Abstract
Objectives
The tongue-to-palate distance influences the volume of the in-mouth air cavity (IMAC), thus conditioning the entry of aromatic compounds to the olfactory mucosa site. This study was set out to record the IMAC volume by measuring tongue-to-palate distance at rest.
Materials and methods
Twelve young adults in good general health were tested—lips contacting, with at-rest posture of the tongue and jaw during a silent reading task. Observations in this study were limited to pre- and post-swallowing sequences. The tongue-to-palate distance was measured using three electromagnetic sensors placed on the tongue upper surface. IMAC volume was evaluated from a geometrical model, taking into account the tongue-to-palate distance, the IMAC transversal distance measured from dental casts and historic data giving the anterior–posterior distance of the oral cavity.
Results
(1) In the at-rest posture, the tongue-to-palate distance was significantly greater at the posterior sensor level. (2) A vertical shift in tongue posture at rest frequently appeared following deglutition. The upward shifts were of larger amplitude and more frequent than the downward shifts. (3) Evaluation of the IMAC volume gave an approximate value of 12 ml at rest. (4) The chin sensor at rest was 2.8 ± 0.8 mm below its position when in occlusion.
Conclusion
The tongue and mandible contribute to shaping the IMAC volume. Clinical relevance: These and other results suggest that deglutition changes tongue-to-palate distance and influences aroma release during mastication/deglutition acts through modulation of the IMAC volume.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aroma perception depends on expired nasal aromatic compounds reaching the olfactory mucosa. Aromatic compounds are released during mastication and deglutition via the retronasal route. It is known that changes in the air volume of the oral cavity and fluctuation of airflow towards the nasal pharynx are major factors in carrying aromatic compounds to the olfactory mucosa [1]. Large interindividual variations have been observed when recording nasal aromatic compounds [2]. These variations may be explained, at least partly, by parallel individual changes in both oral air volume and movements. Fuller knowledge of the volume of the in-mouth air cavity (IMAC) and of its individual and functional variations would improve our understanding on the transport of aromatic compounds during eating. Although there are data based on imaging techniques that give quantitative values for the tongue volume [3, 4] and qualitative description of tongue movements [5–7], only preliminary data are available on the determination of IMAC volume during mastication and deglutition [8]. To determine the available volume of the IMAC for aromatic compound transport, the tongue position was evaluated via a direct quantitative measure of the distance between tongue upper surface and hard palate taken as a fixed reference. The tongue-to-palate distance, by reflecting the vertical variations in the tongue position, appears to be the predominant variable of the in-mouth air content volume [1, 8–11]. The present experiment was designed to evaluate the tongue-to-palate distance at rest during tongue habitual posture and so determine the habitual volume of the IMAC at rest irrespective of its variations. This work precedes a study of the swallowing events, which will be observed in the same sample.
There are other methods to visualise the upper part of the tongue such as the magnetic resonance imaging [3] or videoradiography [12]. They are appropriate for visualising solid structures but do not suit the visualisation of empty space like IMAC. In addition, the articulograph allows monitoring of the tongue-to-palate distance fluctuations during a complete recording sequence without exposing the patient to X-rays (AG 101®; Carstens, Göttingen, Germany). The recordings were made outside spontaneous deglutition, i.e. deglutition of saliva or dry swallow. The IMAC values calculated from these tongue–palate distance measures are discussed in the context of an evolving eating process.
Materials and methods
Subjects
Twelve subjects aged between 22 and 25 years (six males and six females, mean age 23.2 ± 1.1) were recruited among the students of the Dental Faculty of the University of Auvergne (Clermont-Ferrand, France). They all displayed good oral health with normal interarch relationship and complete dentition except for the third molars. Crowns, bridges, and other fixed restorations were accepted. None had undergone any orthodontic or dental treatment in the 3 months before experimentation. They had a score below 10 in the Hospital Anxiety and Depression Scale [13]. Oral breathers were excluded. The subjects were not receiving any psychotropic treatment that could have influenced salivation or deglutition. None had a deep sagittal groove along the dorsal surface of the tongue that would hinder the sensor placement. Written informed consent for participation in the trial was obtained from all the subjects, who received a financial compensation. Approval was obtained from the Local and National Ethical Committees (DGS2008-0031).
