Abstract
Objective
Compositional modifications may alter the biological and physicochemical characteristics of calcium silicate-based sealers (CSBS) and, ultimately, their bioactivity. The main objective of this study was to evaluate the biological properties of three CSBS: EndoSequence BC Sealer, Ceraseal, and Endoseal mineral trioxide aggregate.
Materials and methods
Human periodontal ligament stem cells (hPDLSCs) were exposed to several eluates of CSBS. The ion release profile and pH were determined, and metabolic activity and cell migration were assessed using the MTT and wound healing assays. hPDLSCs were cultured in direct contact with the surface of each material, and cell morphology and attachment were analyzed by scanning electron microscopy (SEM). Bioactivity potential was assessed by RT-qPCR and mineralization assays. Statistical differences between biomaterials were assessed using one- or two-way ANOVA (α < 0.05).
Results
All materials showed an alkaline pH, although Endoseal exhibited a significantly higher pH compared with the other CSBS (p < 0.05). Ceraseal released significantly more Ca2+ (p < 0.05) than EndoSequence BC Sealer and Endoseal. Interestingly, Endoseal induced a significant reduction in cell viability and cell migration compared with the control (p < 0.001). Moreover, SEM showed abundant cells adhering to EndoSequence BC Sealer and Ceraseal surfaces, whereas very few round cells were detected on the surface of Endoseal. Finally, Ceraseal and EndoSequence induced ALP, CAP, and CEMP-1 expression and a significantly higher mineralization capacity than Endoseal (***p < 0.001).
Conclusions
The eluates from EndoSequence BC Sealer and Ceraseal displayed higher cell viability, cell attachment, cell migration rates, and ion release rates than Endoseal. Ceraseal and EndoSequence BC Sealer exhibited significantly more gene expression and mineralization capacity than Endoseal.
Clinical relevance
The results obtained in the present work suggest that EndoSequence BC Sealer and Ceraseal possess biological properties that make them suitable materials for root canal treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the first clinically approved formulation of mineral trioxide aggregate (MTA), several products have been developed for use in clinical procedures including endodontic sealers [1, 2]. New formulations and additives have been proposed to improve problems such as working time, cost, and handling difficulties [3]. These new sealers, named calcium silicate-based sealers (CSBS), also facilitate biomineralization [4], the process by which a living organism synthesizes mineral substance. One important challenge facing endodontic today is precisely the correct formation of hard mineral tissue. To achieve this, it is essential that the materials used in endodontic treatments be bioactive [5, 6]. Bioactivity involves the synthesis of calcium phosphate deposits on the material surface placed in a mimic body fluid, e.g., a buffer solution with an ion concentration similar to the values found in human blood plasma [7].
Compositional modifications could alter the biological and physicochemical characteristics of CSBS and finally their bioactivity [8]. To predict and elucidate potential clinical behaviors of such materials, their biocompatibility and cytocompatibility are usually tested both in vivo and in vitro models [9, 10]. As bioactive materials are assumed to directly interact with pulp and/or periapical cells, or through the diffusion of components within the living periradicular tissue, it is critical to assess their biocompatibility in order to ascertain their potential influence in reparative/regenerative responses [11]. Stem cells from periodontal ligament (hPDLSCs) are considered a good model for cytotoxicity studies involving endodontic sealers because these cells may be in direct contact with unintentional sealer extrusions [12]. hPDLSCs have also been found to have multidirectional differentiation abilities and could be induced into cementoblast-like cells, osteoblast-like cells, adipogenic-like cells, and chondrogenic-like cells. When transplanted into immunocompromised mice or rats, hPDLSCs were seen to generate a cementum/PDL-like structure, contributing to apical healing [13].
EndoSequence BC Sealer (BC; Brasseler USA, Savannah, GA, USA) is a new premixed, injectable CSBS, whose major components include tricalcium silicates, dicalcium silicates, calcium hydroxide, zirconium oxide, and thickening agents. As a sealer, it possesses good biocompatibility and antimicrobial activities, is easy to handle, and has the ability to promote the osteoblastic differentiation of periodontal ligament cells, although our knowledge of this sealer is still limited [14, 15]. Endoseal MTA (ES; Maruchi, Wonju, Korea) is another premixed CSBS that has shown adequate physicochemical properties, good sealing ability, and good bond strength performance, although the reports on its cytocompatibility are contradictory [16, 17]. It contains calcium silicates, calcium sulfates, calcium aluminates, radiopacifier, and thickening agent. Ceraseal (Meta Biomed Co., Cheongju, Korea) is a newly launched premixed endodontic sealer containing calcium silicates, zirconium oxide, and thickening agent. To date, however, there have been no published studies using this new endodontic sealer.
