Abstract
Purpose
We report the results of the intralesional steroid injections for the management of central giant cell granuloma (CGCG) of the jaws.
Methods
Seven CGCGs were treated with intralesional injection of corticosteroids. To accomplish this, 3.5 mL of triamcinolone and 3.5 mL of 0.5 % marcaine with 1/200,000 epinephrine (total 7 mL) were mixed. An adequate amount of steroid was injected into different areas of the lesion. This procedure was repeated on a weekly basis for 6 weeks.
Results
Clinical and radiological examination showed complete resolution and ossification of the lesions in four patients. Partial recovery was achieved in two patients. One patient did not respond to the treatment and underwent surgical curettage.
Conclusions
We suggest that intralesional steroid injection is safe and effective for the treatment of CGCG, especially in non-aggressive lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The central giant cell granuloma (CGCG) is a benign lesion of the maxillofacial area. It was first described in 1953 by Jaffe [1], and it is defined by the World Health Organization as an intraosseous lesion consisting of cellular fibrous tissue. It contains multiple foci of hemorrhage, aggregations of multinucleated giant cells, and occasionally, trabeculae of woven bone [2]. Although the terminology of “giant cell reparative granuloma” has also been used, the word “reparative” has not been preferred by most pathologists because the lesion is typically destructive and sometimes aggressive [2]. CGCG exclusively occurs in the maxilla or in the mandible [3]. The disease mainly affects children and young adults between 2 and 25 years old, and the occurrence is estimated to be 1.1 in 1 million [4]. Clinically, these lesions are characterized by a painless and slow-growing mass. Pain and numbness are rare.
Intraorally, a swelling in the affected area and malocclusion arising from displacement of teeth can be observed. Radiographically, CGCGs appear as unilocular or multilocular radiolucent areas with relatively well demarcated borders. However, in some cases, the overlying cortical bone is perforated and root resorption can be seen [5]. Choung et al. [6] divided the disease in two different groups named non-aggressive and aggressive lesions on the basis of clinical symptoms and radiographic features. Non-aggressive lesions exhibit a slow, almost asymptomatic growth that does not perforate the cortical bone and have a low tendency to recur. Aggressive lesions are characterized by pain, rapid growth, root resorption, cortical perforation and have a high recurrence rate after surgical curettage. These lesions are also larger in size and histologically demonstrated a larger fractional surface area occupied by giant cells [4].
The differential diagnosis for CGCG includes ameloblastoma, fibrous dysplasia, primer or seconder hyperparathyroidism, cherubism, and aneurysmal bone cyst. The microscopic appearance of the lesions is indistinguishable from the brown tumor of hyperparathyroidism. High serum level of parathyroid hormone and alkaline phosphatase indicates hyperparathyroidism [5]. Differential diagnosis of aneurysmal bone cyst is made by determining sinusoidal blood spaces within the tumor mass. Cherubism can be diagnosed by the presence of characteristic facial abnormalities or synchronous symmetric lesions. Furthermore, the cherubism gene (SH3BP2) is not mutated in CGCG. The other rare syndromes with multiple giant cell lesions known as Noonan syndrome (PTPN11 gene) and neurofibromatosis type 1 are diagnosed with DNA analyses [4].
Treatment methods of CGCG include en bloc resection, curettage, radiotherapy, systemic calcitonin, interferon (IFN), osteoprotegerin, AMG 62, imatinib, bisphosphonate, denosumab, and intralesional steroid injections. En bloc resection is recommended for aggressive or recurrent lesions. But it results in large surgical defects in the jaws that can alter the facial contours, which are undesirable in children or young adults [7]. The literature has reported a high recurrence rate of curettage for the treatment of CGCG especially in aggressive lesions [4, 8].
The calcitonin therapy, initially reported by Harris [9], has been used subcutaneously or as nasal spray for the treatment of giant cell lesions. In an immunohistochemical study using osteoclast-specific monoclonal antibodies, it has been demonstrated that the giant cells in CGCGs are osteoclasts. Therefore, it is considered that calcitonin directly inhibits the function of giant cells [10].
IFN is an antiangiogenic and antiviral agent that is mainly used as a therapy for tumors and viral infections. The effects of IFN on the angiogenesis have been mainly attributed to the inhibition of basic fibroblast growth factor production by tumor cells. Because the aggressive CGCGs are considered proliferative vascular lesions, it would possibly respond to antiangiogenic therapy with IFN [11].
