Abstract
Tongue strength has an important role in the swallowing process, and previous research has suggested that tongue position, concerning the craniomandibular region, could affect the oral function. This study aimed to evaluate the strength and endurance of three areas of the tongue in three experimentally induced craniocervical postures. A cross-sectional study with a nonprobabilistic sample of 37 participants (mean age: 3.85 ± 3.64 years; 20 men, 17 women) was performed. Tongue strength and endurance were assessed using a pressure device entitled Iowa Oral Performance Instrument (IOPI), in three different craniocervical positions: neutral head position (NHP), anterior head translation—or forward head position (FHP), and posterior head translation—or retracted head position (RHP). Measurements taken using the IOPI system showed significant differences in tongue strength for the anterior (p = 0.015) and middle areas of the tongue (p = 0.01). Significant differences were observed in analysis of variance (ANOVA) in the FHP (p = 0.02) and NHP (p = 0.009). The results of tongue endurance measurements showed statistically significant differences for FHP (p = 0.001), NHP (p = 0.00), and RHP (p = 0.007). The craniocervical position influences tongue strength, especially in the anterior and middle tongue areas, concerning the posterior, and, in the anterior and neutral head posture, regarding the retracted position. No differences were found in tongue resistance between the various craniocervical positions, but differences were found in resistance between the different tongue areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tongue is part of the stomatognathic system, presenting an intrinsic and extrinsic musculature. It has specific biomechanical characteristics for the performance of functions such as chewing, swallowing, or speech [1]. Extrinsic musculature has its origin in bone structures, while intrinsic musculature forms the body of the tongue [2, 3].
Tongue develops a crucial function in the oral and pharyngeal phases of swallowing [4]. During swallowing and chewing, cyclic movements of the tongue and jaw are produced, allowing communication between the oral cavity and the pharynx [5]. During chewing the tongue performs a coordinated movement forward and downward followed by a backward reversed movement in the late opening of the jaw [6]. For the bolus control and propulsion in the oral phase of swallowing, the tongue, (specifically genioglossus muscle fibers from anterior to posterior regions) pushes the food into the oropharynx [5]. For this movement, there should be a spread of tongue pressures in an anterior–posterior direction, which requires adequate strength and neuromuscular control of the intrinsic musculature [7].
For this reason, the examination of the tongue motor function and oral muscles is part of the clinical evaluation process of the swallowing mechanism [8]. The spatial situation and direction of the tongue’s fibers lead to different roles for each muscle. Nevertheless, correct neuromuscular control is necessary for proper lingual and oral function [2, 9]. Patients with dysphagia show an impaired masticatory performance, possibly due to reduced tongue forces and disturbed oral sensitivity [10]. The improvement of tongue strength may lead to an improvement of swallowing function [11]. Along these lines, it has been suggested that one of the factors that may affect lingual force in the swallowing process is the tongue position in the oral cavity in relation to the craniomandibular region [12].
Some authors suggest that there is a coordinated relation between the mandible, the tongue, and the craniocervical region in the development of various oral functions, while the movements of the jaw and the cervical spine have a spatiotemporal relation.[13]. During the mouth-opening movement or chewing, the craniocervical region extends concurrently with the onset of jaw depression [13]. Therefore, depending on the head position, the movement of the jaw shifts during mouth opening. When head leads forward, the closing path approaches the maximal intercuspal position from the anterior region, and when the head is bent backward, the closing path approaches the maximal intercuspal position from the posterior region [14]. In addition, there is also an anatomical relation between the upper cervical spine and the caudate nuclei of the trigeminal nerve, which receives afferent information from craniofacial regions such as the tongue and influence motor functions [15, 16].