Considering the relevance of the biocompatibility of endodontic sealers for successful root canal treatment, the aim of this work was to test the cytocompatibility, bioactivity, and ion release of these three premixed CSBS. The null hypothesis was that there would be no significant differences in the biological properties among the studied CSBS.
Material and methods
Material extracts
The CSBS used in this study were EndoSequence BC Sealer (Brasseler USA, Savannah, GA, USA), Ceraseal (Meta Biomed Co., Cheongju, Korea), and Endoseal MTA (Maruchi, Wonju, Korea). Their chemical compositions, as supplied by the manufacturers, are listed in Table 1.
Under aseptic conditions, the sealers were mixed according to the manufacturers’ recommendations, placed in cylindrical molds of 2-mm height and 5-mm diameter, and stored in a dark container at 37 °C for 48 h to allow complete setting (n = 30). After this period, sample disks were stored in the culture medium (DMEM) for 24 h at 37 °C, 5% CO2, and humid atmosphere. This procedure was carried out according to the International Organization for Standardization (ISO) guideline 10993-12, and the ratio of the specimen surface area was 1.5 cm2/mL (ISO 10993-5). The extracts obtained were filtered and diluted (undiluted, 1:2, 1:4) before being used in the MTT assay, migration, qPCR analysis, and Alizarin Red experiments.
Isolation and culture of hPDLSCs
Multipotent PDLSCs were isolated from human periodontal ligament tissue (n = 5) as described previously [18]. Informed consent was obtained from all donors, and the experiment was approved by the Ethical Committee of the Faculty of Medicine, University of Murcia, following the Helsinki Declaration guidelines. Upon tooth extraction, periodontal ligament tissue was enzymatically digested with collagenase A for 1 h at 37 °C. The cell suspension was gently mixed every 15 min to facilitate dissociation of the tissue. Then, cells were seeded into T-25-cm2 flasks in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Gibco), 100 U/mL penicillin G, and 100-mg/mL streptomycin (Gibco).
Ion release analysis and pH
Three samples from each material type measuring 5-mm diameter and 2-mm height were prepared in 5 mL Milli-Q water, and the presence of aluminum, silicon, sulfur, calcium, and zirconium was assessed using inductively coupled plasma mass spectrometry (ICP-MS Agilent 7900, Stockport, UK). The pH of the different extracts was determined using a twin pH meter (GLP21+, Crison, Barcelona, Spain). Results are represented as the mean ± standard deviation.
Analysis of expression of mesenchymal immunophenotype and multipotential differentiation properties
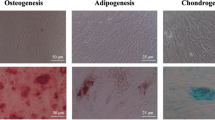
The immunophenotype of hPDLSCs was evaluated by flow cytometry after passage 2, and the expression of mesenchymal stem cell surface markers was analyzed using flow cytometry. Briefly, hPDLSCs were washed with PBS and incubated in the dark for 15 min at 4 °C with the following antibodies: APC-conjugated anti-CD73, FITC-conjugated anti-CD90, PE-conjugated anti-CD105, and PerCP-conjugated anti-CD34, CD45, CD14, and CD20 (Miltenyi Biotec, Bergisch Gladbach, Germany). After labeling, cells were washed twice, resuspended in PBS, and analyzed in a FACS Canto flow cytometer. The results were analyzed using FlowJo software (FlowJo LLC, Ashland, OR, USA). [18]. The medium was refreshed every 3 days. Also, to analyze the in vitro multipotential differentiation ability of hPDLSCs, cells were cultured in OsteoDiff media, AdipoDiff media, and ChondroDiff media (Miltenyi Biotec) for 4 weeks to induce osteogenic, adipogenic, and chondrogenic differentiation, respectively. Osteogenesis was demonstrated by mineralization and assessed by Alizarin Red staining (Sigma-Aldrich, St. Louis, MO, USA) and alkaline phosphatase (ALP) with 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium (BCIP/NBT) (Sigma-Aldrich). Adipogenesis was evaluated with Oil Red O solution (Sigma-Aldrich) to detect accumulation of neutral lipid droplets. Finally, chondrogenic differentiation was verified with Alcian Blue staining (Sigma-Aldrich) to detect glycosaminoglycans.
MTT assay
The cytotoxicity of the extracts toward the hPDLSCs was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The eluates from three discs of each material were obtained after immersing them in culture medium for 24 h. Briefly, 1 × 104 hPDLSCs were added to 96-well plates with 180 μL of DMEM and left for 24 h. Then, extracts of the materials (1:1; 1:2, and 1:4) were added, and the cells were incubated at 37 °C in a 5% CO2 for 24, 48, or 72 h. The samples were incubated with 1 mg/mL of MTT for 4 h at the indicated time points. Then, 0.2 mL of dimethyl sulfoxide (DMSO) was added to each well to solubilize the formazan crystals obtained as a result of MTT reduction by the viable cells. The optical density value was measured by spectrophotometer (Synergy H1, BioTek, Winooski, VT, USA) at 570 nm (Abs570). The data obtained for each group were normalized based on cells + medium.
