Abstract
Objective
This study evaluated the effects of calcium gluconate (CaGlu), sodium fluoride (NaF), sodium trimetaphosphate (TMP), and NaF/TMP added to a 35% hydrogen peroxide (HP) bleaching gel for the reduction in enamel demineralization in vitro, with and without the use of a fluoridated dentifrice.
Design
Enamel blocks (n = 100) were obtained from bovine incisors (n = 200) after flattening and subjected to initial surface hardness (SH) analysis. The blocks were divided according to the bleaching gel (35% HP; 35% HP + 0.05% NaF; 35% HP + 0.25% TMP; 35% HP + 0.05% NaF + 0.25% TMP; 35% HP + 2% CaGlu) and were treated with ether non-fluoridated or fluoridated (1100 ppm) dentifrice. The bleaching gels were applied thrice (40 min/session) at the intervals of 7 days between each application. After 21 days, the final SH for the calculation of the percentage of SH loss (%SH) and cross-sectional hardness for the evaluation of the integrated hardness area (IH) were determined.
Results
Bleaching containing HP + NaF + TMP presented lowest %SH (p < 0.001), regardless of the dentifrice used. HP + NaF + TMP bleaching gel led to lower subsurface enamel mineral loss (IH) compared to the other groups (p < 0.001), and these did not differ from each other (p > 0.05). Daily use of fluoride dentifrice led to higher IH values (p < 0.001), regardless of the bleaching gels.
Conclusion
The addition of NaF/TMP to a 35% HP bleaching gel remarkably reduced the mineral loss compared to the cases of the other bleaching gels, regardless of dentifrice.
Clinical relevance
The association of TMP/NaF can be used as a strategy for reducing mineral loss during the bleaching procedure, even without the daily use of fluoride dentifrice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, with increasing esthetic expectations, dental bleaching has become increasingly important for clinicians and patients [1]. Although dental bleaching is considered to be a conservative and non-invasive cosmetic treatment, many patients report temporary dental sensitivity during and/or after the procedure that requires the patient to stop the bleaching treatment. This phenomenon has been called bleaching sensitivity [2] because dental sensitivity can be a symptom of other oral problems. Bleaching sensitivity is related to the whitening procedure in the vital tooth, and its severity depends on risk factors (sex, age, dietary habits, pre-existing tooth sensitivity, bleaching history), whitener composition (active agents, concentration, and pH), and method of application (e.g., in-office versus home-applied, exposure time) [2, 3].
Despite the controversy about the effects of the whitening products on the tooth structure [4,5,6], it is evident that these adverse effects can be triggered by enamel demineralization and trans-enamel-dentin penetration from peroxide degradation derivatives [7]. Several studies have shown that depending on the peroxide concentration, application time, non-bleach components, and pH, tooth bleaching may give rise to a number of adverse effects such as dental sensitivity, gingival irritation, and alteration of the enamel structure (i.e., demineralization, reduced hardness, and increased surface roughness) [3, 6,7,8]. Nevertheless, the release of H+ from peroxides during the whitening procedure changes the pH of the bleaching products to more acidic values which may lead to the changes in the enamel structure [3]. By contrast, other studies reported that peroxides do not cause any structural alterations in the enamel [9,10,11] and the acidic components present in the formulations are responsible for the observed structural changes of the enamel [6, 8].
Despite the conflicting evidence described above, remineralizing agents (i.e., potassium nitrate-KNO3, calcium chloride-CaCl2, calcium gluconate-CaGlu, and sodium fluoride-NaF) have been added to bleaching agents in order to minimize the mineral loss [12,13,14,15,16,17,18,19,20] and reduce the severity of their side effects. Fluoride regimens with dentifrices or gels have been shown to be effective for increasing enamel hardness and preventing hardness loss during bleaching [16, 21, 22]. Nevertheless, Tschoppe et al. [22] showed that the NaF and/or amorphous calcium phosphate (ACP)-containing bleaching gels had no effect on the remineralization of enamel subsurface. Thus, it is important to elucidate whether the addition of remineralizing agents to whitening gels leads to a demineralization reduction or mineral gain, and it is also important to determine to what degree this effect is manifested in the presence of saliva and for patients who regularly use fluoridated dentifrices.
