Abstract
The aim of this study was to compare the efficacy of elcatonin injections and oral nonsteroidal anti-inflammatory drugs (NSAIDs) for patients with osteoporosis who have acute lumbar pain after experiencing new vertebral compression fractures. Two hundred twenty-eight Japanese female patients (mean age 77.3 years) with acute lumbar pain from osteoporotic vertebral fractures were randomly divided into two groups. Patients in one group were given an NSAID (NSAIDs group) and patients in the other group were given weekly intramuscular injections of 20 units of elcatonin (elcatonin group). All patients underwent follow-up examinations up to 6 weeks from the start of the trial. Outcome measures were the level of functional impairment according to the Japan Questionnaire for Osteoporotic Pain (JQ22), the Roland–Morris Disability Questionnaire (RDQ), and a visual analog scale (VAS) of pain intensity. Statistical analyses focused on (1) the time course of pain and functional level using linear mixed effects models to analyze the longitudinal data and (2) the effectiveness of elcatonin injection with mean difference values and 95 % confidence intervals. Significant differences were seen over time between the initial values and the postintervention values (4 and 6 weeks) in JQ22, RDQ, and VAS scores (effect size d > 0.4) in each group. The mean differences between the elcatonin group and the NSAIDs group in each measure at 4 and 6 weeks were −4.8 and −8.3 for the JQ22, −1.3 and −2.6 for the RDQ, and −11.3 and −11.5 for the VAS, shifted to elcatonin. Once weekly elcatonin injection was more effective than NSAIDs for treating acute lumbar pain and improving mobility in Japanese women with osteoporotic vertebral fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a serious geriatric medicine problem in “super-aging” societies such as Japan. With osteoporosis, fractures are likelier to occur in the vertebrae, proximal part of the femur, distal part of the radius, and proximal part of the humerus because of bone fragility [1]. Osteoporosis-associated fractures often result in impairment of activities of daily living such as walking and housework. These impairments can lead to a person becoming bedridden or isolated in the home, resulting in many elderly people needing further care.
The number of people with osteoporosis is estimated to be about 12.8 million [2] in Japan’s aged society. In addition, the number of elderly people and the population aging rate are continuing to increase. Such an increase in the number of elderly people will naturally mean increases in the incidence of osteoporosis and osteoporosis-associated fractures, which are important public health issues that should be dealt with at national and local levels.
Many elderly people have ambulation symptom complex due to musculoskeletal disability; therefore, it is important for them to be able to get out of bed and walk at an early stage after a compression fracture to prevent the development of disuse syndrome.
Calcitonin is a polypeptide comprising 32 amino acids, and plays a role in the regulation of bone metabolism as a hormone that suppresses bone resorption [3]. Salmon calcitonin and eel calcitonin show high specific activity and have been used as a treatment drug for osteoporosis. Elcatonin is an analog of eel calcitonin and is known to increase bone mineral density [4, 5] and to have an antihyperalgesic effect via the serotonergic system [6, 7] in animal models. Once weekly intramuscular injection of 20 units of elcatonin is widely used in Japan for the treatment of osteoporosis. It was reported that elcatonin administered once weekly significantly increased bone mineral density [8] and alleviated postfracture pain and improved quality of life in patients with osteoporosis [9–11]. However, a comparative study with widely used painkillers focusing on pain management and functional impairments and quality of life as a randomized controlled trial has not been reported.
In this trial we compared the efficacy of elcatonin injections, which are expected to be effective for treatment of acute osteoporotic lumbar pain, with that of nonsteroidal anti-inflammatory drugs (NSAIDs), and investigated whether elcatonin is beneficial in promoting early ambulation and preventing functional deterioration.
Materials and methods
Clinical research design and participants
Clinical research design
This was a nationwide, prospective, multicenter, open-label, randomized controlled trial conducted at private clinics and hospitals that are registered members of the Japanese Clinical Orthopaedic Association. It was a research project of the Japanese Society for Musculoskeletal Medicine (formerly the Japanese Society for Musculoskeletal Rehabilitation).
Ethics review
The internal clinical research review committees at the registered hospitals approved the protocol of this clinical trial. The standard operating protocol was inspected by the Clinical Research Review Committee of the National Research Center for People with Disabilities for private clinics that did not have internal clinical research review committees. Written informed consent was obtained from all patients deemed suitable as participants. This trial was approved by the Japanese Society for Musculoskeletal Medicine and registered in the Clinical Trials Registry operated by the University Hospital Medical Information Network (http://center.umin.ac.jp/cgi-open-bin/ctr/C000000283).
