Abstract
Objective
This study evaluated the effect of toothpastes containing 1100 ppm F associated with nano-sized sodium hexametaphosphate (HMPnano) on enamel demineralization in vitro using a pH-cycling model.
Design
Bovine enamel blocks (4 mm × 4 mm, n = 72) selected by initial surface hardness (SHi) were randomly allocated into six groups (n = 12), according to the test toothpastes: without fluoride or HMPnano (Placebo), 550 ppm F (550F), 1100 ppm F (1100F), 1100F plus HMPnano at concentrations of 0.25% (1100F/0.25%HMPnano), 0.5% (1100F/0.5%HMPnano), and 1.0% (1100F/1.0%HMPnano). Blocks were treated 2×/day with slurries of toothpastes and submitted to five pH cycles (demineralizing/remineralizing solutions) at 37 °C. Next, final surface hardness (SHf), integrated loss subsurface hardness (ΔKHN), integrated mineral loss (gHAp × cm−3), and enamel fluoride (F) concentrations were determined. Data were analyzed by ANOVA and Student-Newman-Keuls test (p < 0.001).
Results
Toothpaste with 1100F/0.5%HMPnano led to the lowest mineral loss and the highest mineral concentration among all groups, which were 26% (SHf) and 21% (ΔKHN) lower and ~58% higher (gHAp × cm−3) when compared to 1100F (p < 0.001). Similar values of enamel F were observed for all fluoridated toothpastes (p > 0.001).
Conclusion
The addition of 0.5%HMPnano to a 1100 F toothpaste significantly enhances its effects against enamel demineralization when compared to its counterpart without HMPnano in vitro.
Clinical significance
Toothpaste containing 1100 ppm F associated with HMPnano has a higher potential to reduce the demineralization compared to 1100 ppm F. This toothpaste could be a viable alternative to patients at high risk of caries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the widespread use of fluorides (F) in different vehicles and modes of application, dental caries is still a very common chronic disease during childhood [1], affecting approximately a quarter of all children and negatively influencing their oral and general health and their quality of life [2, 3] Considering the benefits and extensive use of F toothpastes [4, 5], the increase of their efficacy would be extremely advantageous, especially for individuals with high caries activity [6, 7]. Among the strategies available, the supplementation of toothpastes with organic and inorganic phosphates has been intensively investigated over recent years.
The addition of sodium trimetaphosphate (TMP) to toothpastes containing 500 ppm F [7, 8] or 1100 ppm F [9] was shown to promote a synergistic effect against enamel demineralization using in vitro and in situ protocols. Besides TMP, sodium hexametaphosphate (HMP) has also been shown to enhance the effect of toothpastes containing 250 ppm F [10] or 1100 ppm F [11, 12]. Such effects occur due to specific characteristics of this phosphate, such as its capacity to form complexes with cations and its ability in reducing enamel solubility.
Currently, nano-sized phosphates emerge as an innovative method, aiming to optimize the effect of the F toothpaste on remineralization processes [6]. The effect of nanoparticles is a result of their superior physical and chemical properties when compared to the above-mentioned micrometric phosphates [13]. In this sense, it was recently shown that the addition of nano-sized TMP was more effective to promote enamel remineralization in situ when compared to formulations supplemented with micrometric TMP or not supplemented with any phosphate [6].
Given the positive results obtained by the addition of nano-sized TMP on enamel remineralization and considering the absence of studies assessing the effects of nano-sized sodium hexametaphosphate (HMPnano), the aim of this study was to evaluate the effect of toothpastes containing 1100 ppm F associated with HMPnano on enamel demineralization in vitro using a pH cycling model. The study’s null hypothesis was that the effect of the toothpastes on enamel demineralization would not be influenced by the addition of HMPnano.