When combined with fluoride, sodium trimetaphosphate (TMP) has shown potent effects against demineralization and in promoting the remineralization of subsurface enamel lesions. This combination (TMP/fluoride) has enabled the reduction in fluoride concentration in oral care agents with promising results in dentifrices [23, 24] and varnishes [25] due to its ability to block acid diffusion into the deeper layers of the enamel, so that it also exerts a protective effect against dental erosion [23, 25]. Similar, to the undesired erosive effects, products derived from peroxide degradation lead to undersaturated conditions with respect to hydroxyapatite. Thus, it can be hypothesized that the addition of TMP/fluoride to HP at high concentration (in-office bleaching agents) can minimize structural changes in the enamel during bleaching therapy.
Therefore, the aim of this study was to evaluate the effects of the CaGlu, NaF, TMP, and NaF/TMP added to a 35% HP bleaching gel for the reduction in enamel demineralization in vitro, both with and without the use of a fluoridated dentifrice. The null hypotheses tested were: (1) enamel demineralization will not be affected by exposure to the type of bleaching gel and (2) enamel demineralization associated with the use of the bleaching treatment will not be affected by the use of a fluoridated dentifrice.
Material and methods
Experimental design
For this study, enamel blocks (4 mm × 4 mm, n = 100) were obtained from bovine incisors (n = 200), and were stored in 2% formalin at pH 7.0 for 30 days [23, 24]. The enamel surface of the blocks was ground flat using water-cooled carborundum disks (400, 600, 800, and 1200 grades of Al2O3 papers; Buehler, Lake Bluff, IL, USA) in order to remove approximately 200 μm of the surface enamel. The surface was then polished with a polishing cloth (Polishing Cloth Buehler 40-7618; Buehler, Lake Bluff, IL, USA) and a diamond suspension (MetaDi Diamond Suspension 1 μm Blue Color Polish Spray, Water Base 40-653; Buehler, Lake Bluff, IL, USA). Next, enamel blocks were subjected to the initial surface hardness analysis (SHi), allowing the selection of the blocks with the surface hardness values in the 326–369 Knoop hardness (KHN) range without cracks, scratches, or hypoplasia. The enamel blocks were randomly assigned into five groups (n = 10) with the mean hardness values ranging from 337.8 to 341.0 KHN (p = 0.766) (ANOVA one-way, Student-Newman-Keuls test). The experimental design was randomized, and the blocks were divided according to the bleaching gel: 35% hydrogen peroxide (HP) (35% HP); 35% HP + 0.05% sodium fluoride-NaF (HP + NaF); 35% HP + 0.25% sodium trimetaphosphate (TMP) (HP + TMP); 35% HP + 0.05% NaF + 0.25% TMP (HP + NaF + TMP); 35% HP + 2% calcium gluconate (HP + CaGlu; Whiteness HP Blue, FGM, Joinville, SC, Brazil). A TMP/NaF concentration of 0.05% NaF + 0.25% TMP was used based on the previous studies [23, 24]. The blocks were further divided into two conditions of either non-fluoridated or fluoridated dentifrice treatment. The bleaching gels were applied once (40 min/session) every 7 days for a total of 3 sessions (21 days). Prior to the application of the gels and throughout the experiment, the blocks in the “dentifrice” subgroup were treated with a 1100-ppm fluoride dentifrice slurry (1:3) twice a day. The blocks remained in the artificial saliva between the treatment sessions, and the artificial saliva was renewed on a daily basis. After 21 days, the final surface hardness (SHf), percentage of the surface hardness loss (%SH), and integrated subsurface hardness loss (ΔKHN: hardness values were calculated by the trapezoidal rule in each depth of the demineralized enamel into sound enamel) were evaluated.
Formulation of the bleaching gels and fluoride dentifrice
The experimental bleaching gels were manipulated at each application session because they did not contain a stabilizer agent. The base of the gels consisted of a thickener (15% Carbopol 960, Pharmacy Apothicario, Araçatuba, Brazil), bleaching agent (35% hydrogen peroxide, Pharmacy Apothicario, Araçatuba, Brazil), glycerin (Sigma Aldrich, St. Louis, MO, USA), and deionized water (q.s.p.), to obtain an optimal consistency and homogenous mixing of the gel. Their solid components were weighed on a precision scale (Adventurer, Ohaus, Parsippany, NJ, USA) and placed in a plastic flask to which 35% HP was added with a pipette for weighing, and then were mixed thoroughly. The pH of the bleaching gels was adjusted with 4 mol/L NaOH (Sigma Aldrich, St. Louis, MO, USA), using pH indicator strips (Merck, Darmstadt, Germany), until reaching a final pH of approximately 7.0. In total, four different compositions were used according to the concentrations of TMP (Sigma Aldrich, St. Louis, MO, USA) and NaF (Merck, Darmstadt, Germany).
