Abstract
Background
Tramadol–acetaminophen tablets are currently used to treat pain, including that of degenerative lumbar disease. Although there are many reports on tramadol–acetaminophen tablets, treatment outcomes in low back pain (LBP) patients with depression remain uncertain. This study investigated the outcomes of LBP patients with depression treated with tramadol–acetaminophen tablets.
Methods
Of 95 patients with chronic LBP, 70 (26 men, 44 women; mean age 64 years) who were judged as having depression by the Self-Rating Depression Scale (SDS) were included in this study. In this trial, patients received one of two randomly assigned 8-week treatment regimes: tramadol–acetaminophen (Tramadol group, n = 35) and non-steroidal anti-inflammatory drugs (NSAIDs) (NSAID group, n = 35). In addition to completing self-report questionnaires, patients provided demographic and clinical information. All patients were assessed using a Numerical Rating Scale (NRS), Oswestry Disability Index (ODI), Pain Disability Assessment Scale (PDAS), Hospital Anxiety and Depression Scale (HADS), SDS, and Pain Catastrophizing Scale (PCS).
Results
After 8 weeks’ treatment, the NRS and SDS scores were lower in the Tramadol group than in the NSAID group (p < 0.05). There were no significant differences in the ODI, PDAS, and PCS scores between the groups (p = 0.47, 0.09, 0.47). Although there was no difference in the anxiety component of the HADS between the groups (p = 0.36), the depression component was lower in the Tramadol group than in the NSAID group (p < 0.05). There was no significant difference between groups in the percentage of patients with treatment-associated adverse events.
Conclusions
This investigation found that tramadol–acetaminophen is effective for reducing LBP and provided a prophylactic antidepressant effect in chronic LBP patients with depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low back pain (LBP) is one of the most common musculoskeletal disorders afflicting the adult population. Consequently, patients use health services frequently because of long-term disability. Factors such as the degree of disc degeneration, nutrition, and mechanical stress affect LBP. However, many patients show no physical or radiographic signs of an organic disorder, yet they still present with severe back pain. In these cases, psychological and/or social factors may be linked to LBP and the resultant disability [1]. Because of the inadequate effect of antidepressants and non-steroidal anti-inflammatory drugs (NSAIDs) [1], the treatment of LBP is problematic in these cases. In addition to surgery and rehabilitation, psychiatric approaches can benefit both the mental disorder and the LBP [1]. Chronic LBP is a condition known to be due to both neuropathic and nociceptive pain mechanisms [2]. Patients with neuropathic pain showed higher ratings of pain intensity, with more co-morbidities such as depression, panic/anxiety, and sleep disorders [2].
The results of self-report questionnaires have indicated that the majority of patients with LBP admitted to a university hospital (77 %) were classified as depressed, including 39 % with severe depression [3]. Psychological factors, occupational disability, and somatization disorder have the potential to result in prolonged LBP [4]. Clinicians should bear in mind both the depression and the fear associated with chronic LBP.
Tramcet (TRAMCET Combination Tablets, Janssen Pharmaceutical K.K., Tokyo, Japan) is a combination of two drugs, 37.5 mg tramadol and 325 mg acetaminophen, in a single tablet. Tramadol is a centrally acting analgesic with weak µ-opioid agonist effects and weak inhibition of serotonin and norepinephrine reuptake [5]. This µ-opioid agonist activity may conceivably play a role in mood improvement [6, 7]. Tramadol has antidepressant-like effects in mice, mediated by the noradrenergic system rather than the serotoninergic or opioidergic systems [8]. Acetaminophen acts at both central and peripheral pathways [9]. Acetaminophen-induced antinociception involves a self-synergistic interaction between spinal and supraspinal sites [10]. However, its mechanism of action has not been clearly elucidated. Acetaminophen is often combined with other drugs to enhance its therapeutic efficacy. Acetaminophen and morphine in combination exert their antinociceptive effect through the opioidergic system [11]. Although there are many reports of tramadol–acetaminophen use, the antidepressant effect of tramadol–acetaminophen in chronic LBP patients remains uncertain. The purpose of this study was to determine the therapeutic efficacy of tramadol–acetaminophen as a treatment for pain and disability in LBP patients with depression.