Materials and methods
Experimental design
Enamel blocks (4 mm × 4 mm, n = 72) were obtained from bovine incisors kept in formaldehyde 2%, pH 7.0, for 30 days prior to experimental procedures [14]. The enamel surfaces were sequentially polished using 600, 800, and 1200-grade water-cooled silicon carbide paper disks (Buehler), with a final polish using a felt disk (Buehler Polishing Cloth 40–7618) moistened with a 1-μm diamond polishing suspension (Extec Corp., Enfield,CT, USA), removing ~120 μm of the outer enamel (measurement performed with a digital caliper). Blocks were selected by initial surface hardness test (SHi; 320 to 380 KHN) and randomly divided in six experimental toothpastes (n = 12 each), according to the 95% confidence interval. They were submitted to a pH-cycling regimen (5 days) and to treatments (twice a day) with slurries of the following toothpastes: without fluoride or HMPnano (Placebo), 550 ppm F (550F), 1100 ppm F (1100F), 1100F plus HMPnano at concentrations of 0.25% (1100F/0.25%HMPnano), 0.5% (1100F/0.5%HMPnano), and 1.0% (1100F/1.0%HMPnano). Next, final surface harness (SHf), integrated loss subsurface hardness (ΔKHN), enamel mineral concentration (gHAp × cm−3), and enamel fluoride (F) concentrations were determined.
Synthesis and characterization of nano-sized sodium hexametaphosphate
The synthesis and characterization of nano-sized HMP was based on study of Danelon et al. [6]. In short, 70 g of pure sodium hexametaphosphate (((NaPO3)6) (average size of 31 ± 33 μm), Aldrich, purity ≥95% CAS 7785-84-4, UK) was ball milled using 500 g of zirconia spheres (diameter of 2 mm) in 1 l of hexane. After 48 h, the material was filtered and sealed with aluminum foil, and the vials were dried at 75 °C to evaporate the hexane.
X-ray diffraction (XRD) was used to identify the crystalline structure and estimating the crystallographic coherency domain of HMP, and milled for 48 h (HMPnano). The X-ray diffractograms were obtained from samples in powder form, using Shimadzu XRD 6000 equipment with CuK radiation source (λ = 1.54056 Å), voltage 30 kV, and current of 30 mA. Measurements were made continuously, in the range of 10 ° ≤ 2θ ≤ 80 °, a 2 ° sweep speed/min. The structural identification of the samples was done by comparing the diffraction patterns obtained with tabulated patterns available in databases “Joint Committee on Powder Diffraction Standards - Powder Difraction File (JCPDS - PDF).” The particle morphology of HMP and HMP milled for 48 h (HMPnano) was analyzed by scanning electron microscopy (SEM). The SEM images were collected using a Philips XL-30 FEG.
Toothpaste formulation and fluoride and pH assessment
Toothpastes were produced in the laboratory of Pediatric Dentistry School of Dentistry, Araçatuba (Unesp, Brazil), with the following components: titanium dioxide, carboxymethyl cellulose, sodium methyl-p-hydroxybenzoate, sodium saccharin, mint oil, glycerine, abrasive silica, sodium lauryl sulfate, and water [11]. Toothpastes containing 1100 ppm F (as NaF, Merck, CAS 7681-49-4, Germany) and nano-sized HMP at concentrations of 0.25, 0.5, and 1.0% were prepared. Also, a Placebo toothpaste (without F or HMPnano) and toothpastes with 550 ppm F (550F) and 1100 ppm F (1100F) were prepared with the same ingredients the other toothpastes. The total (TI) and ionic (IF) concentrations of fluoride and the pH of the toothpastes were determined in triplicate [15, 16] prior to the beginning of the study (Table 1).
pH-cycling and treatment with toothpastes
Prior to pH cycling, two layers of acid-resistant nail varnish (Risqué®-Brazil) were applied on the sides (cut surfaces) and on the bottom of each block. Next, the blocks were submitted, in individual vials, to five pH cycles during 5 days (1 cycle/day), and immersed in a fresh remineralizing solution for two additional days [17]. They were immersed under constant stirring in suspensions of toothpastes and deionized water (1:3-weight:weight) when removed from the demineralizing (6 h—Ca and P 2.0 mmol l−1 in acetate buffer 0,075 mol l−1, 0.04 μg F/ml in pH 4.7–2.2 ml/mm2) and from the remineralizing solutions (18 h—Ca 1.5 mmol l−1, P 0.9 mmol l−1, KCl 0.15 mol l−1 in cacodylic buffer 0.02 mol l−1, 0.05 μg F/ml in pH 7.0–1.1 ml/mm2). The blocks were washed with jets of deionized water for 30 s whenever blocks were removed from the pH-cycling solutions or from the toothpaste suspensions.