Wound healing assay
Cell migration and motility were evaluated by a scratch wound assay as previously described [17]. The eluates from six discs of each material were obtained after immersing them in culture medium for 24 h. The hPDLSCs were cultured on 12-well plates and grown to confluence to obtain a cell monolayer. The monolayers were scratched using a sterile 100-μL pipette tip and washed twice with PBS to eliminate detached cells. The healing process was allowed to proceed in the absence (control group) or presence of the different CSBS eluates (1:1; 1:2, and 1:4). Observation of the “wound area” was performed at 24, 48, and 72 h. The wound closure areas were measured with ImageJ (National Institutes of Health, Bethesda, MD, USA) to calculate the percentage of wound area after 24, 48, or 72 h relative to the total wound area measured at 0 h in the same well. Migration distances were analyzed separately during periods 0–24 h (migration during first 24 h period), 24–48 h (during second 24 h period), and 48–72 h (during third 24 h period). In order to avoid scratch width variation, “relative wound closure” area (RWC) was calculated (RWC (%) = wound closure area (pixel) × 100 (%)/× (pixel)).
Scanning electron microscopy
Fifteen disks (2-mm height and 5-mm diameter) of the different CSBS were subdivided into three groups (n = 5). A total of 5 × 104 hPDLSCs were directly added to each disk and cultured for 72 h. The cells were fixed using 4% glutaraldehyde in PBS for 4 h at 4 °C. Subsequently, the samples were dehydrated in increasing ethanol solutions before critical point drying. Specimens were mounted on aluminum stubs, followed by gold-palladium (Au-Pd) coating (Bio-Rad Polaron e5400 SEM Sputter Coating System, Kennett Square, PA, USA). Finally, the samples were observed with scanning electron microscopy (SEM) to elucidate the cell-material interaction.
Bioactivity assays
RT-qPCR gene expression analysis
The expression values of cementoblastic/osteoblastic-related genes (CEMP-1, CAP, and ALP) were measured by RT-qPCR. For this purpose, six discs were immersed in culture medium for 24 h. In order to determine differences in the expression of the above mentioned genes, transcript levels were quantified in cDNA obtained from cell cultures after exposure to the endodontic sealers (dilution 1:1) for 3, 7, 14, and 21 days and from the same cells grown in medium without endodontic sealers (control). The differential expression was calculated with respect to expression in the control using the delta-delta cycle threshold (ΔΔCT) method, normalizing for GAPDH (endogenous control).
For RT-qPCR, total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and cDNA was synthesized from 1 μg of RNA by using iScript™ Reverse Transcription Supermix for RT-qPCR (Bio-Rad).
Real-time PCR was performed in triplicate with an ABI PRISM 7700 instrument (Applied Biosystems) using SYBR® Premix Ex Taq™ (Takara, Clontech) following the supplier’s recommendations as we previously reported [19]. Primer sequences for human genes encoding cementum protein 1 (CEMP1), cementum-derived attachment protein (CAP), alkaline phosphatase (ALP), and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were as follows (forward/reverse): CEMP1 (5′-GGGCACATCAAGCACTGACAG-3′/5′-CCCTTAGGAAGTGGCTGTCCAG-3′); CAP (5′-TTTTTCTGGTCGCGTGGACT-3′/5′-TCACCAGCAACTCCAACAGG-3′); ALP (5′-TCAGAAGCTCAACACCAACG-3′/5′-TTGTACGTCTTGGAGAGGGC-3′); GAPDH (5′-TCAGCAATGCCTCCTGCAC-3′/5′-TCTGGGTGGCAGTGATGG-3′).
Alizarin Red S (ARS) mineralization assay
Alizarin Red S was performed to evaluate cell mineralization. For this purpose, six discs were immersed in culture medium for 24 h. hPDLSCs (2 × 105/well) were seeded in a 24-well plate, and after 90% confluence, the cells were treated with extracts of CBSBS (dilution 1:1) for 21 days, and changing the medium was changed every 3 days. A negative control (without extracts) and a positive control for osteogenic differentiation using OsteoDiff media (Miltenyi Biotech) were carried out. Next, hPDLSCs were fixed with 70% ethanol for 1 h at 4 °C, washed 3 times for 10 min in phosphate-buffered saline, and stained for 30 min with 2% Alizarin Red solution (Sigma AB, Malmö, Sweden) before washing three times with ultrapure water. Absorbance was measured at 550 nm using a spectrophotometer.