Patients and methods
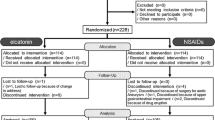
In this 8-week, prospective study performed at our hospital, the efficacies of the antineuropathic drug tramadol–acetaminophen and of NSAIDs in the treatment of chronic LBP patients with depression were compared. A total of 95 patients whose chief complaint was LBP, including both specific and non-specific LBP, were admitted to our hospital. Inclusion criteria included patients whose pain had persisted for more than 3 months, patients whose Self-Rating Depression Scale (SDS) score at recruitment was more than 40 points, and patients who agreed to answer the questionnaire. The SDS assesses the psychological and somatic symptoms of depression. It is commonly used to screen for depression in larger patient groups and to measure the severity of depression [12]. The SDS is a self-report, 20-question instrument with good internal consistency and validity that encompasses most DSM-IV criteria for major depression [13]. The SDS is the primary discriminating variable for distinguishing depressed from non-depressed patients [14]. The SDS index score ranges from 20 to 80. Exclusion criteria included patients with dementia, delirium, or other conditions that made it difficult to complete a self-reported written questionnaire, and patients with severe chronic disease that interfered with treatment (e.g., cardiovascular disease, renal failure, or other disqualifying conditions). Patients were randomly divided into two equal groups to receive either tramadol–acetaminophen tablets (TRAMCET Combination Tablets, Janssen Pharmaceutical K.K., Tokyo, Japan) or celecoxib (Celecox, Astellas Pharma Inc., Tokyo, Japan) for 8 weeks (Fig. 1). Randomization was performed by the second author, and the allocation sequence was generated using a computer program (Microsoft Excel) for simple randomization. Patients provided their demographic and clinical information. Patients in the Tramadol group took two tramadol–acetaminophen tablets/day. The doses of tramadol–acetaminophen tablets were titrated at the 1-week visit up to four tablets/day, unless side effects prevented the dose from being titrated further. Patients in the NSAID group took two celecoxoib tablets/day (200 mg/day) for 8 weeks. No other analgesics or anti-inflammatory medications were administered. Visits were scheduled for days 7, 14, 28, and 56. All patients included in this study gave their written, informed consent, and ethical approval for this study was obtained from the institutional review board.
Pain assessment
The Numeric Rating Scale (NRS) for pain self-assessment is a widely used, valid, and reliable tool to measure chronic pain intensity [15]. The NRS ranges from 0 to 10, with 0 representing no pain and 10 representing the worst pain imaginable. The NRS was obtained at baseline and after 8 weeks of treatment.
Physical disability assessment
Self-reported pain disability was assessed with the Oswestry Disability Index (ODI) [16]. The maximum score is 100 %, with a higher score indicating a high level of disability. The Pain Disability Assessment Scale (PDAS) contains items that assess the negative effects of pain on broad-spectrum pain interference domains [17]. The PDAS consists of 20 items scored using a 4-point Likert scale from 0 to 3 points, with scores ranging from 0 to 60 points. This scale is useful when clinicians require a multidimensional measure of the effects of pain in a patient’s life. Both the ODI and PDAS were obtained at baseline and after 8 weeks of treatment.
Anxiety and depression assessment
Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (HADS) [18]. The HADS is very useful in the assessment of anxiety and depression in patients with physical illness. It is a 14-item scale, seven items assessing anxiety and seven items assessing depression. Each item is scored from 0 to 3 using a Likert scale. Overall scores of either anxiety or depression can take values between 0 and 21, with higher scores indicating greater severity of symptoms. The HADS was obtained at baseline and after 8 weeks of treatment.
Pain catastrophizing assessment
Self-reported pain catastrophizing due to LBP was assessed with the Pain Catastrophizing Scale (PCS) [19]. The PCS is a broad measure of pain catastrophizing and consists of 13 items scored using 5-point Likert scales from 0 (never) to 4 (always) points. The maximum score for the PCS is 52, with higher scores indicating greater pain catastrophizing levels. A score of more than 24 indicates a high level of catastrophizing. The items are divided into three subscales: rumination, helplessness, and magnification. Rumination (items 8–11) “refers to the fact that the patient cannot get the idea of pain out of his/her head and cannot stop thinking about the pain”; helplessness (items 1–5 and 12) “refers to the estimation that the person has of not being able to do anything to influence the pain”; and magnification (items 6, 7, and 13) “refers to the exaggeration of the threatening properties of the painful stimulus.” High internal reliability has been reported in patients with chronic pain with adequate validity and test–retest reliability [20]. The PCS was obtained at baseline and after 8 weeks of treatment.