Analysis of enamel hardness
Surface hardness was determined with Micromet 5114 hardness tester (Buehler, Lake Bluff, USA) and Buehler Omni Met software (Buehler, Lake Bluff, USA) with a Knoop diamond indenter under a 25 g load for 10 s. Five indentations, separated by a distance of 100 μm, were made in the center of each block to analyze initial surface hardness (SHi). After the pH-cycling, final surface hardness (SHf) was calculated by producing five other indentations (100 μm from the baseline indentations). For cross-sectional hardness measurements, blocks were sectioned at the center and one of the halves was included in acrylic resin and gradually polished until the enamel was totally exposed. One sequence of 14 indentations was created at different distances (5, 10, 15, 20, 25, 30, 40, 50, 70, 90, 110, 130, 220, and 330 μm) from the surface of the enamel, in the central region of the blocks, using a Micromet 5114 hardness tester (Buehler Lake Bluff, IL, USA) with a Knoop diamond indenter under a 5-g load for 10 s. Integrated hardness (KHN × μm) for the lesion into sound enamel was calculated by the trapezoidal rule (GraphPad Prism, version 3.02) and subtracted from the integrated hardness for sound enamel to obtain the integrated area of the subsurface regions in enamel, which was named integrated loss of subsurface hardness (ΔKHN; KHN × μm) [18].
Analysis of enamel mineral concentrations
Samples (n = 10/group, 1 mm × 1 mm) were analyzed by micro-computed tomography (MicroCT) operated at 70 kV, 142 mA, aluminum filter of 0.5 mm, at 1.5 mm of spatial resolution, rotation step at 0.400° and random moviment at 10. The projections of the images were rebuilt using the NRecon software (version 1.6.10.2, Skyscan1272, Bruker Micro-CT) and smoothing at 5, ring artifact correction at 7, beam hardening correction at 50%. Following image reconstruction, two-dimension virtual slices in the sagittal and coronal plane were acquired using the Data Viewer software (Skyscan1272). The stacked 2D was imported into ImageJ software to produce an overall mineral concentration (gHAp × cm−3) profile in function of the depth (μm). The mineral concentrations were calculated from of the linear attenuation coefficient (LAC) and expressed as the mass of pure hydroxyapatite (ρ = 3.15 g × cm−3) per unit volume of tissue (gHAp × cm−3) [19, 20].
The integrated area above the curve (cross-sectional profiles of mineral concentration into the enamel), using the mineral concentration values (gHAp × cm−3), was calculated by trapezoidal rule (GraphPad Prism, version 3.02) in each depth (μm) from the lesion up to sound enamel. This value was subtracted from integrated area of sound enamel, to obtain the integrated area of the subsurface regions in enamel, which was named integrated mineral loss (IML).
Analysis of F concentration in the enamel
Blocks (n = 12/group, 2 mm × 2 mm) were obtained from the halves of the original 4 mm × 4 mm specimens not embedded, and fixed with adhesive glue on a mandrel for straight. Self-adhesive polishing disks (diameter, 13 mm) and 400-grit silicon carbide (Buehler) were fixed to the bottom of polystyrene crystal tube (J-10; Injeplast, Sao Paulo, SP, Brazil). One layer of 50.0 ± 0.03 μm each enamel block was removed. The vials, after the addition of 0.5 ml HCl 1.0 mol l−1, were kept under constant stirring for 1 h [21, 22]. For F analysis, specific electrode 9409BN (Thermo Scientific, Beverly, MA, USA) and microelectrode reference (Analyser, São Paulo, Brazil) coupled to an ion analyzer (Orion 720A+, Thermo Scientific, Beverly, MA, USA) was used. The electrodes were calibrated with standards containing from 0.25 to 4.00 μg F/ml (100 ppm F, Orion 940,907), under the same conditions as the samples. The readings were conducted using 0.25 ml of the biopsy solution, buffered with the same volume of TISAB II modified NaOH 1.0 mol l−1. The results were expressed in μg/mm3.
Statistical analysis
For statistical analysis, SigmaPlot software version 12.0 (SigmaPlot, Systat Software Incorporation, San Jose, CA, USA) was used, and the significance limit was set at 5%. The data presented normal (Shapiro-Wilk test) and homogenous (Cochran test) distribution. Data from SHf, ΔKHN and F (log transformation) and gHAp × cm−3 (square root transformation) were submitted to one-way analysis of variance followed by Student-Newman-Keuls test. Pearson’s correlation coefficients between ΔKHN and gHAp × cm−3 were also calculated.
Results
Table 1 presents mean (SD) total and ionic F concentrations, as well as mean pH values of the toothpastes. F concentrations in the products presented only small variations around the expected values (lower than 8%). Similarly, mean pH of toothpastes presented variations (ranging from 6.8 to 7.7).
