especially in cases of aggressive CGCG, surgery, which is the most common treatment, results in marked aesthetic and functional defects [2, 4,5,6]. The condition can be even worse, as these lesions are more frequent in very young patients, including children. Due mainly to the great extent of the lesion and its recurrences, drug treatment has been considered [6, 12, 15, 27, 29] and can be used alone or prior to surgery, in order to reduce the size of the lesion, promote repair, and/or decrease recurrences [6, 12, 27, 29, 34].
Since its first description by Harris in 1993 for the treatment of CGCG, calcitonin has been used frequently [35]. Immunohistochemistry studies have shown that the giant cells present in the CGCG act in a similar way to osteoclasts, in addition to having calcitonin receptors on their membrane. The binding of calcitonin to the receptor causes changes in cell structure, leading to inhibition of DNA synthesis by cells [6, 12, 30, 36], which supports its use in these cases. Sixty-one patients (42.06%) underwent treatment with calcitonin [6, 12, 24,25,26, 30,31,32]. The protocols were similar: human subcutaneous calcitonin 100 I.U. or salmon calcitonin nasal spray 200 I.U. per day. The time of use depends on the results, but according to our findings, in general, the treatment is long, ranging from 6 [26] to 34 months [30]. The largest number of patients who used salmon calcitonin occurred because human calcitonin is no longer available; in addition, salmon calcitonin appears to be more potent [6].
De Lange et al. [12], who performed the only randomized controlled trial included in this review, did not observe complete remission of the lesion in any patient when using this therapy. This was also the only study that compared the results between aggressive and non-aggressive variants, showing borderline differences, as well as was the only one to report an increase in lesion size in two patients with the aggressive variant. In the other studies [6, 24,25,26, 30,31,32], there was total resolution at the end of treatment in 30 of the 61 patients. Although Allon et al. [30] reported no side effects, in other studies, [6, 12, 25, 26, 32] nausea, headache, flushing, diarrhea, and epistaxis were reported. Surgical supplementation was performed in 17 patients [6, 24, 26, 31], and recurrence was reported in two cases [6, 31].
From the 1980s, intralesional corticosteroids started to be used to treat intraosseous and oral mucosa lesions [37] since (1) they inhibit the extracellular production of lysosomal proteases; (2) induce apoptosis in osteoclast-like cells; (3) inhibit transcription factors for intracellular proliferation; and (4) induce anti-angiogenic effects on endothelial cells. All of these factors lead to inhibition of resorption, thus preventing the growth of CGCG [15, 34]. In 1988, Jacoway et al. [38] administered, for the first time, a solution with triamcinolone acetonide and local anesthetic (2% lidocaine with adrenaline 1:100.000) in a CGCG (2 mL/cm once a week for 6 weeks). Of the studies included in this review (68 patients), three modified this protocol, performing two weekly injections instead of one [15, 27, 33].
The advantages of this modality include the low cost and technical simplicity, as well as the preservation of adjacent structures and low patient morbidity [13, 14, 23, 39,40,41,42,43]. Although some authors consider this therapy to be effective in the management of CGCG [44], others claim that the results are controversial [12]. In this review, in 57.35% of the patients, there was a decrease in the size of the lesion. Since complete resolution was reported in only seven patients, supplementary surgery was required in 35 patients. A possible explanation for the need for surgical intervention is bone neoformation caused by the administration of corticosteroids—with successive injections, needle penetration into the lesion is being hampered. Thus, there is not necessarily a complete bone formation, and therefore an area of radiolucency remains and can be observed on the radiograph [15, 23, 27, 28, 33]. Although no study has reported any side effects, the disadvantages of using intralesional corticosteroids include their systemic effects (especially in immunocompromised and diabetic patients), such as peptic ulcers and infections [34], in addition to the discomfort caused by the injections and patient compliance with treatment [12, 14, 39, 43].