Statistical analysis
The statistical analyses were conducted using SPSS version 22.0 software (SPSS, Inc., Chicago, IL, USA). Each experiment was performed with three replicates in three to six separate experiments. Quantitative data are presented as the mean ± standard deviation (SD). After verifying the homogeneity of variances, MTT, cell migration, and qPCR assays were assessed using two-way ANOVA followed by a Tukey post hoc test. Data of ARS were submitted to one-way ANOVA and the Tukey tests. The results were deemed significant when p < 0.05.
Results
Analysis of ion release and pH
At 1 h, Endoseal showed the highest pH (pH 9.66), followed by Ceraseal (pH 8.22) and EndoSequence BC Sealer (pH 6.3). At the final time point (7 days), all endodontic sealers showed an alkaline pH, although the pH of Endoseal was significantly higher (10.18) the values of the other CSBS (p < 0.05) (Table 2). Ca2+ release was higher in the case of Ceraseal than with EndoSequence BC Sealer and Endoseal (p < 0.05). However, the release of Zr was more pronounced in Ceraseal. In addition, the presence of aluminum only was detected in Endoseal (Table 3).
Characterization of hPDLSC immunophenotype
Analysis flow cytometry results indicated that mesenchymal stem cells isolated from periodontal ligament expressed the characteristic mesenchymal stem cell markers CD90, CD105, and CD73, but not the hematopoietic cell markers CD34, CD45, CD14, and CD20. Also, after the induction of osteogenic, adipogenic, and chondrogenic differentiation, hPDLSCs showed positive staining with Oil Red O, Alizarin Red, ALP, and Alcian Blue, thus confirming the multipotentiality of the isolated cells (Fig. 1).
a Expression of the typical mesenchymal stem cell markers CD90, CD73, and CD105 displayed by hPDLSCs. Representative histograms obtained in n = 3 separate experiments are shown. b Multipotential differentiation ability toward the adipogenic (Oil Red O), osteogenic (Alizarin Red, ALP), and chondrogenic (Alcian Blue) mesodermal lineages of hPDLSCs and their specific staining are shown. Scale bar 100 μm
MTT assay
The results presented in Fig. 2 show the effects of the CSBS extracts on cell viability. Extracts of Ceraseal and EndoSequence BC Sealers exhibited similar rates to the control. Interestingly, at 72 h, a small but significant increase in viability was observed for hPDLSCs cultured with undiluted Ceraseal and EndoSequence BC Sealer 1:2 (*p < 0.05), whereas all dilutions of Endoseal produced a statistically significant reduction in cell viability after 24, 48, and 72 h of incubation (***p < 0.001).
Migration assay
The wound area was determined after removal of the scarring insert, and the percentage of open wound area was determined during the course of following 72 h. The results show that after 24 and 48 h, wound healing had progressed in all the experimental conditions, except for a pronounced deceleration in cell migration in the case of Endoseal (**p < 0.01; ***p < 0.001). Interestingly, treatments involving 1:1 and 1:2 dilutions of EndoSequence BC Sealer induced a higher cell migration rate at 72 h than the control complete medium (*p < 0.05) (Fig. 3). Taken together, these results showed that EndoSequence BC Sealer and Ceraseal allowed hPDLSCs migration.
Effect of endodontic cement eluates on cellular migration in wound healing assays. Confluent hPDLSC monolayers were cultured in the absence (control) or presence of different dilutions of the material extracts for up to 72 h. Cell migration was determined and expressed as the open wound area percentage for each condition compared with the control (*p < 0.05; **p < 0.01; ***p < 0.001, respectively)
Cell attachment on materials
As shown in Fig. 4, cells adhering to all the material surfaces were detected after 72 h of culture. At × 100 and × 300 magnifications, flattened cells with multiple prolongations proliferated on the surface of Ceraseal and EndoSequence BC Sealer, whereas only few and rounded cells were detected on Endoseal surfaces.
Ceraseal and EndoSequence induced ALP, CAP, and CEMP-1 expression
The ability of the sealers to produce hPDLSC differentiation was assayed by reverse transcription quantitative PCR (RT-qPCR) of well-known markers. Thus, ALP gen was selected as a marker of osteogenesis and CEMP-1 and CAP as indicators of cementogenesis gene expression.