The dentifrice had the following composition: titanium dioxide, carboxymethyl cellulose, methyl p-hydroxybenzoate sodium, saccharin, peppermint oil, glycerol, abrasive silica, sodium lauryl sulfate, deionized water, and NaF at the concentration of 1100 ppm fluoride (Merck, Darmstadt, Germany) [23, 24]. The total (TF) and ionic (IF) fluoride concentrations were evaluated [24,25,26] using a fluoride ion-specific electrode (Orion 9609 BN, Orion Research Inc., Beverly, Mass., USA) coupled to an ion analyzer (Orion 720 A+, Orion Research Inc.) previously calibrated with five standard solutions (0.25, 0.5, 1.0, 2.0, and 4.0 μg F/mL). The total and ionic fluoride concentrations (TF and IF) (mean [SD]; ppm F−; n = 3) were, respectively, 1102.1 [5.2] and 1102.4 [3.5]. All of the experimental and commercial bleaching gels had a final pH of approximately 7.0. The pH (mean [SD]; n = 3) of the 1100 ppm fluoride dentifrice was 7.0 [0.2].
Treatment with dentifrice and bleaching gels
After the determination of SHi, the blocks were treated with a slurry of the dentifrice prior to the study and during the 21-day time period of the experiment. To prepare the slurry, the dentifrice was weighed daily, placed in a glass beaker, and added to deionized water at a ratio of 1:3, and shaken to obtain a homogeneous suspension. Each enamel block was immersed in the slurry (4 mL) in individual vials under agitation on an orbital shaker (SK300, Nova Analítica, São Paulo, SP, Brazil) for 1 min. Then, bleaching gels were applied to the enamel surface for 40 min. Then, the gels were removed with gauze and rinsed with deionized water for 30 s in order to remove any residue on the blocks. Then, the blocks were stored in individual containers containing artificial saliva (5 mL) [23] and were kept in an incubator at 37 °C until the next day. The blocks were then treated with the dentifrice slurry twice a day, and artificial saliva was renewed daily. The bleaching gels were applied every 7 days for 21 days for a total of 3 sessions. The composition of the artificial saliva was 1.5 mmol/L Ca(NO3)2 × 4 H2O, 0.9 mmol/L NaH2PO × 2 H2O, 150 mmol/L KCl, 0.1 mol/L Tris buffer, 0.03 ppm fluoride, pH 7.0 [24, 25].
Enamel hardness analysis
The surface hardness of the enamel was determined using a Shimadzu HMV-2000 hardness tester (Shimadzu, Kyoto, Japan) under a 25-g load for 10 s. Five indentations at the intervals of 100 μm were made in the center of the enamel block (SHi). After the treatments, five indentations (SHf) spaced at the intervals of 100 μm from the baseline indentations were made to calculate the percentage of surface hardness loss (%SH = [(SHf − SHi)/SHi] × 100) [26]. Then, the blocks were cross-sectioned, and half of each block was embedded in acrylic resin and gradually polished. One sequence of 14 indentations at different distances (5, 10, 15, 20, 25, 30, 40, 50, 60, 80, 100, 120, 140, 160 and 180 μm) [20, 25, 26] was made in the surface of the enamel in the central region. This was achieved using a Micromet 5114 hardness tester (Buehler, Lake Bluff, IL, USA) with a Knoop diamond indenter under a 5-g load for 10 s and the Buehler OmniMet software program (Buehler, Lake Bluff, IL, USA). The integrated area above the curve (KHN × μm) using the hardness values was calculated by the trapezoidal rule [24, 25] at the depths of 5–180 μm in the inner part of the enamel and named the integrated hardness area (IH).
Statistical analysis
For statistical analysis, the values of SHf, %SH, and IH were considered as variables, and the whitening gel and dentifrice were considered as variation factors. The statistical SigmaPlot software version 12.0 (SigmaPlot, Systat Software, San Jose, CA, USA) was used with the significance at 5% level. The variables presented a normal distribution (Shapiro-Wilk test) and were homogeneous (Cochran test) and were subjected to two-way analysis of variance followed by the Student-Newman-Keuls test.