These anatomical and neurophysiological relationships may explain some of the findings previously discovered, relating the cervical spine to the craniomandibular function. It has also been observed that changes in craniocervical posture might influence the activation of extrinsic lingual muscles or that different craniocervical postures might influence the patterns of electromyographic activity of the suprahyoid and infrahyoid muscles [17,18,19]. Other studies have described how the various craniocervical positions affect orofacial functions, such as maximum mouth opening, electromyographic (EMG) activity of the masseter muscles, the position of the mandibular condyle, or disorders of the somatosensory system [20,21,22].
On the other hand, previous studies have also shown that the orofacial function, and specifically tongue strength, may be different according to gender. However, there are controversial data in this regard, some research shows that men have greater tongue strength than women, while other studies found no significant differences [23,24,25,26]. Hence, it is not clear whether there is a real difference between men and women concerning tongue strength, which could represent a clinically relevant difference in the prevention or treatment of orofacial or swallowing disorders.
The hypothesis of this study is that craniocervical and head postures may directly influence muscle and oral functions, which could have an impact on tongue strength or tongue endurance. Understanding the influence of craniocervical posture on tongue strength could help patients with swallowing or chewing disorders which could translate into better therapeutic strategies. The aim of this study was to evaluate tongue strength and tongue endurance of the three areas of the tongue in three experimentally induced craniocervical postures. The secondary aim was to analyze whether there are differences in tongue strength and tongue endurance between men and women in the three tongue areas and craniocervical postures.
Methods
Study Design
A cross-sectional study was performed with a nonprobabilistic sample. The design followed the international recommendations for Strengthening the Reporting of Observational Studies in Epidemiology [27]. All participants received an explanation of the study procedures, which were planned according to the ethical standards of the Declaration of Helsinki and approved by an Ethics Committee (CSEULS-PI 001/2019). Written informed consent was obtained from all participants before their inclusion.
Participants
A sample of 37 asymptomatic individuals was obtained from La Salle University and from the Community of Madrid through media and social networks, posters, brochures, and emails. The participants were recruited between January 2019 and June 2019.
The inclusion criterion was as follows: healthy individuals with no pain. The exclusion criteria were: (a) individuals who presented systemic, cardiorespiratory, central nervous system or rheumatic diseases, or those who presented any musculoskeletal or craniocervical pathology; (b) underage individuals; (c) individuals with orofacial pain or a history of temporomandibular disorders.
Experimental Procedures
Craniocervical Postures
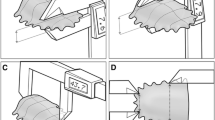
For each participant, tongue strength and resistance were evaluated in the three craniocervical positions described below, and illustrated at the left side of Fig. 1:
-
Neutral head posture (NHP), defined as the position of the head in a vertical position. This position was further confirmed as neutral if the tragus of the ear and acromion were bisected by a plumb line.
-
Forward head posture (FHP), defined as a 2 cm anterior translation of the head, with or without lower cervical flexion [28,29,30].
-
Retracted head posture (RHP), defined as a 2 cm posterior translation of the head over the trunk, associated with upper craniocervical flexion and extension of the low-to-mid-cervical spine [28,29,30].
Craniocervical postures and placement side of the measurement device. a Retracted head posture (RHP); b neutral head posture (NHP); c forward head posture (FHP); d posterior lingual area; e mid-position lingual area; f anterior lingual area. Measurements were taken in the three craniocervical positions and in each lingual region
Procedures for the Establishment of the Craniocervical Posture
The Cervical Range of Motion (CROM) device (Performance Attainment Associates, Roseville, MN; USA) was used to perform the various craniocervical postures. This instrument consists of plastic glasses to which several inclinometers are attached for each plane in space. Another of the components included is a superior arm with a ruler, placed in the horizontal plane, and a second vertical angled arm with a level aimed to locate over the bony prominence of the C7 vertebra which is the reference point used. In terms of reliability and validity, several studies have confirmed the use of this device for craniocervical range of motion measurements [31, 32], as well as the moderate to good intra-evaluator and inter-evaluator reliability [33, 34].