Patients
Patients were defined as elderly women with lumbar pain caused by new fragility vertebral fractures due to primary osteoporosis. Patients were recruited from among outpatients examined by the Japanese Clinical Orthopaedic Association members. Patient selection was conducted continuously during the period of scheduled medical interviews (July 2008 to May 2010).
Inclusion criteria
The inclusion criteria for this clinical trial were as follows:
-
1.
Women aged 65 years or older
-
2.
Acute lumbar pain within the preceding 2 weeks (in the area from the inferior edge of the scapula to the gluteal sulcus)
-
3.
X-ray findings of new fragility fractures in the thoracic or lumbar vertebrae
-
4.
Adequate understanding of this clinical trial and the ability to provide written informed consent
The exclusion criteria were as follows:
-
1.
Patients with secondary osteoporosis
-
2.
Patients with a history of back surgery of the thoracic or lumbar vertebrae
-
3.
Clear neurological deficit associated with vertebral disease
-
4.
Severe scoliosis
-
5.
Contraindications for the drugs used (elcatonin or NSAIDs).
-
6.
Patients with an infectious disease in the vertebrae
-
7.
Patients who used an NSAID continuously within 3 days before consenting to be included in this clinical trial
-
8.
History of treatment for heart failure, renal dysfunction (serum creatinine level greater than 3.0 mg/dL), or hepatic dysfunction (aspartate aminotransferase and alanine aminotransferase levels greater than 100 IU/L)
-
9.
History of treatment for malignant tumor
-
10.
Patients whose primary physician considered them to be inappropriate for this clinical trial
According to the definitions in “Diagnostic criteria for primary osteoporosis: (year 2000 revision)” [12], fragility fractures are fractures attributable to decreased bone volume (osteoporotic changes confirmed by a bone mineral density of less than 80 % of the young adult mean at the vertebrae) and nontraumatic fragility fractures that occur with very mild external force.
Therefore, the diagnostic inclusion criteria for new fragility vertebral fractures in this clinical trial were (1) new fragility vertebral fractures confirmed on radiographs or (2) no new radiologically confirmed fragility vertebral fractures, but clinical findings of (1) acute lumbar pain, (2) severe pain associated with activity and almost no pain during rest, and (3) percussion pain in related areas.
In cases when a cause could be clearly specified, such as falling or lifting a heavy object, clinical decisions were made with reference to that cause. After inclusion criteria and exclusion criteria had been applied and patients who met the two conditions described above had been considered to be suitable as participants in this clinical trial, patients were invited to participate in the clinical trial.
Randomization
Patients who consented were randomly assigned to either the elcatonin group or the NSAIDs group by means of a randomization program. Permuted-block randomization was done with a block size of four, and a randomization sequence was produced with a computer-generated random number table. Blocks were assigned in accordance with the number of patients included at each participating clinic or hospital. This was designed to obtain a balance between each preregistered institution and the entire patient group.
Radiograph-based diagnostic confirmation
As entry criteria for new vertebral fractures, we combined confirmed findings on radiographs and three clinical findings.
Anteroposterior and lateral radiographs of the thoracic vertebrae (focused on T8) and lumbar vertebrae (focused on L3) were obtained with patients in the supine position, and were used in confirming the diagnosis. The radiograph was collected by the review team after the primary physician had explained the findings to the patient.
The radiographic films were sent to the administration office, and the findings were confirmed by an independent team of orthopedic surgeons. Three physicians evaluated the radiographs independently, and a majority decision was made in cases where there was disagreement.
Intervention
Patients who were randomly allocated to the elcatonin group or the NSAIDs group received the treatment described in the following sections.
Elcatonin group
Twenty units of elcatonin were injected intramuscularly once weekly for 6 weeks.
NSAIDs group
Oral NSAIDs were administered daily. Patients were given one of the five most widely sold NSAIDs in Japan today: loxoprofen sodium (three 60-mg tablets, one tablet costs ¥17.5), diclofenac sodium (three 25-mg tablets, one tablet costs ¥13.1), etodolac (two 200-mg tablets, one tablet costs ¥27.6), lornoxicam (three 4-mg tablets, one tablet costs ¥25.8), or zaltoprofen (three 80-mg tablets, one tablet costs ¥19.2). Patients were not permitted to take any other pain medication. Rebamipide (100-mg tablet), sodium azulene sulfonate (500-mg granules), or teprenone (50-mg capsules) was administered to prevent gastrointestinal side effects from the NSAIDs. Patients could cease taking the NSAIDs at any time if treatment was no longer necessary.