Results
Commercial 35% HP bleaching led to lower values of SHf compared to other groups (p < 0.001) when non-fluoridated dentifrice was used (Table 1). SHf increased for the 35% HP and HP + CaGlu bleaching groups with daily use of fluoride dentifrice (p = 0.006). Bleaching containing HP + NaF + TMP presented the highest values of SHf (p < 0.001) regardless of the dentifrice used. Compared to other groups, the surface hardness loss (%SH) was lower for the bleaching containing HP + NaF + TMP and higher for the commercial 35% HP (p < 0.001). The use of fluoridated dentifrice did not influence the SHf and %SH values of the HP + NaF, HP + TMP, and HP + NaF + TMP groups.
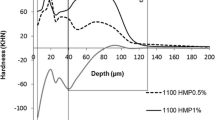
The cross-sectional hardness profiles from the different bleaching gels are displayed in Fig. 1. The hardness values were lower and showed greater oscillations in the depth of enamel when the fluoride-free dentifrice was used. Lower hardness values were more evident at the depth of 5–30 μm in the enamel (Fig. 1). The integrated hardness (IH) was higher when HP + NaF + TMP bleaching gel was applied on enamel (p < 0.001), while the other bleaching agents showed no effect (p = 0.995; Table 1). Daily use with fluoride dentifrice led to higher IH values (p < 0.001) regardless of the bleaching gels.
Discussion
Tooth sensitivity is a possible side effect of whitening gels that can occur due to the mineral loss of enamel because many formulations have active agents with low pH, in addition to the factors specific to each individual patient [2, 3, 6, 8, 9]. The present study showed that the application of a bleaching gel containing NaF and TMP led to lower surface and depth demineralization. Furthermore, the daily use of dentifrice containing 1100 ppm fluoride was shown to promote a protective effect during bleaching. Thus, both null hypotheses were rejected.
In the present study, artificial saliva was used to simulate intraoral conditions, particularly with respect to ionic composition and pH [13, 19, 21]. In this sense, saliva increases the hardness of the bleached enamel through the supply of calcium and phosphate ions, but the remineralization process may not be complete under some conditions [13, 20]. Furthermore, the addition of fluoride to the bleaching gel can contribute to the microstructural repair of defects in the demineralized enamel during gel application, because F ions can decrease the dissolution of enamel minerals and increase crystalline growth [20]. However, in the present study, the effect of fluoride at such low concentrations (0.05% NaF) was only observed on the enamel surface, but not deep in the enamel. It is possible that the deposition of high amounts of calcium fluoride may have occurred due to the availability of calcium from the demineralized enamel resulting from the action of the bleaching agent [27]. Following this rationale, when enamel was immersed in saliva, such calcium fluoride deposits increased the precipitation of calcium and phosphate mainly on the surface enamel layers. Under similar experimental conditions, other studies using higher fluoride concentrations (0.2% or 0.5% NaF) in 35% hydrogen peroxide (in-office bleaching agents) also reported lower surface hardness loss compared to using fluoride-free gels without reversal of the surface demineralization [19, 20], and no effect was observed deep in the enamel [20].
By contrast, despite showing similar results for surface hardness, and lack of an effect on the demineralization in the inner region of the enamel compared to fluoride-containing bleaching gels [19, 20], the CaGlu-based bleaching agent containing 35% HP led to the highest surface hardness loss in the present study. Moreover, the studies reported in the literature have shown that bleaching agents containing 2% of calcium gluconate did not exert a positive effect on the enamel [16, 28], mainly for products containing HP at low concentrations [19]. The results of the present study confirm the previous findings that saturating the medium with a calcium source does not guarantee an effect (Table 1). The addition of remineralizing agents must be able to react with enamel in the presence of hydrogen peroxide and at the same time resist the action of its degradation products. Fluoride or TMP appears to have the ability to be adsorbed to enamel and reduce mineral loss than calcium [29, 30]. According to previous studies [18,19,20], CaGlu is incompatible with strong oxidizing agents and may not be released during the bleaching decomposition [12, 26]. Nevertheless, the addition of calcium or fluoride sources in the bleaching gels did not lead to an additional benefit in the case of the daily exposure to the non-fluoride or fluoride (1100 ppm) dentifrice. Our findings are similar to those reported by Tschoppe et al. [22] who verified that the use of calcium and/or fluoride-containing bleaching gels had only minimal effects on the remineralization of the enamel subsurface and suggested that different concentrations of fluoride in bleaching gels should be tested for improvement in reducing enamel demineralization. Treatment of bleached enamel with fluoridated dentifrice was shown to minimize both surface and cross-sectional hardness loss, in line with previous studies [22] or during bleaching treatment [21]. It is important to note that the cross-sectional hardness shows good correlation with the mineral concentrations when analyzed by transversal microradiography [31, 32], synchrotron microcomputed tomography [29], or X-ray microcomputed tomography [33, 34]. Thus, our findings showed that the SH test is a highly sensitive and reproducible method for studying the early stages of enamel demineralization in vitro.