Craniocervical postures were measured in a sitting position in a comfortable upright position with the thoracic spine in contact with the back of a chair. The feet were positioned flat on the floor with knees and hips at 90°, and arms resting freely alongside. FHP and RHP were achieved by initial placement into the NHP.
FHP was performed with the CROM device after verbal instruction to position the head forward in a horizontal plane, reaching a 2 cm anterior displacement from the NHP measured with the superior arm of the CROM. Participants were instructed to maintain their eyes at the same horizontal level while being told to “slide your jaw and head forward until the examiner tells you to stop”. For the RHP, participants were instructed to position the head posteriorly in a horizontal plane, reaching a 2 cm posterior displacement from the NHP, also measured with the superior arm of the CROM. All participants were instructed to maintain their eyes at the same horizontal level while being told to “slide your jaw and head backward until the examiner tells you to stop” until reaching the target plumb line (Fig. 2).
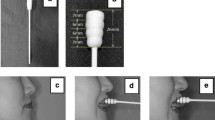
Measurement procedures. a Setting for the forward head position (FHP) and variables measurement outcome. b Setting for the neutral head position (NHP) and outcome variables measurement. c Setting for the retracted head position (RHP) and outcome variables measurement. d Iowa Oral Performance Instrument (IOPI) device used in the assessment of outcome variables (tongue strength and tongue endurance). e Cervical Range of Motion (CROM) device used to perform the craniocervical postures
Randomization
The order of the measurements was randomized for each participant. A second, double randomization was carried out for each participant, establishing a table that showed the order in which the measurements were made for each participant. A random sequence generator application was used to create this order (GraphPad Software version 8.00 for Windows, 2019. GraphPad Inc., CA, USA).
The first randomization established the order in the craniocervical postures (NHP, FHP, or RHP). In the same way, the second randomization generated the measurement sequence of the tongue area: anterior tongue area, middle tongue area, or posterior tongue area. The final result consisted of a combination of both randomizations, indicating the entire measurement sequence. The measures performed on the three tongue areas at each of the craniocervical postures are represented in Fig. 1.
Outcome Variables: Tongue Strength and Tongue Endurance
Measurement Instruments
The IOPI system was used to measure tongue strength and tongue resistance (Iowa Oral Performance Instrument; Northwest, Co., LLC, Carnation, WA, EEUU). It is a portable device that records the pressure made by the tongue, in kilopascals (kPa). This system can record both maximum tongue strength and tongue endurance, using LED light feedback. The instrument has shown good reliability and validity for the measurement of tongue strength [35, 36].
Measurement Procedures
The measurements were obtained through the pressure exerted on the bulb of the IOPI system, which was placed in various positions (anterior area, middle area, and posterior area of the tongue) and numerically recorded by the device. Using images and indications from the researchers, the placement site of the device on the three areas of the tongue was determined as described below and also illustrated on the right side of Fig. 1.
For the placement in the posterior tongue area, the last molars were taken as a reference. For the anterior tongue area, the bulb was placed just behind the incisors (without making contact with them), between the tongue and the palate. For the tongue mid-position, starting from the anterior position, the bulb was placed to coincide with the height of the premolars/anterior molars. For all measurements, the participant was instructed in the correct placement of the mid-point of the tongue in the transverse plane prior to the start of the measurement. In addition, the position of the jaw during the measurement had to be closed but without reaching the dental occlusion, allowing the cable connected to the bulb to pass between the teeth, as described previously [37]. Measurement procedures are illustrated in Fig. 2.
Tongue Strength Measurement
During the tongue strength measurement protocol, after the bulb was correctly placed, the participant was required to perform a maximum contraction during 3 s. After obtaining the maximum tongue strength measurement, a 1-min rest was performed, and the test was repeated until three measurements were obtained; and the highest measured value was used as data [35].
Tongue Endurance Measurement
To measure tongue resistance, 50% of the maximum tongue force previously measured was used. By means of LED light feedback, the participant was instructed to maintain this force for as long as possible, which was measured in seconds [35, 36].