Statistical analysis
Normally distributed variables were compared using Student’s t test, and non-normally distributed variables were compared using the Mann–Whitney U test. Differences of p < 0.05 were considered significant. Statistical analysis was conducted using SPSS software version 13.0 for Windows. We hypothesized that we would observe a 20 % reduction of the SDS in the Tramadol group and a 10 % reduction in the NSAID group after treatment based on the preliminary study. A power analysis [using means of 40 points (Tramadol group) and 45 points (NSAID group) with a standard deviation of 7.0] estimated that 32 patients would be needed in each group to provide a 95 % chance of detecting such a reduction at the 0.05 level of significance.
Results
Participants
A group of 70 patients (26 men, 44 women) with LBP admitted to our hospital was included in this study. The mean duration from the onset of symptoms to consultation was 4 years and 2 months (3 months to 30 years), and the mean age at the time of examination was 64 years (30–84 years) (Table 1). In this series, 27 patients had lumber canal stenosis, 17 patients had lumber disc herniation, 15 patients had osteoarthritis, 5 patients had multiple compression fractures, 2 patients had degenerative spondylolisthesis, 2 patients had scoliosis, and 2 patients were post spine surgery.
Patient demographics
Seventy patients were randomly divided into two equal groups (Tramadol and NSAID groups; n = 35 patients per group). The mean age at the time of examination was 65.4 years (30–84 years) in the Tramadol group and 62.3 years (31–81 years) in the NSAID group. The mean pain duration was 46 months (3–240 months) in the Tramadol group and 54.2 months (3–360 months) in the NSAID group. No significant differences between the two groups were observed with regard to both age (p = 0.37) and pain duration (p = 0.64) (Table 1). There were no significant differences between the two groups in terms of mean SDS (p = 0.79), NRS (p = 0.34), ODI (p = 0.54), PDAS (p = 0.66), HADS (anxiety p = 0.96, depression p = 0.75), and PCS (p = 0.81) scores (Table 1).
Treatment effectiveness with tramadol–acetaminophen or NSAID
Both the SDS and ODI scores in both the Tramadol and NSAID groups were significantly decreased after treatment (p < 0.05, Table 2). A significant difference between the treatment groups was found for the SDS score after treatment, indicating a reduction in level of depression in the Tramadol group (p < 0.05). The NRS scores in both the Tramadol and NSAID groups were significantly decreased after treatment (p < 0.05). Compared with the NSAID group, the NRS score in the Tramadol group was significantly lower after treatment (p < 0.05), indicating enhancement of internal pain control by tramadol–acetaminophen. There were no significant differences between the treatment groups for both PDAS (p = 0.14) and PCS (p = 0.55) scores after 8 weeks of treatment. No significant difference was found between the treatment groups for the mean HADS anxiety scores (p = 0.77) after 8 weeks of treatment, but a significant difference was found for the mean HADS depression score (p < 0.05), indicating an antidepressant effect of tramadol–acetaminophen treatment.
Side effects
Twenty-two patients (62.9 %) reported adverse events during tramadol–acetaminophen treatment. Sixteen patients (45.7 %) reported adverse events during NSAID treatment. The difference in the overall frequency of side effects between the treatment groups was not significant (p = 0.15). The most commonly reported adverse events related to the study medication for both groups are given in Table 3. In the present study, no patients withdrew because of adverse events in either group.
Discussion
In the present study, the combination of tramadol and acetaminophen had efficacy not only for the reduction of pain intensity, but also for the reduction of depressive symptoms, with tolerable adverse events during the course of treatment. Although somnolence (25.7 %), nausea (22.9 %), and constipation (11.4 %) were noted as side effects of tramadol–acetaminophen, all episodes of somnolence and nausea were transient and comparable to those in the NSAID group. No patients had to be dropped from the treatment.
In one study, pain catastrophizing and kinesiophobia were significant predictors of chronic LBP and associated disability 6 months after initial evaluation [21]. Pincus et al. [22] concluded that there was robust evidence for a role of negative mood (distress or depression) on the transition to chronic pain status, along with limited evidence for the facilitating effects of catastrophizing and somatization. Psychological factors are among the causes of the transition to chronic LBP [1, 22]. In addition to long-term symptoms, the association of chronic pain, anxiety/depression, and sleep disorders, also referred to as the triad of pain, causes functional impairment in many areas of life [23]. Thus, not only biological treatment, but also psychological/psychosocial treatment is necessary in these cases. However, there is no clear evidence that antidepressants reduce pain, depression, or functional status in patients with chronic LBP [1]. In the current study, patients were divided into two groups (Tramadol and NSAID groups) to evaluate the outcomes of tramadol–acetaminophen in LBP patients with depression. Although the SDS score in the NSAID group decreased after treatment, this reduction of the SDS score might be explained by the indirect effects attributed to changes in depressive symptoms, not by the direct effects of NSAIDs. On the other hand, the SDS score was significantly lower in the Tramadol group than in the NSAID group. Additionally, although there was no significant difference between the two groups in the mean HADS anxiety score, the mean HADS depression score was significantly lower in the Tramadol group than in the NSAID group. These results indicate that tramadol–acetaminophen might have an antidepressant-like activity. However, there are several types of chronic pain patients, such as those in whom depression causes severe pain, those in whom chronic pain causes depression, and those in whom they both interact. In the present study, the NRS score after treatment was significantly lower in the Tramadol group than in the NSAID group. Therefore, a portion of the significant pain reduction in the Tramadol group may potentially have an effect on the secondary reduction of the depression score. However, some reports supporting our results exist. There are several reports studying the antidepressant-like activity of tramadol in animal models of depression. The antidepressant-like effect of tramadol in mice was mediated through interaction with the noradrenergic system [24, 25] and was comparable with that of fluoxetine (SSRI) [26].