Both osteoprotegerin and AMG 62 inhibit the action of receptor activator for nuclear factor κβ ligand (RANKL). RANKL affects the differentiation of mononuclear precursor cells into mature giant cells, and it also stimulates osteoclast bone resorbing activity. Because the giant cells in CGCG are osteoclasts, the progression of lesion can be obstructed by inhibition of RANKL [4, 12].
Imatinib is a protein tyrosine-kinase inhibitor used in the treatment of multiple cancers such as chronic myelogenous leukemia and gastrointestinal stromal tumors [13, 14]. In an experimental study, it was shown that imatinib inhibits the differentiation and function of osteoclasts at concentrations within the therapeutic dose range. These results suggest that imatinib may be useful in the treatment of skeletal diseases involving excessive osteoclast activity [15].
Intralesional steroid injection (ISI) was first reported in CGCG lesions by Jacoway et al in 1988 [16]. In 1994, Terry and Jacoway [17] introduced the treatment protocol using a weekly intralesional injection of a mixture of equal parts of triamcinolone acetonide (TA) (10 mg/mL) and a local anesthetic (bupivacaine 0.5 % with epinephrine 1:200,000). The recommended dose is 2 mL/2 cm of radiolucency, and the injection should be given in different locations throughout the lesion for at least 6 weeks. It is hypothesized related with the mechanism of corticosteroids in the treatment of these lesions that the extracellular production of bone resorption through lysosomal proteases by the giant cells is inhibited and steroids induce apoptotic action of osteoclast-like cells. These two mechanisms could cause cessation of bone resorption [18].
In this paper, we present seven cases of CGCG of the jaws treated with ISI. Four of them healed completely, two responded partially, and one case did not respond to treatment.
Patients and methods
Seven patients (four males and three females; aged between 11 and 48 years) with CGCG were treated. In four cases, the lesions were located in the mandible while the other three were found in the maxilla. All patients had vestibular expansion. Patients had single lesions and one patient (case 6) had facial asymmetry. Incisional biopsy was performed and all lesions were identified as CGCGs. Laboratory analysis of parathyroid hormone, calcium, and phosphorus values were within normal limits in all patients, ruling out hyperparathyroidism. The patients were managed by using the protocol defined by Terry and Jacoway [17]. For this purpose, 3.5 mL of TA (Kenacort-A) and 3.5 mL of 0.5 % marcaine with 1/200,000 epinephrine (total 7 mL) were mixed. This mixture was injected into the lesion, distributing as equally as possible. This procedure was repeated on a weekly basis for 6 weeks. First injections were performed by using a 10-mL syringe with dental needle because the lesions were mostly fragile. However, the lesions became firmer in the later phases of treatment and injection procedure became more difficult. Therefore, a 21-gauge needle was used with a 10-mL syringe in subsequent injections. The course of treatment was followed by the radiographs taken every 3 months. Mean follow-up of patients was 39 months.
Results
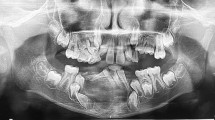
Table 1 provides a summary of the results of treatment with ISIs for the cases. During and after treatment, the side effects were not observed due to the steroid therapy in any patient. However, menstrual irregularities occurred in one patient after six injections (case 6). Radiographic appearance showed complete resolution and ossification of the lesions for four patients (Figs. 1a, b; 2a, b; 3a, b; and 4a, b). In one patient (case 2), the lesion was not totally resolved but it became much smaller. A surgical procedure similar to periodontal flap operation was carried out to remove the residual lesion. The patient healed uneventfully. In case 6, the healing was not achieved at the end of the six injections in the lesion. After that, we performed nasal calcitonin spray. But the patient did not want to maintain calcitonin therapy due to the bleeding of the nose, whereupon curettage was performed on the lesion. Premolars were extracted and the region was grafted simultaneously. No recurrence was seen after 38 months (Fig. 5a–c). Another patient (case 4) had a mass that was in relation to maxillary teeth. Eight months after steroid injections, the lesion became smaller, but it was not completely healed. A new steroid injection therapy was planned, but we lost contact with the patient. Two years later, we reached him. It was seen that the patient was operated in another center. We obtained the histopathological sections of this resection, which had been performed without our knowledge, and re-examined them. Histopathologic examination revealed lobules of giant cell granuloma separated with broad interstitial fibrous connective tissue bands. Lobules of giant cell granuloma were composed from stromal fibrohistiocytic cells and osteoclast-type giant cells. Comparing with the previous histopathologic appearance of the lesion, this new view may suggest maturation of the original background granulation tissue (Fig. 6a–b).