Based on the assumption that the giant cells present in the CGCG are analogous to osteoclasts, therapy with denosumab has been adopted in some patients. It is a monoclonal antibody that binds to the receptor activator of nuclear factor-kappa B (RANK) ligand (RANKL). RANK is expressed on the surface of pre-osteoclasts and RANKL on the surface of osteoblasts. When RANK and RANKL are linked, the precursor cell turns into an osteoclast, which reabsorbs the bone. As denosumab also binds to RANKL, it prevents the RANK-RANKL binding, thus preventing the osteolytic process [17, 29]. Only one long-term retrospective cohort study of medium risk of bias was included in this review [29]. As pharmaceutical therapy with denosumab appears to be successful for giant cell tumors (GCT) of the femur [45], the authors hypothesized its equally successful use for CGCG of the jaws. All patients (n = 4), at the end of treatment, had no active bone metabolism in the region of the lesion, presenting a curative response to treatment and complete metabolic resolution, findings that led the authors to consider this as a successful option. Although in all patients at least one injection of intralesional corticosteroids was performed before the start of treatment, they state that this amount would not be sufficient to interfere with the results of treatment and that denosumab should be considered as a therapeutic option for large CGCG of the jaws. Additionally, the authors recommend a treatment length of not shorter than 12 months [29]. The inclusion of only one study with such a small number of patients highlights the paucity of evidence to support the use of denosumab for this purpose, especially when its possible side effects, such as hypophosphatemia, pain in the extremities, anemia, and jaw osteonecrosis [45], are considered. In short, further studies are needed to determine its real effectiveness in treating CGCG.
The combination of drugs was performed when the lesion did not respond positively to the primary non-surgical treatment with salmon calcitonin nasal spray, regardless of whether the variant was aggressive or non-aggressive [6]. In these cases, intralesional corticosteroid, subcutaneous interferon alpha, beta and-or PEGylate, human subcutaneous calcitonin with interferon alpha or isolated, and oral imatinib were used [6]. The supplementation of a pharmacological treatment with another type of drug makes it difficult to measure the results. In other words, it is difficult to say whether the effects achieved result from one or the other drug or from the combination of them.
Imatinib, for example, was only mentioned in this study [6] and was used prior to enucleation after an unsatisfactory response from previous drug therapy. The treatment with imatinib showed regression in one subject when combined with interferon. In another patient treated with imatinib, there was progression, and it was stopped after 2 months [6].
Interferons have been used in some cases [6]. It is an anti-angiogenic drug used to treat large hemangiomas and vascular tumors [46]. As CGCG is a vascularized lesion, it is believed that it can respond positively to anti-angiogenic therapy [6, 47,48,49]. Although all lesions showed good response after a period of adjuvant interferon alpha [6], the side effects ranged from easily manageable flu-like symptoms to more troublesome complaints, such as hypothyroidism and depression. In some cases, it was necessary to adjust the doses or even stop their administration [6]. In addition to toxicity, treatment is long.
Based on the above, in short, each drug has advantages and disadvantages inherent in cost, treatment time, technique, and side effects, as summarized in Table 7.
This review demonstrated the difficulty in conducting studies to assess the effectiveness of pharmacological therapy in the management of CGCG. Some of the difficulties include casuistry (sample sizes are reduced), patient compliance during treatment, the large number of confounding factors, the need for long periods of follow-up, the different assessment methods (which varied from panoramic radiography to CT, MRI and PET-scan) and the lack of standardization in measuring lesion sizes, as well as the need for supplementation with other drugs or surgery. Patients have a broad spectrum of lesion size and aggressiveness, from relatively small indolent lesions to rapid-growing lesions with aggressive signs and symptoms. All these factors can justify the lack of randomized controlled clinical investigations or even cohort studies. The only randomized controlled trial [12] with medium risk of bias addressed 14 patients and compared the calcitonin therapy with a placebo, this being the largest sample tested for this therapy. These were also the only authors to not achieve complete regression of the lesion in any case and to report size increases in two patients with the aggressive variant at the end of the treatment period, which corresponds to 1.37% of the total sample. The authors themselves emphasize that their results and interpretation are limited by the small sample size and the short placebo-controlled period (3 months).
Most papers were case series and the only study with a low risk of bias was the one by Schreuder et al. [6], which evaluated the combination of therapies. This was also the study that included the largest number of patients (n = 29). For the corticosteroids, the study with the largest sample addressed 21 patients [15].
Meta-analysis and subgroup analysis (age; size of the lesion; aggressive and non-aggressive lesions; treatment of primary lesions versus recurrent lesions) could not be performed in this review due to the lack of data and the heterogeneity of the studies. Regarding the demographic data of the sample, in general, women and men were similarly affected (1.08:1). Mean age was 19.91 years, demonstrating the trend of CGCG in young patients. It is known that, especially in children and adolescents, the aggressive variant is more common [2, 3]. In this review, most were aggressive lesions (64.95%); however, six studies [24,25,26,27,28,29] did not classify the lesions, which prevents us from correlating these data with the findings. Only one study [12] reported the differences found between aggressive and non-aggressive variants when using calcitonin. Possibly, the results of all treatments differ when performed on lesions with different clinical behaviors. Likewise, the outcomes may also differ between younger and older patients.