It is noteworthy that calcium or fluoride-based bleaching agents or the simultaneous use of fluoridated dentifrice do not interfere with the bleaching potential of the gels [14, 17, 22]. Moreover, the addition of TMP and NaF to the bleaching gel led to the lowest mineral loss among all of the test groups, regardless of the use of fluoride dentifrice. Previous studies have shown that TMP is effective for the prevention of enamel demineralization and in promoting enamel remineralization when co-administered with fluoride at the appropriate fluoride/TMP molar ratios [23, 24]. There is evidence that TMP binds to the OH− groups on the hydroxyapatite molecule [30, 35] and, once adsorbed, it has the ability to reduce H+ diffusion, facilitate calcium and phosphate diffusion, and form a TMP-Ca++-PO4− and/or TMP-Ca++-fluoride layer on the enamel [23, 24]. Based on the present results, we hypothesize that anions and cations derived from hydrogen peroxide degradation will bind to the TMP layer deposited on enamel. This may affect the diffusion of the HP-derived products into the enamel by reducing both enamel demineralization and aggression to the pulp tissues. The data from the cross-sectional hardness measurements support the previous hypothesis, since mineral loss was reduced deep in the enamel, regardless of the exposure to the fluoride dentifrice. Nonetheless, two important aspects must be addressed regarding the addition of NaF/TMP to bleaching agents. First, despite similar bleaching effects that were visually observed for all of the gels tested, colorimetric readouts established by the Comission Internacionale de I’Eclairage (CIE) [7, 17] need to be used in order to determine the actual effects of such compositional change on the resulting bleaching effect of the formulations. Furthermore, the effect of NaF/TMP on the diffusion of HP-derived products through tooth hard tissues must be verified through cytotoxic assays or/and trans-enamel/dentin penetration of hydrogen peroxide.
Within the limitations of this in vitro study, we can conclude that the addition of 0.25% TMP and 0.05% NaF to a 35% hydrogen peroxide bleaching gel significantly reduced both the surface and cross-sectional hardness compared to the cases of the other bleaching gels. The calcium-based, fluoride-based, or TMP-based bleaching gels did not provide any benefit in the cases where fluoride dentifrice (1100 ppm fluoride) was used daily.
References
Samorodnitzky-Naveh GR, Geiger SB, Levin L (2007) Patients’ satisfaction with dental esthetics. J Am Dent Assoc 138:805–808. https://doi.org/10.14219/jada.archive.2007.0269
Kielbassa AM, Maier M, Gieren AK, Eliav E (2015) Tooth sensitivity during and after vital tooth bleaching: a systematic review on an unsolved problem. Quintessence Int 46:881–897. https://doi.org/10.3290/j.qi.a34700
Carey CM (2014) Tooth whitening: what we now know. J Evid Based Dent Pract 14:70–76. https://doi.org/10.1016/j.jebdp.2014.02.006
Auschill TM, Hellwig E, Schmidale S, Sculean A, Arweiler NB (2005) Efficacy, side-effects and patients’ acceptance of different bleaching techniques (OTC, in-office, at-home). Oper Dent 30:156–166. https://doi.org/10.1016/S0084-3717(08)70026-6
Reis A, Tay L, Herrera D, Kossatz S, Loguercio AD (2011) Clinical effects of prolonged application time of an in-office bleaching gel. Oper Dent 36:590–596. https://doi.org/10.2341/10-173-C
Magalhães JG, Marimoto ARK, Torres CRG, Pagani C, Teixeira SC, Barcellos DC (2012) Microhardness change of enamel due to bleaching with in-office bleaching gels of different acidity. Acta Odontol Scand 70:122–126. https://doi.org/10.3109/00016357.2011.600704