a OPG shows a multilocular radiolucent lesion of a 13-year-old female patient in the left mandible. b The lesion did not respond to the injections. After 2 months, increased radiolucency was seen. c The curettage was performed on the lesion. Premolars were extracted and the region was grafted simultaneously. No recurrence was seen after 38 months
Discussion
Conventional treatment modalities of CGCG involve surgical interventions such as curettage or bloc resection. Although vigorous surgical curettage is known to yield partially successful outcomes, recurrence rates of up to 70 % have been reported with this technique [6]. Damage to the teeth and their tooth follicle and alveolar bone loss are other possible complications. Resections reduce the recurrence risk, but it require demanding reconstructive procedures along with possible undesirable outcomes especially children and young adults, such as paresthesia, excessive bone loss, or aesthetic problems after the reconstruction [19].
These concerns motivated some researchers to find a reliable conservative technique for the management of CGCGs. Radiotherapy has been suggested as a non-surgical treatment modality, but it can lead to malignant transformations [20]. Interferon-alpha has also been used for CGCGs. Although it causes reduction of the aggressive lesions, it often requires the combined treatment such as imatinib [21]. The other non-surgical method, calcitonin therapy, has also been recommended since it was first suggested [9]. Calcitonin is given as subcutaneous injections or in a nasal spray form, and it has been found to be a viable conservative treatment modality. However, treatment period is quite long (more than 1 year), and if it is received by daily subcutaneous injections, this may be rather displeasing for patients. Furthermore, there is an assertion that the long-term use of calcitonin nasal spray might increase the risk of liver cancer [22]. Additionally, long-term use of salmon calcitonin nasal spray is not recommended by Food and Drug Administration (FDA) because of the increasing risk of cancer [23]. Other applications such as osteoprotegerin, AMG 62, or imatinib are currently being investigated.
After the ISI for the treatment of CGCG was defined by Jacoway et al. [16] in 1988, many authors have reported successful results (Table 2). As we have seen, single case presentations were more common in the literature about the treatment of CGCG with ISI. When the publications of more than one case are investigated, the success rates are encountered in the following way; Terry and Jacoway [17] 75 %, Carlos and Sedano [18] 75 %, Mohanty and Jhamb [33] 50 %, and Nogueira et al. [20] 70 %. On the subject, Marx and Stern [39] also noted that in their experience, 65 % of giant cell tumors had completely resolved with corticosteroid therapy and 35 % recurred more aggressively or did not respond at all. The success rate of our results (almost 60 %) is supported by the literature.
There are several hypotheses about the mechanism of action of steroids in the CGCG lesions. Osteoclasts induce bone resorption by secreting lysosomal proteases (e.g., cathepsin B, cathepsin L, β glucuronidase, lysozyme, and tartrate-resistant acid phosphatase). These proteases mediate osteoclastic bone resorption by creating an acidic extracellular medium. Kramer et al. [40] proved that avian osteoclasts and human osteoclast-like giant cell tumor cells respond in vitro to treatment with 17β-estradiol (17β-E2) by decreased bone resorption activity. To better understand the mechanism, they investigated the effects of 17β-E2 treatment on lysosomal enzyme production and secretion by isolated avian osteoclasts and multinucleated cells from human giant cell tumors in vitro. They concluded a dose-dependent decrease in secreted levels of these enzymes. Kameda et al. [41] also showed that 17β-E2 was able to directly inhibit osteoclastic bone resorption. Furthermore, E2 also directly induced osteoclast apoptosis in a dose- and time-dependent manner at concentrations effective for inhibiting bone resorption. Hirayama et al. [42] reported that the dexamethasone which is a glucocorticoid has a direct effect on inhibiting the bone-resorbing activity of mature osteoclasts. On the basis of the experimental evidence, it is possible to hypothesize that the effect of intralesional corticosteroids for the treatment of CGCGs may be due to inhibition of the extracellular production of lysosomal proteases as well as steroidal apoptotic action on the osteoclast-like cells [20].
The success criteria of the treatment with intralesional steroid injections include absence of symptoms such as pain or swelling, and increase in radiopacity can be considered. Therefore, we believe that the patients should be monitored for a long time in the postoperative period. In this case series, patients were followed for a mean of 39 months clinically and radiographically. We did not need to perform more than six injections in any patients. We planned a second protocol implementation in only one patient (case 4) because the lesion was not completely healed. But we lost contact with the patient.