Movement recordings
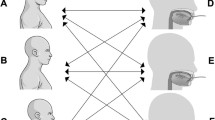
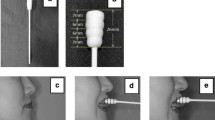
The articulograph was developed to record tongue movements in speech and language research (AG 101®). It has been validated to record jaw movements during chewing [14, 15]. Each subject sat with their head in a sustainable individual position to accept relative corporal immobility during a maximal recording sequence of 45 min. The subject’s head was placed in the middle of a magnetic field generated by three coils (Fig. 1a). Three miniature magnetic receiving sensors (Self SMS2-220J®; TDK, Corel Electronique, France) embedded in biological silicone were glued with cyanoacrylate adhesive (Cyanolite®) to the dorsal surface of the tongue on the sagittal midline (Fig. 1a). The anterior sensor was 15 mm from the tip of the tongue, and each sensor was 15 mm from the adjacent one. The sensor, when embedded, formed a sphere 3 mm in diameter. Displacements of the sensors in the magnetic field generated a current that allowed evaluation of the corresponding vertical and anterior–posterior positions and movements, i.e. two dimensions. Two supplementary embedded sensors were used. One was glued to the chin to monitor jaw movements, and another was glued to an upper central incisor to record head movements to subtract them from jaw and tongue movements. The sensors were connected to wires crossing the dental arch at the level of the anterior teeth. Swallowing was recorded by capturing the Adam’s apple displacement with a piezoelectric sensor (KPE-165®) fixed to the cricoid protuberance with adhesive tape. Signals were recorded and analysed using Spike® 2 software (C.E.D.®). The acquisition frequency was 100 Hz (Fig. 2).
A 170-s sequence recording of the anterior–posterior and vertical displacements of the four sensors glued on the tongue and chin. The first upper line indicates swallowing events (AAD) recorded by capturing the Adam’s apple displacement. The second line represents (in millimetres) the anterior posterior mandibular position (AP Mand) recorded by a sensor glued to the chin. The next three lines represent (in millimetres) the anterior–posterior displacements of the tongue recorded by three sensors (AP Ant, AP Cent, and AP Post: denoting anterior, central, and posterior parts of the tongue, respectively). The next four lines represent the vertical displacements (Vert) of the corresponding previous sensors. A 5-mm increment scale unit on the left indicates displacement amplitude
Experimental design
The subjects took part in two recording sessions beginning between 9:30 and 10 a.m. to avoid spontaneous change in cortical levels due to daily rhythms. The subject was comfortably seated, looking in front at eye level with the head centred in the magnetic field. Feet were isolated from the ground to reduce background noise during recordings. The first session was devoted to familiarising the subject with the experimental environment, and the corresponding recordings were not analysed. To train the subject to achieve good control of the tongue position, several tasks were performed during the first session. Several successive tongue positions against the palate were followed by tongue resting positions in which the subject was relaxed and comfortable.
During the second session, the tongue position was recorded while the subject read silently, for 3 min, a text they had chosen. This reading task was performed to distract the subject from the experimental conditions by focusing their attention on the meaning of the text. No instructions were given concerning the interarch relationship during the recording process except at the beginning and end of each sequence, when subjects were asked to close their mouth and place their tongue against their hard palate to record both the mandible uppermost position and palate outline as references.