The expression of these selected genes was analyzed by RT-qPCR in total RNA extracted from cells grow in the presence of EndoSequence or Ceraseal and compared with total RNA isolated from cells grown without sealers as a negative control. Cells were grown during 3, 7, 14, and 21 days because of the analysis included early (ALP) and late markers (CEMP-1 and CAP). The RT-qPCR assays showed an early significant overexpression of ALP gen at 3 and 7 days in the presence of EndoSequence and Ceraseal (p < 0.001, two-way ANOVA test) (Fig. 5). Moreover, the sealers induced an upregulation of cementogenesis gene expression. Thus, these assays showed a significantly increase of relative fold change expression compared with the control of CAP both in the presence of EndoSequence (p < 0.05 at 3 days, p < 0.001 at 7, 14, and 21 days, two-way ANOVA test) and Ceraseal (p < 0.001 at 3, 7, 14, and 21 days, two-way ANOVA) (Fig. 5). As regards CEMP-1, EndoSequence induces a statistically significant increase in its expression at 7 and 14 days (p < 0.05, two-way ANOVA test). In the presence of Ceraseal, the result showed a statistically significant increase in CEMP-1 expression from 3 to 21 days (p < 0.001 at 3, 7, 14 days; p < 0.05 at 21 days, two-way ANOVA test). These results demonstrated that the three genes, ALP, CAP, and CEMP-1, are strongly induced in the presence of both sealers.
ARS for matrix calcium deposition analysis
Matrix calcium deposition was verified by Alizarin Red staining. EndoSequence BC Sealer, Ceraseal, and OsteoDiff groups exhibited a significantly higher level of Alizarin Red staining than the control after only after 21 days of cultures (***p < 0.001; Fig. 6). It should be noted that the highest degree of mineralization was observed with the EndoSequence BC Sealer group compared with Ceraseal, OsteoDiff, and Endoseal (*p < 0.05; **p < 0.01; ***p < 0.001, respectively). Indeed, no mineralization was detected in the Endoseal group.
Mineralization assay. Quantification of Alizarin Red staining after 21 days. Data are presented as the mean ± standard deviation percentage of staining compared with the control (*p < 0.05; **p < 0.01; ***p < 0.001, by one-way ANOVA and Tukey’s post hoc test). Highest mineralization was observed in EndoSequence BC Sealer group in comparison with Ceraseal, OsteoDiff, Endoseal, and control (Scale bar × 100)
Discussion
Cytocompatibility, biocompatibility, and regenerative/reparative potential are assumed to be inherent to calcium silicate-based materials [20, 21]. However, the development of new CSBS with different formulations may alter their biological properties, jeopardizing apical healing. In this study, we tested the biological properties of three CSBS in terms of cell viability, cell migration, cell adhesion, and ion release. In addition, their bioactivity potential in terms of gene expression and mineralization capacity was evaluated.
Previous reports have used other types of cell culture to investigate CSBS cytotoxicity in vitro, most commonly fibroblasts [22, 23]. However, we considered it necessary to evaluate the reaction of these new materials when in direct contact with cell types that are more closely associated with a clinical situation, such as hPDLSCs or osteoblasts.
Regarding ion release, Ceraseal induced a high degree of Ca2+release, followed by EndoSequence BC Sealer and Endoseal. Bioactive cements have the ability to release ions and an acid neutralization capability that favors tissue healing. In addition, it is well known that Ca2+ is actively involved in the differentiation of MSCs and tissue mineralization [24, 25]. All calcium silicate-based sealers evaluated in this study had zirconium oxide as radiopacifying agent. These radiopacifiers, as components of calcium silicate-based sealers, have been demonstrated to have acceptable radiopacity and physicochemical properties, in agreement with the ISO 6876/2001 specifications [16, 24, 26, 27]. Previous reports have been shown that materials containing zirconium oxide induced the proliferation of fibroblasts and accelerated the regression of inflammatory reactions [28], while studies point to the cytotoxic effects of bismuth oxide in odontoblast-like cells [29] and in human dental pulp cells [30]. However, high levels of aluminum were detected in Endoseal, and previous reported have associated the presence of aluminum in dental materials with genotoxicity or toxicity in animals [16, 31].
The cytotoxicity results obtained in the present work suggest that EndoSequence BC Sealer and Ceraseal had a comparable effect to that of the control. In agreement with our results, previous studies found that EndoSequence BC Sealer promoted an adequate biological response on hPDLSCs in terms of cell proliferation, morphology, migration, and attachment [32], whereas Endoseal showed a certain degree of cytotoxicity compared with BioRoot RCS on human PDL cells and better cytocompatibility than AH Plus on mouse osteoblasts [17, 32]. However, there are no current studies evaluating Ceraseal which makes it difficult to compare results.
Wound healing involves complex interactions among inflammatory mediators and cells, whose angiogenesis and tissue remodeling capacity play an important role in this process [33]. Correlating the cell cytotoxicity assays with the wound healing data, we found that EndoSequence BC Sealer and Ceraseal displayed similar cell migration rates to the control, while Endoseal induced a lower level of cell migration. This phenomenon was evident in a previous study, which pointed to lower cell viability and cell migration in the presence of Endoseal [17]. Conversely, some authors have reported that Endoseal is cytocompatible using other types of cells, e.g., gingival cells [16].