Cintra LTA, Benetti F, Ferreira LL, Gomes-Filho JE, Ervolino E, Gallinari MO, Rahal V, Briso ALF (2016) Penetration capacity, color alteration and biological response of two in-office bleaching protocols. Braz Dent J 27:169–175. https://doi.org/10.1590/0103-6440201600329
Zantner C, Beheim-Schwarzbach N, Neumann K, Kielbassa AM (2007) Surface microhardness of enamel after different home bleaching procedures. Dent Mater 23:243–250. https://doi.org/10.1016/j.dental.2006.06.044
Eimar H, Siciliano R, Abdallah MN, Nader SA, Amin WM, Martinez PP, Celemin A, Cerruti M, Tamimi F (2012) Hydrogen peroxide whitens teeth by oxidizing the organic structure. J Dent 40:e25–e33. https://doi.org/10.1016/j.jdent.2012.08.008
White DJ, Kozak KM, Zoladz JR, Duschner H, Götz H (2002) Peroxide interactions with hard tissues: effects on surface hardness and surface/subsurface ultrastructural properties. Compend Contin Educ Dent 23:42–48
Sasaki RT, Arcanjo AJ, Flório FM, Basting RT (2009) Micromorphology and microhardness of enamel after treatment with home-use bleaching agents containing 10% carbamide peroxide and 7.5% hydrogen peroxide. J Appl Oral Sci 17:611–616. https://doi.org/10.1590/S1678-77572009000600014
Basting R, Amaral F, França F, Flório F (2012) Clinical comparative study of the effectiveness of and tooth sensitivity to 10% and 20% carbamide peroxide home-use and 35% and 38% hydrogen peroxide in-office bleaching materials containing desensitizing agents. Oper Dent 37:464–473. https://doi.org/10.2341/11-337-C
Wiegand A, Schreier M, Attin T (2007) Effect of different fluoridation regimes on the microhardness of bleached enamel. Oper Dent 32:610–615. https://doi.org/10.2341/06-171
Chen HP, Chang CH, Liu JK, Chuang SF, Yang JY (2008) Effect of fluoride containing bleaching agents on enamel surface properties. J Dent 36:718–725. https://doi.org/10.1016/j.jdent.2008.05.003
Cavalli V, Rodrigues LK, Paes-Leme AF, Brancalion ML, Arruda MA, Berger SBGM (2010) Effects of bleaching agents containing fluoride and calcium on human enamel. Quintessence Int 41:157–165
da Costa Soares MUS, Araújo NC, Borges BCD, Sales WS, Sobral APV (2013) Impact of remineralizing agents on enamel microhardness recovery after in-office tooth bleaching therapies. Acta Odontol Scand 71:343–348. https://doi.org/10.3109/00016357.2012.681119
Alexandrino L, Gomes Y, Alves E, Costi H, Rogez H, Silva C (2014) Effects of a bleaching agent with calcium on bovine enamel. Eur J Dent 08:320–325. https://doi.org/10.4103/1305-7456.137634
Basting RT, Antunes EV, Turssi CP, do Amaral FL, Franca FM, Florio FM (2015) In vitro evaluation of calcium and phosphorus concentrations in enamel submitted to an in-office bleaching gel treatment containing calcium. Gen Dent 63:52–56
Furlan IS, Bridi EC, Amaral FLBD, França FMG, Turssi CP, Basting RT (2017) Effect of high- or low-concentration bleaching agents containing calcium and/or fluoride on enamel microhardness. Gen Dent 65:66–70
Cavalli V, Rosa DAD, Silva DPD et al (2018) Effects of experimental bleaching agents on the mineral content of sound and demineralized enamels. J Appl Oral Sci 26:26. https://doi.org/10.1590/1678-7757-2017-0589
Vieira-Junior W, Lima D, Tabchoury C, Ambrosano GMB, Aguiar FHB, Lovadino JR (2016) Effect of toothpaste application prior to dental bleaching on whitening effectiveness and enamel properties. Oper Dent 41:E29–E38. https://doi.org/10.2341/15-042-L
Tschoppe P, Neumann K, Mueller J, Kielbassa AM (2009) Effect of fluoridated bleaching gels on the remineralization of predemineralized bovine enamel in vitro. J Dent 37:156–162. https://doi.org/10.1016/j.jdent.2008.11.001
Cruz NVS, Pessan JP, Manarelli MM, Souza MDB, Delbem ACB (2015) In vitro effect of low-fluoride toothpastes containing sodium trimetaphosphate on enamel erosion. Arch Oral Biol 60:1231–1236. https://doi.org/10.1016/j.archoralbio.2015.05.010