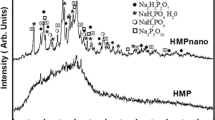
The X-ray diffraction pattern (XRD) of commercial micrometric HMP in Fig. 1 shows few and broad peaks, revealing a material of low crystallinity. On the other hand, the XRD of nano-sized HMP after ball milling for 48 h (HMPnano) shows a mixture of several crystalline polymorphs of phosphates, as assigned in Fig. 1. Grinding altered HMP, causing particles to fragment, and reducing their specific surface area and size distribution. It also induced structural changes such as crystallization of the material, as seen in micrographs of samples in Fig. 2a, which shows the SEM images of HMP with large aggregates and particles of smaller sizes (average size of 31 ± 33 μm). Figure 2b shows MEV images of HMPnano particles with low size distribution and average size of 91 ± 34 nm.
X-ray diffraction patterns of HMP before (HMP) and after (HMPnano) grinding of powder for 48 h in ball mill. The possible phases (NaPO3)6 PDF# 3643 Sodium hexametaphosphate, NaPO3 PDF# 76788 Sodium metaphosphate , Na2H2P2O7 PDF# 10187 Disodium dihydrogen diphosphate, NaH2PO4 (H2O) DF#11651 Sodium Dihydrogen Phosphate Monohydrate, NaH2PO4 PDF#11657 Sodium dihydrogen phosphate and Na5P3O10 PDF# 11652 Pentasodium triphosphate
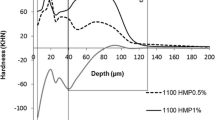
Mean (SD) SHi considering all blocks was 375 (1.2) KHN. No significant differences were observed among the groups after random allocation (p = 0.474). SHf of blocks treated with 550F and 1100F were approximately 182 and 306% higher compared to Placebo (p < 0.001). The addition of 0.5% HMPnano to 1100F led to SHf ~26% higher when compared with1100F (p < 0.001), while concentrations of 0.25% and 1.0% were shown to significantly reduce the protective effect of the toothpastes when compared with 1100F (Table 2). A similar pattern was observed for the integrated loss subsurface hardness. ΔKHN values were approximately 17 and 60% lower, respectively, for 550F and 1100F, when compared with the placebo. The addition of 0.5%HMPnano to 1100F significantly reduced the ΔKHN when compared with 1100F (p < 0.001), while HMP at 0.25 and 1.0% were shown to increase subsurface hardness loss by approximately 13 and 34% when compared with 1100F (p < 0.001), (Table 2).
Table 2 shows the mean IML (gHAp × cm−3 × μm) for the different treatments obtained from the profile of mineral concentration in function of depth (Fig. 3). Significantly higher IML (~58%) was observed in enamel treated with 1100F/0.5%HMPnano compared to 1100F (p < 0.001); no significant differences were observed among groups treated with 550F, 1100F/0.25%HMPnano and 1100F/1.0%HMPnano (p > 0.001). Positive and significant correlations were observed between ΔKHN and IML (Pearson’s r = 0.874; p < 0.001) (Fig. 4).
A dose-response pattern was observed between F concentrations in the toothpastes not supplemented with HMPnano and enamel fluoride uptake. The supplementation of 1100F with HMPnano did not significantly alter enamel fluoride levels (p > 0.001) (Table 2).
Discussion
To optimize conventional F toothpastes (1100 ppm F), this study evaluated the efficacy of a 1100 ppm F toothpaste associated with different concentrations of HMP (administered as nano-sized particles) in reducing enamel demineralization. The results showed that HMPnano added at concentration of 0.5% promoted a superior efficacy when compared with a conventional toothpaste, leading to the rejection of the study’s null hypothesis.
The evaluation of the dose-response relationship is an important step in assessing the effects of any supplement added to toothpastes, as well as to validate the experimental protocol used [23]. Such relationship was indeed confirmed, given that data from Placebo, 550F and 1100F were dose-dependent for all variables assessed (Table 2). Anbar et al. [24] demonstrated that the adsorption of a polyphosphate to enamel surface is highly fast and can compete with the absorption of ionic F, which would, in turn, decrease F diffusion into the enamel, thus leading to greater mineral loss. The addition of HMPnano at concentrations of 0.25 to 1.0%, however, did not affect enamel F concentrations, suggesting that the presence of HMPnano does not impair enamel F uptake, in opposition to the findings of Anbar et al. [24]. Moreover, although the 1100F/0.5HMPnano toothpaste had a slightly lower pH than the other toothpastes, it is noteworthy that it was very close to a neutral value, so that it is likely that it had negligible or no effect on the response variables assessed.