Cell adhesion is another good indicator of cytocompatibility since this process plays a crucial role during periradicular repair [34] and is closely related with cell viability, migration, and differentiation for tissue repair [35, 36]. At 72 h, a large number of cells were detected spread over the surface of EndoSequence BC Sealer and Ceraseal disks. Other studies reported similar degrees of cell attachment using EndoSequence BC Sealer and others CSBS [16, 17, 37]. In fact, human mesenchymal bone marrow cells exposed to EndoSequence BC Sealer and BioRoot RCS presented well extending morphologic conditions [38].
The expression of ALP, CAP, and CEMP-1 is related to the formation of cementum and bone tissue and has been previously reported to promote osteoblastic and/or cementoblastic differentiation in PDLSCs in vitro. Its expression is restricted to cementoblasts and mesenchymal stem cells of the human periodontium, and it regulates cell viability, differentiation, deposition rate, composition, and morphology of the hydroxyapatite crystals formed by these cells [19]. In our study, Ceraseal induced significant upregulation of CEMP-1 compared with the control, OsteoDiff, and EndoSequence BC extracts, especially at 3, 7, and 14 days. In the context of tissue regeneration, cement production is essential for the formation of replacement tissue, also known as a biological seals, and considered the “ideal scenario” for endodontic treatment repair. Thus, the induction of cement deposition by endodontic materials is considered a beneficial process. In our study, EndoSequence BC Sealer and Ceraseal upregulated the gene expression of CAP and ALP, in agreement with numerous studies that have demonstrated the repair potential stimulated by bioceramic-containing materials [15]. Furthermore, the Alizarin Red staining revealed that Ceraseal and EndoSequence BC Sealer had a stronger mineralization capacity than the control group, OsteoDiff, and Endoseal MTA. In the same line, Giacomino et al. [15] showed that EndoSequence BC Sealer promoted mineralization of osteoblast precursor cells.
Conclusions
EndoSequence BC Sealer and Ceraseal eluates displayed higher cell viability and induced greater cell attachment and migration rates than those obtained using Endoseal. Also, Ceraseal and EndoSequence BC Sealer released significantly more Ca2+ and favored hPDLSC differentiation and mineralization compared with Endoseal.
References
Khalil I, Naaman A, Camilleri J (2016) Properties of tricalcium silicate sealers. J Endod 42:1529–1535. https://doi.org/10.1016/j.joen.2016.06.002
Donnermeyer D, Burklein S, Dammaschke T, Schafer E (2018) Endodontic sealers based on calcium silicates: a systematic review. Odontology. https://doi.org/10.1007/s10266-018-0400-3
Marciano MA, Duarte MA, Camilleri J (2016) Calcium silicate-based sealers: assessment of physicochemical properties, porosity and hydration. Dent Mater 32(2):e30–e40. https://doi.org/10.1016/j.dental.2015.11.008
Silva Almeida LH, Moraes RR, Morgental RD, Pappen FG (2017) Are premixed calcium silicate-based endodontic sealers comparable to conventional materials? A systematic review of in vitro studies. J Endod 43(4):527–535. https://doi.org/10.1016/j.joen.2016.11.019
Dubey N, Rajan SS, Bello YD, Min KS, Rosa V (2017) Graphene nanosheets to improve physico-mechanical properties of bioactive calcium silicate cements. Materials (Basel) 10(6). https://doi.org/10.3390/ma10060606
Jimenez-Sanchez MDC, Segura-Egea JJ, Diaz-Cuenca A (2019) Higher hydration performance and bioactive response of the new endodontic bioactive cement MTA HP repair compared with ProRoot MTA white and NeoMTA plus. J Biomed Mater Res B Appl Biomater 107:2109–2120. https://doi.org/10.1002/jbm.b.34304
Benetti F, Gomes-Filho JE, de Araujo Lopes JM, Barbosa JG, Jacinto RC, Cintra LTA (2018) In vivo biocompatibility and biomineralization of calcium silicate cements. Eur J Oral Sci 126(4):326–333. https://doi.org/10.1111/eos.12539
Natu VP, Dubey N, Loke GC, Tan TS, Ng WH, Yong CW, Cao T, Rosa V (2015) Bioactivity, physical and chemical properties of MTA mixed with propylene glycol. J Appl Oral Sci 23(4):405–411. https://doi.org/10.1590/1678-775720150084