Missel EMC, Cunha RF, Vieira AEM, Cruz NVS, Castilho FCN, Delbem ACB (2016) Sodium trimetaphosphate enhances the effect of 250 p.p.m. fluoride toothpaste against enamel demineralization in vitro. Eur J Oral Sci 124:343–348. https://doi.org/10.1111/eos.12277
Manarelli MM, Moretto MJ, Sassaki KT, Martinhon CC, Pessan JP, Delbem AC (2013) Effect of fluoride varnish supplemented with sodium trimetaphosphate on enamel erosion and abrasion. Am J Dent 26:307–312
Pancote LP, Manarelli MM, Danelon M, Delbem ACB (2014) Effect of fluoride gels supplemented with sodium trimetaphosphate on enamel erosion and abrasion: in vitro study. Arch Oral Biol 59:336–340. https://doi.org/10.1016/j.archoralbio.2013.12.007
Burgmaier GM, Schulze IM, Attin T (2002) Fluoride uptake and development of artificial erosions in bleached and fluoridated enamel in vitro. J Oral Rehabil 29:799–804. https://doi.org/10.1046/j.1365-2842.2002.00966.x
Basting RT, Rodrigues AL, Serra MC (2003) The effects of seven carbamide peroxide bleaching agents on enamel microhardness over time. J Am Dent Assoc 134:1335–1342. https://doi.org/10.14219/jada.archive.2003.0047
Vieira AEM, Danelon M, Camara DMD et al (2017) In vitro effect of amorphous calcium phosphate paste applied for extended periods of time on enamel remineralization. J Appl Oral Sci 25:596–603. https://doi.org/10.1590/1678-7757-2016-0513
Amaral JG, Pessan JP, Souza JAS, Moraes JCS, Delbem ACB (2018) Cyclotriphosphate associated to fluoride increases hydroxyapatite resistance to acid attack. J Biomed Mater Res B Appl Biomater 106:2553–2564. https://doi.org/10.1002/jbm.b.34072
Featherstone JDB, ten Cate JM, Shariati M, Arends J (1983) Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res 17:385–391. https://doi.org/10.1159/000260692
Kielbassa AM, Wrbas KT, Schulte-Mönting J, Hellwig E (1999) Correlation of transversal microradiography and microhardness on in situ-induced demineralization in irradiated and nonirradiated human dental enamel. Arch Oral Biol 44:243–251. https://doi.org/10.1016/S0003-9969(98)00123-X
Dalpasquale G, Delbem ACB, Pessan JP, Nunes GP, Gorup LF, Neto FNS, de Camargo ER, Danelon M (2017) Effect of the addition of nano-sized sodium hexametaphosphate to fluoride toothpastes on tooth demineralization: an in vitro study. Clin Oral Investig 21:1821–1827. https://doi.org/10.1007/s00784-017-2093-3
Danelon M, Garcia LG, Pessan JP, Passarinho A, Camargo ER, Delbem ACB (2019) Effect of fluoride toothpaste containing nano-sized sodium hexametaphosphate on enamel remineralization: an in situ study. Caries Res 53:260–267. https://doi.org/10.1159/000491555
Delbem ACB, Souza JAS, Zaze ACSF, Takeshita EM, Sassaki KT, Moraes JCS (2014) Effect of trimetaphosphate and fluoride association on hydroxyapatite dissolution and precipitation in vitro. Braz Dent J 25:479–484. https://doi.org/10.1590/0103-6440201300174
Funding
This study was supported by FAPESP (The State of São Paulo Research Foundation, grant 2016/26132-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors Alberto Carlos Botazzo Delbem and Mirela Sanae Shinohara and hold a patent request for a product used in the study, by the National Institute of Industrial Property—INPI/SP, on 11 Nov. 2014 under number BR 102013 006761-0 A2.
Ethical approval
This paper does not contain any studies with human subjects, so that no ethical approval was required.
Informed consent
Same as above.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Júnior, R.A.T.P., Danelon, M., Pessan, J.P. et al. Effect of daily use of fluoridated dentifrice and bleaching gels containing calcium, fluoride, or trimetaphosphate on enamel hardness: an in vitro study. Clin Oral Invest 25, 883–889 (2021). https://doi.org/10.1007/s00784-020-03375-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03375-5