HMPnano concentrations tested in this study were based on F/HMP ratio from previous studies showing that the addition of micrometric particles of HMP at 1.0% to a 1100 ppm F toothpaste promoted a higher effect against enamel demineralization when compared with its counterpart without HMP [11, 12]. Previous in vitro and in situ studies using higher HMP concentrations, on the other hand, failed to demonstrate a synergistic effect between fluoride and HMP. Pfarrer et al. [25] observed in an in vitro study using pH cycling that treatment with 1100 ppm F toothpaste supplemented with 7% HMP resulted in integrated mineral loss equivalent to that of Crest® and no significant difference in the incorporation of F by enamel. In an in situ study, Wefel et al. [26] reported that toothpastes containing 0.243% NaF with 7% HMP or 0.454% SnF2 with 13% HMP showed results similar to the respective controls without HMP with respect to enamel lesion analyzed by polarized light microscopy. All of these studies evaluated the effect of HMP at high concentrations, which differ from the studies by da Camara et al. [11, 12], in which HMP was added at 1% to a 1100 ppm F toothpaste, promoting a synergistic effect on enamel demineralization both in vitro and in situ. However, as the effect of HMPnano (as well as other phosphates) is related to its ability to adsorb on the enamel, the study clearly demonstrates that the HMPnano:F molar ratio has a strong influence on the reducing enamel demineralization effect.
In the present study, the addition of 0.5%HMPnano to a 1100 ppm F toothpaste significantly increased surface hardness (~26%) and reduced subsurface hardness (~21%) in comparison with 1100F, justifying the use of nano-sized HMP. The procedure used to synthesize HMPnano promoted more reactive particles with increased adsorption on enamel, due to the reduction in size and increase in surface area (in proportion to its volume), which leads to a higher number of atoms on the surface (Fig. 2). Also, the effect of 0.5%HMPnano concentration on ΔKHN suggests that, under clinical conditions, the subsurface lesion would take longer to develop when compared to conventional toothpaste.
Besides the analysis of enamel by traditional hardness measurements (surface and cross sectional), the use of micro-computed tomography (microCT) was of great importance to explain how the treatments modified the mineralization patterns throughout the subsurface lesions, and how these patterns were influenced by treatments with F, especially when associated with HMPnano. In the analysis of integrated mineral loss (IML, gHAp × cm3) by microCT at different depths, mineral profiles varied widely among the treatment groups; the 1100F/0.5%HMPnano toothpaste promoted the lowest IML, approximately 58% lower than 1100F group (Table 2). The mineral profile of the lesion in groups treated with F/HMPnano was different in relation to the group containing only F, confirming previous findings that the F and HMP have a different action mode on the dynamics of caries process (Fig. 3) [10, 11].
The present findings can also be explained by the characteristics of the studied salt. HMP is considered a non-hydrolyzable and cyclic phosphate [27, 28] which forms strong complexes with metal ions [29, 30] in the oral environment. Based in previously studies [11, 12], HMP interacts with the enamel and adsorbed into the enamel surface retain charged ions (CaF+ and Ca2+) through the replacement of Na+ in the cyclic structure, leading to a reticular formation [31] through the binding of Ca2+ with one or more HMP molecules. As a result of these multiple connections, HMP molecules form a layer of condensed phosphates, changing the selective permeability of the enamel [32] and, in this case, increasing cation selectivity. These data are in agreement with findings of da Camara et al. [11] showing that the ionic activity of species such as CaF+ and Ca2+, as well as neutral species of HF0 and CaHPO4 0 in the dental biofilm formed in situ was significantly higher when compared to the 1100 ppm F toothpaste.
Although it is well known that the pH-cycling models are appropriate for evaluating the impact of new active ingredients in fluoride toothpastes as well as their association with other anticaries treatments, in vitro protocols have limitations, including (1) the absence of variables that mimic intra oral conditions and interaction of HMPnano with the enamel surfaces, (2) the faster periods of de/remineralization when compared to in vivo conditions, and (3) the lower reactivity of fluoride than in vivo [33]. Moreover, this study did not include a toothpaste supplemented with conventional (micrometric) HMP, what does not allow the determination of the additional benefit of nano-sized particles over their micrometric counterparts. Therefore, the results should be evaluated with caution, and future in situ and clinical studies should be conducted in order to better understand the effect of this cyclic phosphate on the de/remineralization processes.