Vouzara T, Dimosiari G, Koulaouzidou EA, Economides N (2018) Cytotoxicity of a new calcium silicate endodontic sealer. J Endod 44(5):849–852. https://doi.org/10.1016/j.joen.2018.01.015
Cintra LTA, Benetti F, de Azevedo Queiroz IO, de Araujo Lopes JM, Penha de Oliveira SH, Sivieri Araujo G, Gomes-Filho JE (2017) Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA material. J Endod 43(5):774–778. https://doi.org/10.1016/j.joen.2016.12.018
Sequeira DB, Seabra CM, Palma PJ, Cardoso AL, Peca J, Santos JM (2018) Effects of a new bioceramic material on human apical papilla cells. J Funct Biomater 9(4). https://doi.org/10.3390/jfb9040074
Taraslia V, Anastasiadou E, Lignou C, Keratiotis G, Agrafioti A, Kontakiotis EG (2018) Assessment of cell viability in four novel endodontic sealers. Eur J Dent 12(2):287–291. https://doi.org/10.4103/ejd.ejd_9_18
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364(9429):149–155. https://doi.org/10.1016/S0140-6736(04)16627-0
Souza GL, Rosatto CMP, Silva MJB, Silva MV, Rocha Rodrigues DB, Moura CCG (2018) Evaluation of apoptosis/necrosis and cytokine release provoked by three root canal sealers in human polymorphonuclears and monocytes. Int Endod J 52:629–638. https://doi.org/10.1111/iej.13036
Giacomino CM, Wealleans JA, Kuhn N, Diogenes A (2019) Comparative biocompatibility and osteogenic potential of two bioceramic sealers. J Endod 45(1):51–56. https://doi.org/10.1016/j.joen.2018.08.007
Kebudi Benezra M, Schembri Wismayer P, Camilleri J (2018) Interfacial characteristics and cytocompatibility of hydraulic sealer cements. J Endod 44(6):1007–1017. https://doi.org/10.1016/j.joen.2017.11.011
Collado-Gonzalez M, Garcia-Bernal D, Onate-Sanchez RE, Ortolani-Seltenerich PS, Lozano A, Forner L, Llena C, Rodriguez-Lozano FJ (2017) Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int Endod J 50(9):875–884. https://doi.org/10.1111/iej.12703
Collado-Gonzalez M, Tomas-Catala CJ, Onate-Sanchez RE, Moraleda JM, Rodriguez-Lozano FJ (2017) Cytotoxicity of GuttaFlow Bioseal, GuttaFlow2, MTA Fillapex, and AH Plus on human periodontal ligament stem cells. J Endod 43(5):816–822. https://doi.org/10.1016/j.joen.2017.01.001
Rodriguez-Lozano FJ, Collado-Gonzalez M, Tomas-Catala CJ, Garcia-Bernal D, Lopez S, Onate-Sanchez RE, Moraleda JM, Murcia L (2019) GuttaFlow Bioseal promotes spontaneous differentiation of human periodontal ligament stem cells into cementoblast-like cells. Dent Mater 35(1):114–124. https://doi.org/10.1016/j.dental.2018.11.003
Torabinejad M, Parirokh M, Dummer PMH (2018) Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part II: other clinical applications and complications. Int Endod J 51(3):284–317. https://doi.org/10.1111/iej.12843
Parirokh M, Torabinejad M, Dummer PMH (2018) Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part I: vital pulp therapy. Int Endod J 51(2):177–205. https://doi.org/10.1111/iej.12841
Willershausen I, Wolf T, Kasaj A, Weyer V, Willershausen B, Marroquin BB (2013) Influence of a bioceramic root end material and mineral trioxide aggregates on fibroblasts and osteoblasts. Arch Oral Biol 58(9):1232–1237. https://doi.org/10.1016/j.archoralbio.2013.04.002
Poggio C, Riva P, Chiesa M, Colombo M, Pietrocola G (2017) Comparative cytotoxicity evaluation of eight root canal sealers. J Clin Exp Dent 9(4):e574–e578. https://doi.org/10.4317/jced.53724
Li X, Yoshihara K, De Munck J, Cokic S, Pongprueksa P, Putzeys E, Pedano M, Chen Z, Van Landuyt K, Van Meerbeek B (2017) Modified tricalcium silicate cement formulations with added zirconium oxide. Clin Oral Investig 21(3):895–905. https://doi.org/10.1007/s00784-016-1843-y
Rajasekharan S, Vercruysse C, Martens L, Verbeeck R (2018) Effect of exposed surface area, volume and environmental pH on the calcium ion release of three commercially available tricalcium silicate based dental cements. Materials (Basel) 11(1). https://doi.org/10.3390/ma11010123
Candeiro GT, Correia FC, Duarte MA, Ribeiro-Siqueira DC, Gavini G (2012) Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J Endod 38(6):842–845. https://doi.org/10.1016/j.joen.2012.02.029