Data extraction and analysis
Our goal was to determine the habitual or most frequent position of the tongue at rest by monitoring the distance separating tongue sensors from the palate. All acquisition values were averaged for each 5-s block, giving one value (in millimetres) for the vertical dimension (Fig. 3) and another for the anterior–posterior one. These values were measured by reference to the position of each tongue sensor when in contact with the palate and, at the same time, in its highest and most forward position at any time during the whole session. For each tongue sensor, the vertical and anterior–posterior values were calculated for each 5-s block as follows:
Three individual examples of tongue position recording while reading for about 170 s. Each point represents the position averaged for 5 s (e.g. the 9 value corresponds to the 5 × 9 = 45-s time point of the recorded sequence) of the anterior, central and posterior sensors (as described in Figs. 1 and 2). The first and last points of each recording represent the positions of the sensors during the first and last 5-s period when the subjects were asked to place their tongue against their hard palate. Black arrows indicate swallowing events. Letters inside white circles show the highest instantaneous position extracted from the raw recording (with a sampling frequency of 100 Hz). These instantaneous positions were transiently reached by the anterior (a), central (c) and posterior (p) sensors either during deglutition or when the subjects were asked to place their tongue against their palate
It is important to consider the anterior–posterior distance because the hard palate slopes. Any anterior–posterior shift of the tongue therefore had an effect on the tongue-to-palate distance. To compensate for anterior–posterior palatal slope, the corrected vertical tongue-to-palate distance was calculated as the sum of the vertical value (V) and the vertical outcome of the anterior–posterior shift (V AP) of the tongue, derived from the formula V AP = AP.tg α, where α is the angle made by the hard palate with the occlusal plane. This angle was determined from an elastomeric impression of the plaster cast of the maxillary dental arch previously obtained from each subject. When the palate presented different changes in the sagittal outlines, different slopes were determined to take into account the location of the different sensors. Finally, the vertical tongue-to-palate distance was V + V AP.
To track the position of the mandible, the postural interarch distance was inferred from the position of the sensor glued to the chin and calculated by reference to the highest chin position, generally reached at the time of a swallowing. The parts of the recorded sequence corresponding to the swallowing events were discarded from 1 s before and after the onset and offset of the accompanying motor events seen from the cricoid movement recordings.
Determination of tongue-to-palate contacts
The tongue was considered as contacting the hard palate when the measured distance between the tongue and palate lay between 0 and 2.5 mm. This value was chosen, taking into account the thickness of each silicone-embedded sensor, to obtain a cylindrical shape 3 mm in diameter by 2.5 mm in length.
Estimation of the mean IMAC volume at rest
The IMAC value calculated outside swallowing time was evaluated from a geometrical model formed by a pyramidal shape placed on a horizontal lozenge base (Fig. 1b). Three dimensions had to be determined. The height of the model viewed in the median sagittal plane is maximal at the level of the first molar, and its value was given by the third sensor. It reduces to zero at the anterior limit of the IMAC, considering that the anterior part of the tongue contacts the posterior face of the incisors and canines. It also reduces to zero at the posterior limit of the IMAC, considering that the velum rests on the rear part of the tongue. This is the normal position of the tongue and surrounding tissues at rest during nose breathing. The width of the lozenge is also maximal at the level of the first molar, and its value was measured from dental casts of our 12 subjects as described previously [16]. The anterior–posterior dimension of the IMAC corresponded to the length of the lozenge base. It was obtained from another experiment by using an acoustic pharyngometer device [8]. The volume as defined above is a fourth of the parallelepiped volume in which the above lozenge-based pyramid is included.
Statistical analysis
The measured variable was the calculated sensor–palate vertical distance. The explicative variables were the sensors and the subjects. One-way or one-way repeated measure analyses were performed using SPSS software (version 11.5 for Windows, 2002) with general linear models for analysis of variance (ANOVA). A post hoc Student–Newman–Keuls test was carried out for comparison of means (with a risk of 5 %). Values are given as mean (in millimetres) ± SEM. The Pearson’s correlation coefficients were calculated between anterior–posterior and vertical distances of the sensors glued to the tongue and chin.
Results
Tongue posture during reading
The time course of tongue position during the 3 min of the reading task ranged widely from one subject to another. Figure 3 gives several examples of the position of the three sensors. Letters inside white circles show the highest instantaneous position extracted from the raw recording. The figure shows that most parts of the tongue contacted the palate at the beginning and end of the session when the subjects were asked to place their tongue against their hard palate. At swallowing time, there was a tongue–palate contact at one sensor site but never at all three sites. In some cases, the swallowing movement was not upwards as generally observed, and the change in the mean tongue-to-palate distance triggered by swallowing was not always the same: it could be upward, downward or with no change, depending on both the sensor and the subject. The mean distance (± SEM) between the palate and the anterior central and posterior sensors were 7.6 ± 0.8, 8.5 ± 1.3 and 16.5 ± 4.1 mm, respectively (n = 12). The distance at the posterior sensor was significantly different from the two others (p < 0.05, one-way ANOVA). Hence, most of the time, there was a space between the tongue and palate at rest.