Sample Size Calculation
A pilot study was carried out with 11 participants to calculate the sample size, from which an effect size of 0.23 was obtained after performing an ANOVA of repeated measurements for the variable tongue strength. Using the program G * Power 3.1.9.2 for Mac (G * Power © by the University of Dusseldorf, Germany), the calculation of the sample size was made, considering an α error of 0.05 and 1 − β of 0.95, for one group and three measurements. This calculation indicated that the sample should be 37 participants.
Statistical Analysis
We used the Statistical Package for Social Sciences (SPSS 22, SPSS Inc., Chicago, IL, USA) for the statistical analysis. The level of significance for all tests was p < 0.05.
The Shapiro–Wilk test was applied to determine the normal distribution of the variables. A one-way repeated measured analysis of variance (ANOVA), followed by three pairwise comparisons, was used to determine differences in tongue strength and tongue endurance among the three head postures. For our secondary objective, a secondary analysis by one-way ANOVA followed by three pairwise comparisons was used to determine differences in tongue strength and tongue endurance among men and women for the three tongue regions in the three head postures. Post hoc comparisons were conducted with the Bonferroni test. We calculated the effect size (Cohen’s d) to compare the study variables. Based on the Cohen method, the effect was considered small (0.20–0.49), medium (0.50–0.79), or large (> 0.8) [38].
Results
The total sample was 37 participants with a mean age of 23.85 ± 3.64 years. All variables analyzed had a normal distribution (p > 0.05). Any of the participants had pain or adverse symptoms during the procedure.
Tongue Strength
ANOVA revealed significant differences in the anterior tongue area, depending on the craniocervical postures (F = 4.42; p = 0.015; ηp2 = 0.11). The post hoc analysis showed significant differences between NHP and RHP, with a small effect size (p < 0.01; d = 0.25). The ANOVA also showed significant differences between medium tongue area and the craniocervical postures (F = 4.32; p = 0.01; ηp2 = 0.10). The post hoc analysis showed significant differences between FHP and RHP (p = 0.01; d = 0.23) and between NHP and RHP (p = 0.05; d = 0.27), both with a small effect size. The ANOVA did not reveal significant differences in the posterior tongue area (F = 0.91; p = 0.41; ηp2 = 0.02).
In addition, the ANOVA revealed significant differences in the FHP and tongue area (F = 4.48; p = 0.015; ηp2 = 0.11). The post hoc analysis showed significant differences only between the medium tongue area and the posterior tongue area, with a small effect size (p = 0.01; d = 0.23). The ANOVA also showed significant differences in the NHP (F = 5.76; p = 0.005; ηp2 = 0.138). The post hoc analysis showed significant differences between the anterior tongue area and the posterior tongue area (p = 0.03; d = 0.38), as well as between the medium tongue area and the posterior tongue area (p = 0.01; d = 0.27), both with a small effect size (Table 1). ANOVA did not reveal differences in RHP (F = 2.92; p = 0.06; ηp2 = 0.07).
Tongue Endurance
The ANOVA revealed significant differences in the FHP and tongue areas (F = 7.17; p = 0.001; ηp2 = 0.166). The post hoc analysis showed significant differences between the anterior and the posterior tongue areas, with a medium effect size (p = 0.005; d = 0.58). ANOVA also revealed significant differences for the NHP (F = 9.77; p < 0.001; ηp2 = 0.213). The post hoc analysis showed significant differences between the anterior and the posterior tongue areas, with medium effect size (p = 0.001; d = 0.60) and between the medium and the posterior tongue areas, with small effect size (p = 0.01; d = 0.39). Finally, ANOVA revealed significant differences for the RHP (F = 5.39; p = 0.007; ηp2 = 0.13). The post hoc analysis showed significant differences between the anterior tongue area and the posterior tongue area, with medium effect size (p = 0.04; d = 0.50), as well as between the medium and the posterior tongue areas, with small effect size (p = 0.001; d = 0.45) (Table 2).