To sum up, the addition of 0.5%HMPnano to a 1100F toothpaste significantly enhances its effects against enamel demineralization when compared to its counterpart without HMP under in vitro conditions.
References
Nunes AM, da Silva AA, Alves CM, Hugo FN, Ribeiro CC (2014) Factors underlying the polarization of early childhood caries within a high-risk population. BMC Public Health 14:988
Ferreira SH, Béria JU, Kramer PF, Feldens EG, Feldens CA (2007) Dental caries in 0- to 5-year-old Brazilian children:prevalence, severity, and associated factors. Int J Paediatr Dent 17:289–296
Tomar SL, Reeves AF (2009) Changes in the oral health of US children and adolescents and dental public health infrastructure since the release of the healthy people 2010 objectives. Acad Pediatr 9:388–395
Rølla G, Ogaard B, Cruz Rde A (1991) Clinical effect and mechanism of cariostatic action of fluoride-containing toothpastes: a review. Int Dent J 41:171–174
Vanichvatana S, Auychai P (2013) Efficacy of two calcium phosphate pastes on the remineralization of artificial caries: a randomized controlled double-blind in situ study. Int J Oral Sci 5:224–228
Danelon M, Pessan JP, Neto FN, de Camargo ER, Delbem AC (2015) Effect of toothpaste with nano-sized trimetaphosphate on dental caries: in situ study. J Dent 43:806–813
Takeshita EM, Danelon M, Castro LP, Sassaki KT, Delbem AC (2015) Effectiveness of a toothpaste with low fluoride content combined with trimetaphosphate on dental biofilm and enamel demineralization in situ. Caries Res 49:394–400
Takeshita EM, Castro LP, Sassaki KT, Delbem AC (2009) In vitro evaluation of dentifrice with low fluoride content supplemented with trimetaphosphate. Caries Res 43:50–56
de Castro LP, Delbem ACB, Danelon M, Passarinho A, Percinoto C (2015) In vitro effect of sodium trimetaphosphate additives to conventional toothpastes on enamel demineralization. Clin Oral Investig 19:1683–1687
da Camara DM, Miyasaki ML, Danelon M, Sassaki KT, Delbem AC (2014) Effect of low-fluoride toothpastes combined with hexametaphosphate on in vitro enamel demineralization. J Dent 42:256–262
da Camara DM, Pessan JP, Francati TM, Santos Souza JA, Danelon M, Delbem AC (2015) Synergistic effect of fluoride and sodium hexametaphosphate in toothpaste on enamel demineralization in situ. J Dent 43:1249–1254
da Camara DM, Pessan JP, Francati TM, Souza JA, Danelon M, Delbem AC (2016) Fluoride toothpaste supplemented with sodium hexametaphosphate reduces enamel demineralization in vitro. Clin Oral Investig
Xu HH, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC et al (2010) Strong nanocomposites with Ca, PO(4) and F release for caries inhibition. J Dent Res 89:19–28
Delbem AC, Cury JA (2002) Effect of application time of APF and NaF gels on microhardness and fluoride uptake of in vitro enamel caries. Am J Dent 15:169–172
Delbem AC, Sassaki KT, Vieira AE, Rodrigues E, Bergamaschi M, Stock SR et al (2009) Comparison of methods for evaluating mineral loss: hardness versus synchrotron microcomputed tomography. Caries Res 43:359–365
Moretto MJ, Magalhaes AC, Sassaki KT, Delbem AC, Martinhon CC (2010) Effect of different fluoride concentrations of experimental dentifrices on enamel erosion and abrasion. Caries Res 44:135–140
Vieira AE, Delbem AC, Sassaki KT, Rodrigues E, Cury JA, Cunha RF (2005) Fluoride dose response in pH-cycling models using bovine enamel. Caries Res 39:514–520
Spiguel MH, Tovo MF, Kramer PF, Franco KS, Alves KM, Delbem AC (2009) Evaluation of laser fluorescence in the monitoring of the initial stage of the de−/remineralization process: an in vitro and in situ study. Caries Res 43:302–307
Dowker SE, Elliott JC, Davis GR, Wassif HS (2003) Longitudinal study of the three-dimensional development of subsurface enamel lesions during in vitro demineralization. Caries Res 37:237–245