Change in tongue and mandible postures before and after swallowing
Swallowing is frequently preceded and followed by a change in tongue posture. Considering the tongue’s steady posture before and after swallowing, the mean vertical shift amplitude of the tongue observed during the 170-s recording was 3.8 ± 1.6 mm calculated from the three sensors in 23 swallows (n = 69). The values were different for the three sensors, i.e. 2 ± 0.4, 3 ± 0.6 and 6.4 ± 2.6 mm for anterior, central and posterior sensors, respectively. Although these large variations occurred in both upward and downward vertical directions, the shifts upwards were of larger amplitude (3.3 ± 1.6 mm) and more frequent (n = 34 out of 69) than the shifts downwards (1.3 ± 0.2 mm, n = 19 out of 69) (p > 0.05). In some cases, the tongue sensors (n = 16 out of 69) did not appear to move vertically. As shown in Fig. 3, the tongue positions did not always vary in the same vertical direction at the different tongue loci.
Anterior–posterior movements of the three tongue sensors were intercorrelated and were associated with the mandible vertical displacements (Table 1). For instance, an anterior tongue displacement corresponded to a mandible elevation, and a posterior tongue displacement corresponded to a mandible downward movement (Table 1). The vertical movements of the three sensors did not occur at exactly the same time, as suggested by the significant mild or weak correlations between vertical movements of the three sensors (Table 1). This vertical tongue movement organisation was probably related to the time interval separating the vertical movements of the three sensors.
The postural interarch distance recorded from the chin sensor did not vary with time except during swallowing, an effect that is to be the subject of another experimental study. Swallowing apart, the mandible adopted a slight downward posture, which amounted to a mean value of 2.8 ± 0.8 mm at the level of the chin sensor. This downward position never exceeded 5 mm.
Estimation of the mean IMAC volume at rest
The mean length of the in-mouth cavity from the central maxillary gingival limit to the uvula was taken as 70 mm [8], and the palatal vault width that we measured at the level of the posterior sensor, i.e. middle of second molar, was 42 ± 0.92 mm. The highest tongue–palate distance at the posterior sensor was 16.5 mm. The calculation described in “Materials and methods” section gave an estimated IMAC volume of 12.13 ml.
Discussion
Advantages and limits of the study
This study is based on an accurate, quantitative measure of displacements of the anterior part of the tongue observed in real time relative to a fixed structure, the palate. Mandible displacement was also recorded relative to its uppermost position. This offers an advantage over most of the common imaging techniques, which do not allow quantitative measures and movement recordings without superimposition of neighbouring structures. Articulography has been extensively used in the study of tongue movements during speech and oral articulation for its accuracy and reliability [17–19]. For the same reasons, its use has been extended to mastication and swallowing studies, in which its reliability has been repeatedly demonstrated [14, 15, 17]. However, in the present experiment, we did not repeat the recording session but instead averaged a long recording of about 170 s.
In our experiment, the presence of the sensors could have influenced the position of the tongue and mandible and possibly hindered tongue positioning or movement. The introduction in the mouth of solid objects, such as small coils or sensors, is known to increase the freeway space [20]. The wires in the mouth could have had such an effect. However, the fact that the final freeway space was similar to the values usually reported suggests that this was not the case. The sensors may have been partly absorbed by the median depression of the tongue, frequently forming a sagittal groove. The sensors could also have been caught in the uppermost hollow part of the hard palate sagittal band and prevented intimate contact between tongue sensors and palate. No sensors were positioned on the rear part of the tongue upper surface because of (a) poor accessibility for sticking on the sensors and (b) nausea reflex in the subjects. In addition, the sensor locations chosen in this study focusing on the midsagittal line corresponded to the most active portion of the tongue in terms of precision, force control and palatal contacts during swallowing initiation [17, 21–25].
Tongue and jaw positions in habitual postures
This study shows that the tongue is distant from the palate in most of the circumstances studied except for deglutition and that the tongue–palate distance is greater at the median/posterior part of the tongue than in the front part. Although in this study large interindividual and intra-individual variations of tongue position were seen, a space between the tongue and the palate was the most frequent occurrence. However, full tongue–palate contact was reported [16] in ten out of 50 subjects in a survey based on lateral radiographic cephalograms.