The ANOVA did not reveal significant differences in tongue endurance for the anterior, posterior, or medium tongue areas and the different craniocervical postures (F = 0.59; p = 0.55; ηp2 = 0.02/F = 0.78; p = 0.46; ηp2 = 0.02/F = 0.32; p = 0.73; ηp2 = 0.01, respectively).
Secondary Analysis
For the secondary analysis, the total sample was distributed according to gender (20 men, 17 women).
Tongue Strength
Anterior Area
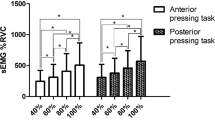
The ANOVA revealed significant differences in the anterior tongue area between men and women, depending on the craniocervical postures (F = 4.79; p = 0.015; ηp2 = 0.22). The post hoc analysis showed significant differences in FHP (p = 0.04; d = 1.02), NHP (p = 0.01; d = 1.24), and RHP (p = 0.05; d = 0.98) (Fig. 3).
Medium Area
The ANOVA revealed significant differences in the anterior tongue area between men and women, depending on the craniocervical postures (F = 5.09; p = 0.012; ηp2 = 0.23). The post hoc analysis showed significant differences in FHP (p = 0.01; d = 0.87), NHP (p < 0.01; d = 1.20), and RHP (p = 0.01; d = 0.88) (Fig. 4).
Posterior Area
The ANOVA did not reveal significant differences in the posterior tongue area between men and women, depending on the craniocervical postures (F = 0.98; p = 0.39; ηp2 = 0.05). However, the post hoc analysis showed significant differences in FHP (p = 0.01; d = 0.95), NHP (p = 0.01; d = 0.88), and RHP (p < 0.0; d = 1.01) (Fig. 5).
Tongue Endurance
The ANOVA did not reveal significant differences in tongue endurance for the anterior, medium or posterior tongue areas and the different craniocervical postures (F = 0.66; p = 0.52; ηp2 = 0.03/F = 0.96; p = 0.39; ηp2 = 0.02/F = 0.31; p = 0.74; ηp2 = 0.02, respectively).
Discussion
The main objective of the study was to determine differences in tongue strength and tongue endurance in different experimentally induced craniocervical postures. The results confirm our hypothesis, showing significant differences between the various craniocervical positions and the different tongue areas. Our results showed (1) that participants demonstrated more tongue strength in NHP than in RHP with the anterior area of the tongue and (2) demonstrated more tongue strength with NHP and FHP than with RHP in the medium tongue area. The results also suggest that by using the anterior and middle areas of the tongue, it is possible to develop greater strength and endurance than using the posterior tongue area.
These results are consistent with previous studies that showed that there were differences in the strength and resistance developed with the anterior area with respect to the posterior area [39]. It has been suggested that the variations in anatomical structure of the tongue in each area might justify these findings, but it might also be due to the specific function of each tongue area [7, 40].
With regard to craniocervical postures, it has been suggested that a greater ability to exert maximum strength performed with the tongue in NHP could be related to the fact that this position generates a greater biomechanical advantage in the production of strength of the tongue musculature [41]. The RHP might influence the hyoid bone, generating a poorer disposition for muscle contraction, which impairs the generation of strength [42]. NHP places the fibers in a stretching situation, but the length–force ratio is more relevant in sub-maximum and maximum stretching positions [41].
Moreover, previous studies have shown that the craniocervical position modifies the EMG activity of masticatory muscles [21]. FHP increases the EMG activity of the genioglossal muscle, which contributes in a substantial way to tongue strength production during the isometric contraction of the protrusion movement of the tongue or to the larger amplitude EMG activity of the mylohyoid muscle during swallowing [17, 43]. These studies suggest that FHP could entail a more effortful recruitment of the suprahyoid muscle that can impair tongue strength production. However, the present study is the first to provide direct information on a functional variable such as tongue strength and endurance in relation to the craniocervical posture.