It is evident that some questions cannot yet be answered and that future studies are encouraged, using more standardized protocols and samples, separating them, for example, between young and older individuals, aggressive and non-aggressive lesions, and primary and recurrent lesions. Certainly, this is not an easy task, considering the infrequency and challenge that CGCG management can be. A methodology that guarantees a balanced distribution in terms of characteristics between treatment groups would be ideal. Multicenter studies may be the path to these answers.
Despite all the limitations, the careful methodology of this review, with strict inclusion criteria, peer review and a thorough and consistent quality analysis, supports that non-surgical treatment can be effective as an alternative in the management of CGCG. It should be considered especially when surgery is contraindicated and in young patients, where the aggressive variant is more frequent. These therapies could avoid or minimize mutilating approaches and aesthetic, functional, and emotional losses. Side effects, patient adherence to treatment, costs, and treatment time are some factors to be considered before choosing the treatment.
Conclusion
Non-surgical treatment modalities, such as calcitonin, intralesional corticosteroids, denosumab, and interferons, can be effective as an alternative in the management of CGCG. Although 40% of patients required additional surgical treatment, in general all substances could provide positive results, especially with regard to reducing the size of the lesion. More and less side effects were found for interferon and corticosteroids, respectively. Side effects and the need for surgical supplementation should be considered when any drug therapy is chosen for the management of CGCG.
References
Speight PM, Takata T (2018) New tumor entities in the 4th edition of the World Health Organization Classification of Head and Neck tumours: odontogenic and maxillofacial bone tumours. Virchows Archiv 472:331–339. https://doi.org/10.1007/s00428-017-2182-3
Chrcanovic BR, Gomes CC, Gomez RS (2018) Central giant cell lesion of the jaws: An updated analysis of 2270 cases reported in the literature. J Oral Pathol Med 47:731–739. https://doi.org/10.1111/jop.12730
Stavropoulos F, Katz J (2002) Central giant cell granulomas: A systematic review of the radiographic characteristics with the addition of 20 new cases. Dentomaxillofac Radiol 31:213–217. https://doi.org/10.1038/sj.dmfr.4600700
Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A (1986) Central giant cell lesions of the jaws: a clinicopathologic study. J Oral Maxillofac Surg 44:708–713. https://doi.org/10.1016/0278-2391(86)90040-6
Jaffe HL (1953) Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-osseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol 6:159–175. https://doi.org/10.1016/0030-4220(53)90151-0
Schreuder WH, van den Berg H, Westermann AM, Peacock ZS, de Lange J (2017) Pharmacological and surgical therapy for the central giant cell granuloma: A long-term retrospective cohort study. J Craniomaxillofac Surg 45:232–243. https://doi.org/10.1016/j.jcms.2016.11.011
Balaji P, Balaji SM (2019) Central giant cell granuloma - A case report. Indian J Dent Res 30:130–132. https://doi.org/10.4103/ijdr.IJDR_61_19
Gupta B, Stanton N, Coleman H, White C, Singh J (2015) A novel approach to the management of a central giant cell granuloma with denosumab: A case report and review of current treatments. J Craniomaxillofac Surg 43:1127–1132. https://doi.org/10.1016/j.jcms.2015.04.011
Suárez-Roa MDL, Reveiz L, Rivera LMR et al (2009) Interventions for central giant cell granuloma (CGCG) of the jaws. Cochrane Database Syst Rev 7:CD0007404. https://doi.org/10.1002/14651858.CD007404.pub2
Eisenbud L, Stern M, Rothberg M, Sachs SA (1988) Central giant cell granuloma of the jaws: experiences in the management of thirty-seven cases. J Oral Maxillofac Surg 46:376–384. https://doi.org/10.1016/0278-2391(88)90221-2
Terry BC, Jacoway J (1996) Management of central giant cell lesions: An alternative to surgical therapy. Oral Maxillofac Surg Clin North Am 6:579–601