Recording of the mandible position confirmed the presence of a postural interarch space (or freeway space). The near 3-mm freeway space value found in this study is close to those reported in many previous studies [26, 27].
Influence of tongue positions and displacements on IMAC values
Our geometrical model of IMAC is a rough approximation for several reasons. The IMAC is not a solid. The mouth is not bounded by straight lines either in its frontal part or in its rear part. There are, however, extreme anterior and posterior points of IMAC with the maxillary incisor papilla and the uvula, respectively. The dorsal surface of the tongue and the hard palate are not plane but curved. The model, however, fits the dihedral shape of the IMAC external limits formed by the tongue margins against the dental and palatal walls and by the rear part of the tongue against the soft palate. Although at rest, the tongue and the cheeks lay on the mouth floor and against the dental arches; IMAC varies from zero to a very large volume [16] because of the motility of the jaw, tongue and velum, even at rest. Because the intra-individual variations far exceed the interindividual variations, we have not considered variations due to gender, age or individual.
Because of this marked variability, we devised three simultaneous studies. In addition to the present experiment based on tongue movement recording, a second one was based on anatomical measures [16] and a third on acoustic pharyngometer recording [8]. The three experiments must be considered simultaneously to provide information on the IMAC and possible inference for involvement in aroma perception. The volume of 12 ml found in this study can be compared to the 8.5 ml in that of Bourdiol et al. [16], determined by anatomical measurements, and the 11.18 ml recorded 27 s after empty deglutition in a parallel experiment [8]. However, in this last experiment, the IMAC was 22.82 ml for a first subgroup of subjects who lowered their tongue after an empty swallow. The subjects in the second subgroup had almost no IMAC, as they kept their tongues against their palate. Taking into consideration only the subjects who displayed an IMAC, the value of 8.5 ml obtained with the anatomical method was also underestimated when deduced (volume of oral cavity − tongue volume) from the magnetic resonance imaging studies [3]. This is because the tongue was observed while the jaws were in interarch contact position. Conversely, the value of 22.82 ml was overestimated, because the lips were held apart by the mouthpiece of the measuring device. In another study, Hodgson et al. [10] integrated the expired nasal air volume with and without ongoing mastication: the difference of 13 ml was considered to represent the IMAC. Land [9] proposed a range of 5–15 ml. A value of IMAC volume in the range 10–18 ml can, thus, be inferred from these several studies.
A major implication of the IMAC is its possible role in the transport of aromatic compounds during eating. Recently, two modes of aroma release during a mastication/deglutition sequence were described [2]. In one mode, the aromatic compounds released at the nostrils were continuous during mastication, whereas it was observed only for the deglutition time in the second mode. When studying on the release of aromatic compounds during chewing, the air volume that can be transferred from the mouth to the pharynx is crucial. At mastication/swallowing time, the odorants come from the oral cavity, and their access to olfactory mucosa depends on tongue and velum relationships. The two patterns of change in IMAC after saliva deglutition that were observed closely parallel to the two modes of aroma release that were described [8]. It was suggested that the two main types of deglutition pattern (tongue elevation of the first pattern and tongue hollowing of the second and third patterns) observed by Dodds et al. [12] corresponded to two different modes of aroma release.
References
Buettner A, Beer A, Hannig C, Settles M, Schieberle P (2002) Physiological and analytical studies on flavour perception dynamics as induced by the eating and swallowing process. Food Qual Prefer 13:497–504
Gierczynski I, Laboure H, Guichard E (2008) In vivo aroma release of milk gels of different hardnesses: inter-individual differences and their consequences on aroma perception. J Agric Food Chem 56:1697–1703
Lauder R, Muhl ZF (1991) Estimation of tongue volume from magnetic resonance imaging. Angle Orthod 61:175–184
Ludescher B, Knebel C, Hoffmann S, Claussen CD, Küper K (2006) MR-Volumetrie der Zunge zur Abshätzung des individuellen Zungengrösse. Mund Kiefer GesichtsChir 10:101–105
Hiiemae KM, Palmer JB (1999) Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia 14:31–42
Hiiemae KM, Palmer JB (2003) Tongue movements in feeding and speech. Crit Rev Oral Biol Med 14:413–429
Matsuo K, Hiiemae KM, Palmer JB (2005) Cyclic motion of the soft palate in feeding. J Dent Res 84:39–42
Mishellany-Dutour A, Woda A, Bourdiol P, Labouré H, Guichard E, Feron G (2012) Retro-nasal olfaction is correlated with the variation of the in-mouth air cavity volume after empty deglutition. PlosOne 7(7):e41276
Land D (1996) Perspectives on the effects of interactions on flavour perception: an overview. In: McGorin RJ, Leland JV (eds) Flavour-food interactions. ACS Symp Ser. 633, Washington; pp 2–11.