Although our results suggest that NHP produces the most tongue force that can be generated, certain exercise methodologies for swallowing and speech disorders use modifications of the cervical and craniocervical postures to improve swallowing. These methodologies are based on the increased electromyographic activity of the suprahyoid musculature, compared to resting positions [44, 45]. Despite this, the use of these exercises is not only promoted for its ability to generate strength with the tongue, but also for its ability to generate changes in the structures of the larynx and pharynx compared to neutral positions [46].
Regarding the secondary aim of this study, the results showed that men were able to produce higher tongue strength than women in all three craniocervical positions, nonethless, no difference in tongue endurance was found. These results are consistent with those obtained by Youmans et al. and Stierwalt and Youmans who found that maximum tongue strength was greater in men. However, their results showed no significant differences in mean tongue strength exerted during shallowing between both genders [24, 25]. The anatomical differences between men and women may explain these differences, and the greater muscle mass, that men usually have, may translate into greater tongue strength [47]. Yet, the proportion of slow fibers may be similar between genders, which could explain the absence of differences in muscle endurance, similar to the changes associated with aging [48].
Finally, the fact that there is a relationship between the craniocervical posture, and the maximum tongue strength and tongue endurance suggests that the relationship should be kept in mind when measuring these variables clinically and for maximizing the results of training and rehabilitation of orolingual function. Furthermore, this study provides information on the influence of the craniocervical posture on the strength and resistance of the tongue. This information could help maximize the results of therapeutic exercise that encourage better training of tongue strength and resistance in patients with alterations in the lingual, oral, or craniomandibular function.
Limitations
The results of the study should be interpreted with caution, given it has certain limitations. First, the craniocervical posture was induced experimentally; future studies could evaluate the strength of the tongue considering the habitual posture of the participants. Additionally, the position of the hyoid, which plays an important role in the generation of tongue force, was not measured in this study. It is also possible that there was intra-individual variability. Besides, the sample is comprised of relatively young participants in a certain age range, which is difficult to extrapolate to other ages, because the variables of tongue strength and resistance tend to decrease with age [49, 50]. Finally, it is necessary to contrast these data with patients with craniomandibular alterations, as these results could be different and should be considered with caution in their transfer to clinical practice.
Conclusions
The findings of this study suggest that craniocervical posture influences tongue force, more significantly in the anterior and middle tongue areas of the tongue with respect to the posterior, and in the NHP and FHP postures with respect to the RHP. These data suggest that craniocervical posture should be a controlled factor, both for the measurement of tongue strength and tongue resistance, and to maximize the results of training and rehabilitation of orolingual function.
No differences were found in terms of resistance between the various craniocervical positions, but differences were found between the tongue areas studied. More studies are needed that extend the age range and include patient populations who have speech, swallowing or chewing disorders.
References
Zhao L, Monahan R. Functional assessment of the stomatognathic system. Clin Plast Surg. 2007;34:e1–e9.
Stål P, Marklund S, Thornell LE, et al. Fibre composition of human intrinsic tongue muscles. Cells Tissues Organs. 2003;173:147–61.
Sakamoto Y. Configuration of the extrinsic muscles of the tongue and their spatial interrelationships. Surg Radiol Anat. 2017;39:497–506.
Clark HM, Henson PA, Barber WD, et al. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. Am J Speech-Lang Pathol. 2003;12:40–50.
Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19(691–707):vii.
Mioche L, Hijemae K, Palmer J. A postero-anterior videofluorographic study of the intra-oral management of food in man. Arch Oral Biol. 2002;47:267–80.
Gingrich LL, Stierwalt JAG, Hageman CF, et al. Lingual propulsive pressures across consistencies generated by the anteromedian and posteromedian tongue by healthy young adults. J Speech Lang Hear Res. 2012;655:960–72.
Robinovitch SN, Hershler C, Romilly DP. A tongue force measurement system for the assessment of oral-phase swallowing disorders. Arch Phys Med Rehabil. 1991;72:38–42.