de Lange J, van den Akker HP, Veldhuijzen van Zanten GO et al (2006) Calcitonin therapy in central giant cell granuloma of the jaw: a randomized double-blind placebo-controlled study. Int J Oral Maxillofac Surg 35:791–795. https://doi.org/10.1016/j.ijom.2006.03.030
de Lange J, van den Akker HP, van den Berg H (2007) Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Endod 104:603–615. https://doi.org/10.1016/j.tripleo.2007.04.003
Sezer B, Koyuncu B, Gomel M, Güngay T (2005) Intralesional corticosteroid injection for central giant cell granuloma: a case report and review of the literature. Turk J Pediatr 47:75–81
Nogueira RLM, Faria MHG, Osterne RLV, Cavalcante RB, Ribeiro RA, Rabenhorst SHB (2012) Glucocorticoid and calcitonin receptor expression in central giant cell lesions: implications for therapy. Int J Oral Maxillofac Surg 41:994–1000. https://doi.org/10.1016/j.ijom.2012.01.017
O’Connell JE, Kearns GJ (2013) Aggressive giant cell granuloma of the jaws treated with interferon alpha: a report of two cases. Int J Med Sci 82:163–170. https://doi.org/10.1007/s11845-012-0858-x
Thomas D, Henshaw R, Skubitz K et al (2010) Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 11:275–280. https://doi.org/10.1016/S1470-2045(10)70010-3
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 18:e1003583. https://doi.org/10.1371/journal.pmed.1003583
Higgins JPT, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Chandler J, McKenzie J, Boutron I, Welch V (editors) (2016) Cochrane Methods. Cochrane Database of Syst Rev 10 (Suppl 1).https://doi.org/10.1002/14651858.CD201601
Wells GA, Shea B, O`Connell D, Peterson J, Welch V, Losos M, Tugwell P (2021) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 06 May 2021
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Lisy K, Qureshi R, Mattis P, Mu P (2020) Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. The Joanna Briggs Institute. https://doi.org/10.46658/JBIMES-20-08. Accessed 06 May 2021
Crestanello Nese JP, Fernandez Luzardo CF, Robano Navatta AR (2004) Corticoides intralesionales en lesiones a células gigantes. Rev Esp Cirug Oral y Maxilofac 25:351–360
Borges HO, Machado RA, Vidor MM, Beltrão RG, Heitz C, Sant’Ana MF, (2008) Calcitonin: a non-invasive giant cells therapy. Int J Pediatr Otorhinolaryngol 72:959–963. https://doi.org/10.1016/j.ijporl.2008.03.016
de Lange J, Rosenberg AJ, van den Akker HP, Koole R, Wirds JJ, van den Berg H (1999) Treatment of central giant cell granuloma of the jaw with calcitonin. Int J Oral Maxillofac Surg 28:372–376. https://doi.org/10.1034/j.1399-0020.1999.285280513.x
Rosenberg AJ, Bosschaart AN, Jacobs JW, Wirds JJ, Koole R (1997) Calcitonine therapie bij grote of recidiverende centrale reuzencelgranulomen van de onderkaak [Calcitonin therapy in large or recurrent central giant cell granulomas of the lower jaw]. Ned Tijdschr Geneeskd 141:335–339
Cavalcante IL, Barros CCS, Rodrigues KAM et al (2018) Quantification of bone gain in central giant cell granuloma of the jaws submitted to intralesional corticotherapy. J Bras Patol Med Lab 54:183–188. https://doi.org/10.5935/1676-2444.20180032
Dolanmaz D, Esen A, Mihmanli A, Işık K (2016) Management of central giant cell granuloma of the jaws with intralesional steroid injection and review of the literature. Oral Maxillofac Surg 20:203–209. https://doi.org/10.1007/s10006-015-0530-5
Bredell M, Rordorf T, Kroiss S, Rüker M, Zweifel DF, Rostetter C (2018) Denosumab as a Treatment Alternative for Central Giant Cell Granuloma: A Long-Term Retrospective Cohort Study. J Oral Maxillofac Surg 76:775–784. https://doi.org/10.1016/j.joms.2017.09.013
Allon DM, Anavi Y, Calderon S (2009) Central giant cell lesion of the jaw: nonsurgical treatment with calcitonin nasal spray. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107:811–818. https://doi.org/10.1016/j.tripleo.2009.02.013
Pogrel MA (2003) Calcitonin therapy for central giant cell granuloma. J Oral Maxillofac Surg 61:649–654. https://doi.org/10.1053/joms.2003.50129
Vered M, Shohat I, Buchner A, Dayan D, Taicher S (2007) Calcitonin nasal spray for treatment of central giant cell granuloma: clinical, radiological, and histological findings and immunohistochemical expression of calcitonin and glucocorticoid receptors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:226–239. https://doi.org/10.1016/j.tripleo.2006.05.020