There are several case reports demonstrating the effect of tramadol as an antidepressant in vivo. Shapira et al. [27] reported a chronic major depressive disorder patient with facial pain who had failed antidepressant medications and several anxiolytic medications. Tramadol improved his depressive symptoms by 60–70 %. Reeves and Cox [28] reported that a chronic LBP patient who underwent a laminectomy for a lumbar disc herniation developed significant depression following cessation of tramadol after several years of therapy. These clinical results might indicate that tramadol may exert an antidepressant effect in patients with chronic pain and support the results of the current study.
Since most pain conditions involve a large number of different pathways, analgesic therapy with a single agent may be insufficient to reduce chronic pain. Combination analgesics with two or more agents may have synergistic analgesic effects and could provide more effective pain relief for a broader spectrum of pain [29]. The combination of individually ineffective doses of tramadol and acetaminophen may provide adequate pain relief through the actions of different pathways and may reduce adverse events [29]. Tramadol is popular because of its low potential for addiction and quick-acting properties compared with other opioid analgesics [30]. Furthermore, acetaminophen is a short-acting and rapidly acting analgesic compared to tramadol [31]. Consequently, tramadol–acetaminophen was superior to tramadol alone with respect to onset of pain relief in the treatment of dental pain [32]. In the present study, tramadol–acetaminophen provided pain relief over the 2-month duration, and the mean NRS score was significantly lower in the Tramadol group than in the NSAID group. This might be explained by the two different pain pathways affected by tramadol and acetaminophen.
The current study has some limitations. First, in order to reduce the side effects of tramadol–acetaminophen tablets, the doses of tramadol–acetaminophen tablets were titrated at the 1-week visit from two tablets/day to four tablets/day, unless side effects prevented the dose from being titrated further. On the other hand, patients in the NSAID group stayed on two celecoxoib tablets/day (200 mg/day) for the whole study. Thus, the results may be attributed not to the drugs themselves, but rather to the difference in dose. Second, this study included patients with LBP caused by various degenerative lumbar diseases containing a high proportion of lumbar canal stenosis. To evaluate the outcomes of tramadol–acetaminophen tablets more precisely, it is desirable to study patients with certain lumbar disease. Finally, tramadol–acetaminophen tablets were given to chronic pain patients in this study. In order to evaluate strictly the anti-depressive effects of tramadol–acetaminophen tablets, tramadol–acetaminophen tablets should be administered to depressed patients without chronic pain. Although this study has these limitations, not only NRS, but also SDS and HADS depression scores in the Tramadol group were significantly decreased after treatment (p < 0.05). The results of the current study suggest that tramadol–acetaminophen might be an attractive alternative treatment option for chronic LBP patients with depression not responding to NSAIDs and/or antidepressants.
References
Chan HN, Fam J, Ng BY. Use of antidepressants in the treatment of chronic pain. Ann Acad Med Singap. 2009;38(11):974–9.
Freynhagen R, Baron R, Gockel U, Tolle TR. Pain detect: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–20.
Tetsunaga T, Misawa H, Tanaka M, Sugimoto Y, Tetsunaga T, Takigawa T, Ozaki T. The clinical manifestations of lumbar disease are correlated with self-rating depression scale scores. J Orthop Sci. 2013;18(3):374–9.
Carragee EJ, Chen Y, Tanner CM, Hayward C, Rossi M, Hagle C. Can discography cause long-term back symptoms in previously asymptomatic subjects? Spine (Phila Pa 1976). 2000;25(14):1803–8.
Adams EH, Breiner S, Cicero TJ, Geller A, Inciardi JA, Schnoll SH, Senay EC, Woody GE. A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. J Pain Symptom Manage. 2006;31(5):465–76.
Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15(1):49–57.
Stoll AL, Rueter S. Treatment augmentation with opiates in severe and refractory major depression. Am J Psychiatry. 1999;156(12):2017.
Rojas-Corrales MO, Gibert-Rahola J, Mico JA. Tramadol induces antidepressant-type effects in mice. Life Sci. 1998;63(12):PL175–80.
Bonnefont J, Courade JP, Alloui A, Eschalier A. Antinociceptive mechanism of action of paracetamol. Drugs. 2003;63(Spec No 2):1–4.
Raffa RB, Stone DJ Jr, Tallarida RJ. Discovery of “self-synergistic” spinal/supraspinal antinociception produced by acetaminophen (paracetamol). J Pharmacol Exp Ther. 2000;295(1):291–4.
Sandrini M, Romualdi P, Capobianco A, Vitale G, Morelli G, Pini LA, Candeletti S. The effect of paracetamol on nociception and dynorphin. A levels in the rat brain. Neuropeptides. 2001;35(2):110–6.
Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70.
Zung WW. A cross-cultural survey of symptoms in depression. Am J Psychiatry. 1969;126(1):116–21.
Thurber S, Snow M, Honts CR. The Zung self-rating depression scale: convergent validity and diagnostic discrimination. Assessment. 2002;9(4):401–5.
Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–26.
Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–3.
Yamashiro K, Arimura T, Iwaki R, Jensen MP, Kubo C, Hosoi M. A multidimensional measure of pain interference: reliability and validity of the pain disability assessment scale. Clin J Pain. 2011;27(4):338–43.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The pain catastrophizing scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23(4):351–65.
Morris LD, Grimmer-Somers KA, Louw QA, Sullivan MJ. Cross-cultural adaptation and validation of the South African pain catastrophizing scale (SA-PCS) among patients with fibromyalgia. Health Qual Life Outcomes. 2012;10:137.
Picavet HS, Vlaeyen JW, Schouten JS. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156(11):1028–34.
Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976). 2002;27(5):E109–20.
Tarride JE, Gordon A, Vera-Llonch M, Dukes E, Rousseau C. Cost-effectiveness of pregabalin for the management of neuropathic pain associated with diabetic peripheral neuropathy and postherpetic neuralgia: a Canadian perspective. Clin Ther. 2006;28(11):1922–34.
Chowta MN, Manjunath M, Gopalakrishna HN, Gokul P. Evaluation of role of noradrenergic system in the antidepressant activity of tramadol using tail suspension test in albino mice. J Pharmacol Pharmacother. 2011;2(4):281–2.
Yalcin I, Aksu F, Bodard S, Chalon S, Belzung C. Antidepressant-like effect of tramadol in the unpredictable chronic mild stress procedure: possible involvement of the noradrenergic system. Behav Pharmacol. 2007;18(7):623–31.
Kalra BS, Tayal V, Chawla S. Antidepressant-like activity of tramadol in mice. Indian J Psychiatry. 2008;50(1):51–3.
Shapira NA, Verduin ML, DeGraw JD. Treatment of refractory major depression with tramadol monotherapy. J Clin Psychiatry. 2001;62(3):205–6.
Reeves RR, Cox SK. Similar effects of tramadol and venlafaxine in major depressive disorder. South Med J. 2008;101(2):193–5.
Raffa RB. Pharmacology of oral combination analgesics: rational therapy for pain. J Clin Pharm Ther. 2001;26(4):257–64.
Barber J. Examining the use of tramadol hydrochloride as an antidepressant. Exp Clin Psychopharmacol. 2011;19(2):123–30.
Mejjad O, Serrie A, Ganry H. Epidemiological data, efficacy and safety of a paracetamol–tramadol fixed combination in the treatment of moderate-to-severe pain. SALZA: a post-marketing study in general practice. Curr Med Res Opin. 2011;27(5):1013–20.
Medve RA, Wang J, Karim R. Tramadol and acetaminophen tablets for dental pain. Anesth Prog. 2001;48(3):79–81.
Acknowledgments
The authors would like to thank sincerely Yoshihisa Sugimoto, MD, Shinya Arataki, MD, and Tomoyuki Takigawa, MD, for their advice and expertise in the production of this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tetsunaga, T., Tetsunaga, T., Tanaka, M. et al. Efficacy of tramadol–acetaminophen tablets in low back pain patients with depression. J Orthop Sci 20, 281–286 (2015). https://doi.org/10.1007/s00776-014-0674-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-014-0674-4