Sakamoto Y. Structural arrangement of the intrinsic muscles of the tongue and their relationships with the extrinsic muscles. Surg Radiol Anat. 2018;40:681–8.
Schimmel M, Ono T, Lam OLT, et al. Oro-facial impairment in stroke patients. J Oral Rehabil. 2017;44:313–26.
Kim HD, Choi JB, Yoo SJ, et al. Tongue-to-palate resistance training improves tongue strength and oropharyngeal swallowing function in subacute stroke survivors with dysphagia. J Oral Rehabil. 2017;44:59–64.
Valentim AF, Furlan RM, Perilo TV, et al. Relação entre a percepção da posição de língua pelo indivíduo e medidas de força da língua nos dentes. CoDAS. 2016;28:546–50.
Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Biol Med. 2003;14:413–29.
Yamada R, Ogawa T, Koyano K. The effect of head posture on direction and stability of mandibular closing movement. J Oral Rehabil. 1999;26:511–20.
Sanders RD. The trigeminal (V) and facial (VII) cranial nerves: head and face sensation and movement. Psychiatry (Edgmont). 2010;7:13–6.
Liu Y, Broman J, Zhang M, et al. Brainstem and thalamic projections from a craniovascular sensory nervous centre in the rostral cervical spinal dorsal horn of rats. Cephalalgia. 2009;29:935–48.
Milidonis MK, Kraus SL, Segal RL, et al. Genioglossi muscle activity in response to changes in anterior/neutral head posture. Am J Orthod Dentofacial Orthop. 1993;103:39–44.
Hanamoto H, Kadono K, Boku A, et al. Both head extension and mouth opening impair the ability to swallow in the supine position. J Oral Rehabil. 2014;41:588–94.
Sakuma T, Kida I. Relationship between ease of swallowing and deglutition-related muscle activity in various postures. J. Oral Rehabil. 2010;37:583–9.
La Touche R, París-Alemany A, von Piekartz H, et al. The influence of cranio-cervical posture on maximal mouth opening and pressure pain threshold in patients with myofascial temporomandibular pain disorders. Clin J Pain. 2011;27:48–55.
Ballenberger N, von Piekartz H, Paris-Alemany A, et al. Influence of different upper cervical positions on electromyography activity of the masticatory muscles. J Manip Physiol Ther. 2012;35:308–18.
Ohmure H, Miyawaki S, Nagata J, et al. Influence of forward head posture on condylar position. J Oral Rehabil. 2008;35:795–800.
Nicosia MA, Hind JA, Roecker EB, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A. 2000;55:M634–M640640.
Youmans SR, Stierwalt JAG. Measures of tongue function related to normal swallowing. Dysphagia. 2006;21:102–11.
Stierwalt JAG, Youmans SR. Tongue measures in individuals with normal and impaired swallowing. Am J Speech-Lang Pathol. 2007;16:148–56.
Youmans SR, Youmans GL, Stierwalt JAG. Differences in tongue strength across age and gender: is there a diminished strength reserve? Dysphagia. 2009;24:57–655.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9.
Lentell G, Kruse M, Chock B, et al. Dimensions of the cervical neural foramina in resting and retracted positions using magnetic resonance imaging. J Orthop Sport Phys Ther. 2002;32:380–90.
Enwemeka CS, Bonet IM, Ingle JA, et al. Postural correction in Persons with neck pain (II. integrated electromyography of the upper trapezius in three simulated neck positions). J Orthop Sports Phys Ther. 1986;8:240–2.
Hanten WP, Lucio RM, Russell JL, et al. Assessment of total head excursion and resting head posture. Arch Phys Med Rehabil. 1991;72:877–80.
Dunleavy K, Neil J, Tallon A, et al. Reliability and validity of cervical position measurements in individuals with and without chronic neck pain. J Man Manip Ther. 2015;23:188–96.
Audette I, Dumas J-P, Côté JN, et al. Validity and between-day reliability of the cervical range of motion (CROM) device. J Orthop Sports Phys Ther. 2010;40:318–23.