Ha JH, Kim HC, Kim YK, Kwon TY (2018) An evaluation of wetting and adhesion of three bioceramic root canal sealers to intraradicular human dentin. Materials (Basel) 11(8). https://doi.org/10.3390/ma11081286
Silva GF, Guerreiro-Tanomaru JM, da Fonseca TS, Bernardi MIB, Sasso-Cerri E, Tanomaru-Filho M, Cerri PS (2017) Zirconium oxide and niobium oxide used as radiopacifiers in a calcium silicate-based material stimulate fibroblast proliferation and collagen formation. Int Endod J 50(Suppl 2):e95–e108. https://doi.org/10.1111/iej.12789
Chiang TY, Ding SJ (2010) Comparative physicochemical and biocompatible properties of radiopaque dicalcium silicate cement and mineral trioxide aggregate. J Endod 36(10):1683–1687. https://doi.org/10.1016/j.joen.2010.07.003
Min KS, Chang HS, Bae JM, Park SH, Hong CU, Kim EC (2007) The induction of heme oxygenase-1 modulates bismuth oxide-induced cytotoxicity in human dental pulp cells. J Endod 33(11):1342–1346. https://doi.org/10.1016/j.joen.2007.07.012
Moon HJ, Lee JH, Kim JH, Knowles JC, Cho YB, Shin DH, Lee HH, Kim HW (2018) Reformulated mineral trioxide aggregate components and the assessments for use as future dental regenerative cements. J Tissue Eng 9:2041731418807396. https://doi.org/10.1177/2041731418807396
da Silva EJ, Zaia AA, Peters OA (2016) Cytocompatibility of calcium silicate-based sealers in a three-dimensional cell culture model. Clin Oral Investig 21:1531–1536. https://doi.org/10.1007/s00784-016-1918-9
Leprince JG, Zeitlin BD, Tolar M, Peters OA (2012) Interactions between immune system and mesenchymal stem cells in dental pulp and periapical tissues. Int Endod J 45(8):689–701. https://doi.org/10.1111/j.1365-2591.2012.02028.x
Agrafioti A, Taraslia V, Chrepa V, Lymperi S, Panopoulos P, Anastasiadou E, Kontakiotis EG (2016) Interaction of dental pulp stem cells with Biodentine and MTA after exposure to different environments. J Appl Oral Sci 24(5):481–486. https://doi.org/10.1590/1678-775720160099
Zhu Y, Shang L, Chen X, Kong X, Liu N, Bai Y, Fang J, Dang J, Wang X, Jin Y (2013) Deciduous dental pulp stem cells are involved in osteoclastogenesis during physiologic root resorption. J Cell Physiol 228(1):207–215. https://doi.org/10.1002/jcp.24122
Zhu L, Yang J, Zhang J, Peng B (2014) A comparative study of BioAggregate and ProRoot MTA on adhesion, migration, and attachment of human dental pulp cells. J Endod 40(8):1118–1123. https://doi.org/10.1016/j.joen.2013.12.028
Zhou HM, Du TF, Shen Y, Wang ZJ, Zheng YF, Haapasalo M (2015) In vitro cytotoxicity of calcium silicate-containing endodontic sealers. J Endod 41(1):56–61. https://doi.org/10.1016/j.joen.2014.09.012
Alsubait SA, Al Ajlan R, Mitwalli H, Aburaisi N, Mahmood A, Muthurangan M, Almadhri R, Alfayez M, Anil S (2018) Cytotoxicity of different concentrations of three root canal sealers on human mesenchymal stem cells. Biomolecules 8(3). https://doi.org/10.3390/biom8030068
Funding
This work was supported by the Spanish Network of Cell Therapy (TerCel), RETICS subprograms of the I+D+I 2013-2016 Spanish National Plan, and project “RD16/0011/0001” funded by the Instituto de Salud Carlos III to JMM and co-funded by the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Baek Myong-Hyun has a commercial interest in one of the tested products. The other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was approved by the Clinical Research Ethics Committee of the University of Murcia (procedure number: 1528/2017). Likewise, permission was obtained from the Health Department authorities to use the information contained in the CDHs, previously anonymized by one of the investigators belonging to the medical staff of the Health Department in order to protect patient confidentiality. All the information was processed in abidance with the confidentiality regulations defined under Act 15/1999 referred to personal data protection.
Informed consent
Informed consent was obtained from the parents of all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
López-García, S., Myong-Hyun, B., Lozano, A. et al. Cytocompatibility, bioactivity potential, and ion release of three premixed calcium silicate-based sealers. Clin Oral Invest 24, 1749–1759 (2020). https://doi.org/10.1007/s00784-019-03036-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03036-2