Hodgson M, Linforth R, Taylor A (2003) Simultaneous real-time measurements of mastication, swallowing, nasal airflow and aroma release. J Agr Food Chem 5:5052–5057
Haahr AM, Bardow A, Thomsen CE, Jensen SB, Nauntofte B, Bakke M et al (2004) Release of peppermint flavour compounds from chewing gum: effect of oral functions. Physiol Behav 82:531–40
Dodds WJ, Taylor AJ, Stewart ET, Kern MK, Logemann JA, Cook IJ (1989) Tipper and dipper types of oral swallows. Am J Roentgenol 153:1197–1199
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370
Peyron MA, Mioche L, Renon P, AbouElKaram S (1996) Masticatory jaw movement recordings: a new method to investigate food texture. Food Qual Prefer 7:229–237
Lassauzay C, Peyron MA, Albuisson E, Dransfield E, Woda A (2000) Variability of the masticatory process during chewing of elastic model foods. Eur J Oral Sci 108:484–492
Bourdiol P, Mishellany-Dutour A, Abou-El-Karam S, Nicolas E, Woda A (2010) Is the tongue position influenced by the palatal vault dimensions? J Oral Rehabil 37:100–106
Steele CM, Van Lieshout PHHM (2004) Use of electromagnetic midsagittal articulography in the study of swallowing. J Speech Lang Hear Res 47:342–352
Byrd D, Browman CP, Goldstein L, Honorof D (1999) Magnetometer and X-ray microbeam comparison. In: Ohala JJ, Hasegawa Y, Ohala M, Granville D, Bailey AC (eds) Proceedings of the 14th International Congress of Phonetic Sciences. American Institute of Physics, New York, pp 627–630
Hasegawa-Johnson M (1998) Electromagnetic exposure safety of the Cartens articulograph AG100. J Acoust Soc Am 104:2529–2532
Woda A, Pionchon P, Palla S (2001) Regulation of mandibular postures: mechanisms and clinical implications. Crit Rev Oral Biol Med 12:166–178
Kieser J, Bolter C, Raniga N, Waddell JN, Swain M, Kennedy D, Foster K, Farland G (2011) Tongue-palate interactions during swallowing. J Texture Stud 42:95–102
Pouderoux P, Kahrilas P (1995) Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology 108:1418–1426
Kahrilas PJ, Lin S, Chen J, Logemann JA (1995) Three-dimensional modelling of the oropharynx during swallowing. Radiology 194:575–579
Chi-Fishman G, Stone M (1996) A new application for electropalatography: swallowing. Dysphagia 11:239–247
Steele CM, Bailey GL, Molfenter SM (2010) Tongue pressure modulation during swallowing: water versus nectar-thick liquids. J Speech Lang Hear Res 53:273–283
Carlsson GE, Ingervall B, Kocak G (1979) Effect of increasing vertical dimension on the masticatory system in subjects with natural teeth. J Prosthet Dent 41:284–289
Rugh JD, Drago CJ (1981) Vertical dimension: a study of clinical rest position and jaw muscle activity. J Prosthet Dent 45:670–675
Acknowledgments
ANR (SensInMouth,ANR 07-PNRA-O14) supported this study. We thank Dr Féron for valuable contributions during data analysis and discussion, and Richard Ryan for language editing.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bourdiol, P., Mishellany-Dutour, A., Peyron, MA. et al. Contributory role of the tongue and mandible in modulating the in-mouth air cavity at rest. Clin Oral Invest 17, 2025–2032 (2013). https://doi.org/10.1007/s00784-012-0897-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-012-0897-8