Dowker SE, Elliott JC, Davis GR, Wilson RM, Cloetens P (2004) Synchrotron X-ray microtomographic investigation of mineral concentrations at micrometer scale in sound and carious enamel. Caries Res 38:514–522
Weatherell JA, Robinson C, Strong M, Nakagaki H (1985) Micro-sampling by abrasion. Caries Res 19:97–102
Alves KM, Pessan JP, Brighenti FL, Franco KS, Oliveira FA, Buzalaf MA, Sassaki KT, Delbem AC (2007) In vitro evaluation of the effectiveness of acidic fluoride dentifricies. Caries Res 41:263–267
ten Cate JM, Mundorff-Shrestha SA (1995) Working group report 1: laboratory models for caries (in vitro and animal models). Adv Dent Res 9:332–334
Anbar M, Farley EP, Denson DD, Maloney KR (1979) Localized fluoride release from fluorine-carrying polyphosphonates. J Dent Res 58:1134–1145
Pfarrer AM, White DJ, Rapozo-Hilo FID (2002) Anticaries and hard tissue abrasion effects of a “dual action” whitening, sodium hexametaphosphate tartar control dentifrice. J Clin Dent 13:50–54
Wefel JS, Stanford CM, Ament DK, Hogan MM, Harless JD, Pfarrer AM, Ramsey LL, Leusch MS, Biesbrock AR (2002) In situ evalution of sodium hexametaphosphate-containing dentifrices. Caries Res 36:122–128
Choi IK, Wen WW, Smith RW (1993) Technical note the effect of a long chain phosphate on the adsorption of collectors on kaolinite. Mineral En 6:1191–1197
Castellini E, Lusvardi G, Malavasi G, Menabue L (2005) Thermodynamic aspects of the adsorption of hexametaphosphate on kaolinite. J Colloid Interface Sci 292:322–329
Andreola F, Castellini E, Manfredini T, Romagnoli M (2004) The role of sodium hexametaphosphate in the dissolution process of kaolinite and kaolin. The Journal of the European Ceramic Society 24:2113–2124
Cochrane NJ, Saranathan S, Cai F, Cross KJ, Reynolds EC (2008) Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res 42:88–97
van Wazer JR, Campanella DA (1950) Structure and properties of the condensed phosphates. IV Complex ion formation in polyphosphate solutions Journal of the American Chemical Society 72:655–663
van Dijk JW, Borggreven JM, Driessens FC (1980) The effect of some phosphates and a phosphonate on the electrochemical properties of bovine enamel. Arch Oral Biol 25:591–595
Buzalaf MA, Hannas AR, Magalhães AC, Rios D, Honório HM, Delbem AC (2010) pH-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: strengths and limitations. J Appl Oral Sci 18:316–334
Acknowledgements
We thank CAPES (Coordination for the Improvement of Higher Education Personnel) and FAPESP (The State of São Paulo Research Foundation, 2014/06676-9) for the concession of a scholarship to the first and fourth authors, respectively. The authors also thank the Multi-user laboratory of FOA-UNESP and to FINEP (FINEP/CT-INFRA—Agreement FINEP: 01.12.0530.00—PROINFRA 01/2011) for the use of the high-resolution micro-computed tomography equipment (SkyScan 1272 Model).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors Marcelle Danelon, Alberto Carlos Botazzo Delbem, Juliano Pelim Pessan, and Emerson Rodrigues de Camargo hold a patent request for a product used in the study, by the National Institute of Industrial Property—INPI/SP, on 04/29/2008 under number 018080026091, PI0801811-1, and published on January 11, 2011. All authors approved the publishing of the manuscript.
Funding
This study was funded by the CAPES and FAPESP (scholarships to the first and fourth authors, respectively).
Ethical approval
This paper does not contain any studies with human subjects, so that no ethical approval was required.
Informed consent
Same as above.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00784-017-2116-0.
Rights and permissions
About this article
Cite this article
Dalpasquale, G., Delbem, A.C.B., Pessan, J.P. et al. Effect of the addition of nano-sized sodium hexametaphosphate to fluoride toothpastes on tooth demineralization: an in vitro study. Clin Oral Invest 21, 1821–1827 (2017). https://doi.org/10.1007/s00784-017-2093-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2093-3