Hickey ER, Rondeau MJ, Corrente JR, et al. Reliability of the cervical range of motion (CROM) device and plumb-line techniques in measuring resting head posture (RHP). J Man Manip Ther. 2000;8:10–7.
Garrett TR, Youdas JW, Madson TJ. Reliability of measuring forward head posture in a clinical setting. J Orthop Sport Phys Ther. 1993;17:155–60.
Adams V, Mathisen B, Baines S, et al. Reliability of measurements of tongue and hand strength and endurance using the iowa oral performance instrument with healthy adults. Dysphagia. 2014;29:83–95.
Adams V, Mathisen B, Baines S, et al. A systematic review and meta-analysis of measurements of tongue and hand strength and endurance using the Iowa oral performance instrument (IOPI). Dysphagia. 2013;28:350–69.
Solomon NP, Munson B. The effect of jaw position on measures of tongue strength and endurance. J Speech Lang Hear Res. 2004;47:584–94.
Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum Associates Inc.; 1988.
Vanderwegen J, Guns C, Van Nuffelen G. The influence of age, sex, bulb position, visual feedback, and the order of testing on maximum anterior and posterior tongue strength and endurance in healthy belgian adults. Dysphagia. 2013;28:159–66.
Kennedy D, Kieser J, Bolter C, et al. Tongue pressure patterns during water swallowing. Dysphagia. 2010;25:11–9.
Rassier DE, MacIntosh BR, Herzog W. Length dependence of active force production in skeletal muscle. J Appl Physiol. 1999;86:1445–577.
Rocabado M. Biomechanical relationship of the cranial, cervical, and hyoid regions. J Craniomandib Pract. 1983;1:61–6.
Carroll B, Hunt S, Sheeleigh K, Wnukowski M. The influence of forward head posture on suprahyoid activity during oropharyngeal swallowing: a surface electromyographic analysis. CUNY Academic Works. 2015. https://academicworks.cuny.edu/gc_etds/802.
Yoon WL, Khoo JKP, Rickard Liow SJ. Chin tuck against resistance (CTAR): new method for enhancing suprahyoid muscle activity using a shaker-type exercise. Dysphagia. 2014;29:243–8.
Sze WP, Yoon WL, Escoffier N, et al. Evaluating the training effects of two swallowing rehabilitation therapies using surface electromyography—chin tuck against resistance (CTAR) exercise and the shaker exercise. Dysphagia. 2016;31:195–205.
Leigh J-H, Oh B-M, Seo HG, et al. Influence of the chin-down and chin-tuck maneuver on the swallowing kinematics of healthy adults. Dysphagia. 2015;30:89–988.
Adamo ML, Farrar RP. Resistance training, and IGF involvement in the maintenance of muscle mass during the aging process. Ageing Res Rev. 2006;5:310–31.
Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol. 2004;91:450–72.
Utanohara Y, Hayashi R, Yoshikawa M, et al. Standard values of maximum tongue pressure taken using newly developed disposable tongue pressure measurement device. Dysphagia. 2008;23:286–90.
Crow HC, Ship JA. Tongue strength and endurance in different aged individuals. J Gerontol A. 1996;51:M247–M250250.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: AP-A, RLT, JC-S. Methodology: AP-A, RLT, AP-A. Formal analysis and investigation: AP-A, RLT, DA-J, LS-M. Writing-original draft preparation: AP-A, RLT, AP-A, DA-J, LS-M, JC-S. Writing-review and editing: AP-A, RLT, AP-A, DA-J, LS-M, JC-S. Supervision: AP-A, RLT.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paris-Alemany, A., Proy-Acosta, A., Adraos-Juárez, D. et al. Influence of the Craniocervical Posture on Tongue Strength and Endurance. Dysphagia 36, 293–302 (2021). https://doi.org/10.1007/s00455-020-10136-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-020-10136-9