Nogueira RLM, Osterne RLV, Lima Verde RMB, Azevedo NO, Teixeira RC, Cavalcante RB (2020) Intralesional injection of triamcinolone hexacetonide as an alternative treatment for central giant cell lesions: a prospective study. Br J Oral Maxillofac Surg 58:e283–e289. https://doi.org/10.1016/j.bjoms.2020.07.032
Tawfik MA, Gaballah ETM, Bilal M (2004) Treatment of central giant cell granuloma of the mandible with intralesional injection of corticosteroid. Egypt Dent J 50:687–699
Harris M (1993) Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg 31:89–94. https://doi.org/10.1016/0266-4356(93)90168-v
Maeda A, Matsui H, Kanamori M, Yudoh K, Tsuji H (1994) Calcitonin receptors on neoplastic mononuclear cells cultured from a human giant-cell tumor of the sacrum. J Cancer Res Clin Oncol 120:272–278. https://doi.org/10.1007/BF01236383
Esen A, Işık K, Dolanmaz D (2015) Treatment of mouth and jaw diseases with intralesional steroid injection. World J Stomatol 4:87–95. https://doi.org/10.5321/wjs.v4.i2.87
Jacoway JR, Howell FV, Terry BC (1988) Central giant cell granuloma: an alternative to surgical therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 66:572
Abdo EN, Alves LC, Rodrigues AS, Mesquita RA, Gomez RS (2005) Treatment of a central giant cell granuloma with intralesional corticosteroid. Br J Oral Maxillofac Surg 43:74–76. https://doi.org/10.1016/j.bjoms.2004.08.015
Adornato MC, Paticoff KA (2001) Intralesional corticosteroid injection for treatment of central giant-cell granuloma. J Am Dent Assoc 132:186–190. https://doi.org/10.14219/jada.archive.2001.0153
Carlos R, Sedano HO (2002) Intralesional corticosteroids as an alternative treatment for central giant cell granuloma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:161–166. https://doi.org/10.1067/moe.2002.119971
Kermer C, Millesi W, Watzke IM (1994) Local injection of corticosteroids for central giant cell granuloma. A case report. Int J Oral Maxillofac Surg 23:366–368. https://doi.org/10.1016/s0901-5027(05)80057-8
Rajeevan NS, Soumithran CS (1998) Intralesional corticosteroid injection for central giant cell granuloma: A case report. Int J Oral Maxillofac Surg 27:303–304. https://doi.org/10.1016/S0901-5027(05)80620-4
Osterne RL, Araújo PM, Souza-Carvalho AC, Cavalcante RB, Sant’Ana E, Nogueira RLM, (2013) Intralesional corticosteroid injections in the treatment of central giant cell lesions of the jaws: A meta-analytic study. Med Oral Patol Oral Cir Bucal 18:e226–e232. https://doi.org/10.4317/medoral.18345
Chawla S, Blay J, Rutkowski P et al (2019) Denosumab in patients with giant-cell tumour fo bone: a multicentre, open-laber, phase 2 study. Lancet Oncol 20:P1719-1729. https://doi.org/10.1016/S1470-2045(19)30663-1
Folkman J, Mulliken JB, Ezekowitz RAB (1997) Antiangiogenic therapy of haemangiomas with interferon A. In: Stuart-Harris R, Penny R (eds) The Clinical Applications of the Interferons. Chapman & Hall Medical, London, pp 255–265
Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB (2002) Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg 60:1103–1113. https://doi.org/10.1053/joms.2002.34975
Kaban LB, Troulis MJ, Wilkinson MS, Ebb D, Dodson TB (2007) Adjuvant antiangiogenic therapy for giant cell tumors of the jaws. J Oral Maxillofac Surg 65:2018–2024. https://doi.org/10.1016/j.joms.2007.03.030
Kaban LB, Mulliken JB, Ezekowitz RA, Ebb D, Smith PS, Folkman J (1999) Antiangiogenic therapy of a recurrent giant cell tumor of the mandible with interferon alfa-2a. Pediatrics 103:1145–1149. https://doi.org/10.1542/peds.103.6.1145
Funding
This work was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior)—n° 88882.448733/2019–01—Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
Author Camila Camarini declares that she has no conflict of interest. Author Elen de Souza Tolentino declares that she has no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Camarini, C., de Souza Tolentino , E. Non-surgical treatment as an alternative for the management of central giant cell granuloma: a systematic review. Clin Oral Invest 26, 2111–2132 (2022). https://doi.org/10.1007/s00784-021-04193-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04193-z