The bony cortex overlying the lesion is generally quite thin, and it can be easily perforated with a needle. In these cases, first injections were applied by using a 10-mL syringe and a dental needle due to the lesions which were mostly fragile. Our aim in this procedure was to avoid damage to the soft tissues and dental follicle within the lesion and to prevent the escape of the solutions.
According to literature, ISI is an effective method in patients with CGCG. However, it is not always possible to obtain a positive response to the treatment in the multilocular or aggressive lesions. Hence, in such cases, it is necessary to apply surgical or combined treatment methods. In addition, serum calcium, phosphorus, and parathyroid hormone levels should be examined on suspicion of hyperparathyroidism after a definitive diagnosis result of the incisional biopsy. It should be noted that the images of brown tumor and CGCG cannot be distinguished histologically. And before starting the ISIs, possible diabetes mellitus and the presence of peptic ulcers or any infection should be questioned.
Apparently, the treatment with intralesional steroid injections is advantageous for large CGCG in order to reduce the size of the lesion and thus minimize the need for extensive bone resection and loss of teeth that can result in functional and aesthetic defects. The present study suggests that intralesional injection of steroids is safe and effective for the treatment of CGCG in children and young adults. The treatment method can be considered an alternative to surgical resection. More long-term controlled studies are needed to determine if intralesional injection of steroids may serve as an alternative to surgery in this setting.
References
Jaffe HL (1953) Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-oseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol 6:159–175
Motamedi MH, Eshghyar N, Jafari SM, et al. (2007) Peripheral and central giant cell granulomas of the jaws: a demographic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:e39–e43
Kaffe I, Ardekian L, Taicher S, Littner MM, Buchner A (1996) Radiologic features of central giant cell granuloma of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81:720–726
de Lange J, van den Akker HP, van den Berg H (2007) Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:603–615
Regezi JA, Sciubba JJ, Jordan RCK (2012) Oral pathology: clinical pathologic correlations. Elsevier Sounders, St.Louis, Missouri
Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A (1986) Central giant cell lesions of the jaws: a clinicopathologic study. J Oral Maxillofac Surg 44:708–713
Shirani G, Abbasi AJ, Mohebbi SZ, Shirinbak I (2011) Management of a locally invasive central giant cell granuloma (CGCG) of mandible: report of an extraordinary large case. J Craniomaxillofac Surg 39:530–533
Whitaker SB, Waldron CA (1993) Central giant cell lesions of the jaws. A clinical, radiologic, and histopathologic study. Oral Surg Oral Med Oral Pathol 75:199–208
Harris M (1993) Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg 31:89–94
Flanagan AM, Nui B, Tinkler SM, Horton MA, Williams DM, Chambers TJ (1988) The multinucleate cells in giant cell granulomas of the jaw are osteoclasts. Cancer 62:1139–1145
de Lange J, van den Akker HP, van den Berg H, Richel DJ, Gortzak RA (2006) Limited regression of central giant cell granuloma by interferon alpha after failed calcitonin therapy: a report of 2 cases. Int J Oral Maxillofac Surg 35:865–869
Bekker PJ, Holloway DL, Rasmussen AS, et al. (2004) A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19:1059–1066
Fausel C (2007) Targeted chronic myeloid leukemia therapy: seeking a cure. J Manag Care Pharm 13:8–12
De Giorgi U, Verweij J (2005) Imatinib and gastrointestinal stromal tumors: where do we go from here? Mol Cancer Ther 4:495–501
Dewar AL, Farrugia AN, Condina MR, et al. (2006) Imatinib as a potential antiresorptive therapy for bone disease. Blood 107:4334–4337
Jacoway J, Howell F, Terry B (1988) Central giant cell granuloma—an alternative to surgical therapy [abstract]. Oral Surg Oral Med Oral Pathol 66:572
Terry B, Jacoway J (1994) Management of central giant cell lesions: an alternative to surgical therapy. Oral Maxillofac Surg Clin N Am 6:579–601
Carlos R, Sedano HO (2002) Intralesional corticosteroids as an alternative treatment for central giant cell granuloma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:161–166
Tosco P, Tanteri G, Iaquinta C, et al. (2009) Surgical treatment and reconstruction for central giant cell granuloma of the jaws: a review of 18 cases. J Oral Maxillofac Surg 37:380–387
Nogueira RL, Teixeira RC, Cavalcante RB, Ribeiro RA, Rabenhosrt SH (2010) Intralesional injection of triamcinolone hexacetonide as an alternative treatment for central giant-cell granuloma in 21 cases. Int J Oral Maxillofac Surg 39:1204–1210
de Lange J, van Rijn RR, van den Berg H, van den Akker HP (2009) Regression of central giant cell granuloma by a combination of imatinib and interferon: a case report. Br J Oral Maxillofac Surg 47:59–61
Sun LM, Lin MC, Muo CH, Liang JA, Kao CH (2014) Calcitonin nasal spray and increased cancer risk: a population-based nested case-control study. J Clin Endocrinol Metab 99:4259–4264
In brief (2013) cancer risk with salmon calcitonin. Med Lett Drugs Ther 55:29
Kermer C, Millesi W, Watzke IM (1994) Local injection of corticosteroids for central giant cell granuloma. A case report. Int J Oral Maxillofac Surg 23:366–368
Rajeevan NS, Soumithran CS (1998) Intralesional corticosteroid injection for central giant cell granuloma. A case report. Int J Oral Maxillofac Surg 27:303–304
Khafif A, Krempl G, Medina JE (2000) Treatment of giant cell granuloma of the maxilla with intralesional injection of steroids. Head Neck 22:822–825
Kurtz M, Mesa M, Alberto P (2001) Treatment of a central giant cell lesion of the mandible with intralesional glucocorticosteroids. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91:636–637
Adornato MC, Paticoff KA (2001) Intralesional corticosteroid injection for treatment of central giant-cell granuloma. J Am Dent Assoc 132:186–190
Abdo EN, Alves LC, Rodrigues AS, Mesquita RA, Gomez RS (2005) Treatment of a central giant cell granuloma with intralesional corticosteroid. Br J Oral Maxillofac Surg 43:74–76
Sezer B, Koyuncu B, Gomel M, Gunbay T (2005) Intralesional corticosteroid injection for central giant cell granuloma: a case report and review of the literature. Turk J Pediatr 47:75–81
Comert E, Turanli M, Ulu S (2006) Oral and intralesional steroid therapy in giant cell granuloma. Acta Otolaryngol 126:664–666
Wendt FP, Torriani MA, Gomes AP, de Araujo LM, Torriani DD (2009) Intralesional corticosteroid injection for central giant cell granuloma: an alternative treatment for children. J Dent Child (Chic) 76:229–232
Mohanty S, Jhamb A (2009) Central giant cell lesion of mandible managed by intralesional triamcinolone injections. A report of two cases and literature review. Med Oral Patol Oral Cir Bucal 14:E98–102
Ferretti C, Muthray E (2011) Management of central giant cell granuloma of mandible using intralesional corticosteroids: case report and review of literature. J Oral Maxillofac Surg 69:2824–2829
Rachmiel A, Emodi O, Sabo E, Aizenbud D, Peled M (2012) Combined treatment of aggressive central giant cell granuloma in the lower jaw. J Craniomaxillofac Surg 40:292–297
da Silva NG, Carreira AS, Pedreira EN, Tuji FM, Ortega KL, de Jesus Viana Pinheiro J (2012) Treatment of central giant cell lesions using bisphosphonates with intralesional corticosteroid injections. Head Face Med 8:23
Choi JW, Kraut RA (2013) Management of central giant granuloma of mandible with intralesional triamcinolone injections: a case report. N Y State Dent J 79:34–36
Fonseca FP, Ribeiro AC, Santos-Silva AR, Vargas PA, Lopes MA (2013) Fine needle aspiration cytology and intralesional steroid injection in a central giant cell granuloma affecting the gingiva: a new clinical approach. Braz Dent J 24:420–427
Marx RE, Stern D (2012) Oral and maxillofacial pathology: a rationale for diagnosis and treatment. Quintessence Publishing Company, Hanover Park
Kremer M, Judd J, Rifkin B, Auszmann J, Oursler MJ (1995) Estrogen modulation of osteoclast lysosomal enzyme secretion. J Cell Biochem 57:271–279
Kameda T, Mano H, Yuasa T, et al. (1997) Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med 186:489–495
Hirayama T, Sabokbar A, Athanasou NA (2002) Effect of corticosteroids on human osteoclast formation and activity. J Endocrinol 175:155–163
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dolanmaz, D., Esen, A., Mihmanlı, A. et al. Management of central giant cell granuloma of the jaws with intralesional steroid injection and review of the literature. Oral Maxillofac Surg 20, 203–209 (2016). https://doi.org/10.1007/s10006-015-0530-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-015-0530-5