Patients were also asked to undergo outpatient examinations at least once a week. Dosages and adverse effects were closely checked by means of an activity diary along with an NSAID diary.
Outcome measures
Outcome measures were assessed at week 0 (baseline, i.e., start of intervention) and weeks 4 and 6 (completion of intervention and follow-up). The results were not disclosed to the primary physicians until the clinical trial was completed.
Pain was assessed with a visual analog scale (VAS) from 0 (minimum) to 10 (strongest pain). Patients were asked to score their pain during the past several days. Functional impairment, and thereby quality of life, was assessed with the validated Japanese version of the Roland–Morris Disability Questionnaire (RDQ) [13, 14] and the Japan Questionnaire for Osteoporotic Pain (JQ22) [15]. The JQ22 consists of 22 self-rated questions specialized to the disease, developed with consideration of the cultural differences between Japan and Western countries. Its validity and reliability have been demonstrated by computational psychological analysis. The JQ22 incorporates a psychological model concept for lumbar pain and reflects unique Japanese cultural aspects related to osteoporetic pain. The 22 questions in four domains are scored on a five-point scale from no disability (0 points) to severe disability (4 points), summed to obtain a total score (maximum 88 points).
Patients were asked to complete the questionnaires themselves.
Calculation of sample size
We estimated the sample size in nonparametric statistics using the level of significance (1 minus confidence) and demonstration level for reliability [16].
To achieve an 80 % power for detection of a clinically meaningful difference, 54 participants were needed to confirm the observed difference between the two groups. Considering the possibility of dropout or other problems, we set 100 participants in each group in this trial.
Minimal clinically important difference
Patients were asked to subjectively assess each intervention at week 6. This general self-assessment was divided into three categories: (1) improvement with no need to continue treatment, (2) condition improved, but would like to continue part of treatment, and (3) no change, or exacerbation. On the basis of this assessment, patients were divided into two groups of improved condition (categories 1 and 2) or no change/exacerbation (category 3). These results were used as data to calculate the minimal clinically important difference of the assessment scales.
Statistical analysis
The baseline JQ22, RDQ, and VAS results for the 228 randomly assigned patients in the elcatonin and NSAIDs groups were analyzed by means of the Mann–Whitney U test. Student’s t test analysis was applied for patient age.
As a first step to check the difference between the two groups regarding vertebral fractures, we examined the degree of pain at each of 13 levels (T5 to L5) of vertebrae from both groups. Nonparametric comparisons with the JQ22 for all possible pairs (13 levels; 13 C 2 = maximum 78 pairs) of the spine from the two groups were conducted with the Steel–Dwass method. As a second step we checked the distribution of the confirmed sites of fractured vertebrae using Cochran–Mantel–Haenszel statistics calculations.
Comparisons of patients who had completed the interventions were made between the baseline and week 4 or week 6 in each group by means of a linear mixed effects model.
Assessment items for drug effectiveness were compared on the basis of the difference in the change between the two groups at week 4 and week 6. First Levene’s test for equality of variances was conducted, and then a t test for equality of means was performed and the 95 % confidence interval for the mean difference between the baseline (week 0) and week 4 or week 6 for each assessment item was calculated. The 95 % confidence interval for the mean difference for each assessment item was calculated according to the method reported by Altman et al. [17].
Statistical analysis was done with SPSS Statistics for Windows version 17.0 (SPSS, Chicago, IL, USA) and G*Power (http://www.gpower.hhu.de/fileadmin/redaktion/Fakultaeten/Mathematisch-Naturwissenschaftliche_Fakultaet/Psychologie/AAP/gpower).
Role of funding
The trial was selected as a grant study as part of the 2008 academic research projects of the Japanese Society for Musculoskeletal Medicine. Neither the research group nor the physicians participating in this clinical trial had any vested financial interests in the trial.
The organization entrusted with the clinical trial administration did not play any role in the design of the trial, the collection, analysis, and interpretation of data, or the writing of this article. The contact author had complete access to the clinical trial data and had the ultimate responsibility with regard to decisions on the submission and publication of this article.
Results
Clinical trial profile
This multicenter clinical trial was conducted at 92 clinics and hospitals in all parts of Japan. Only orthopedic surgeons who were members of the Japanese Clinical Orthopaedic Association and who had received official certification from an expert committee of the Japanese Clinical Orthopaedic Association participated in this trial.
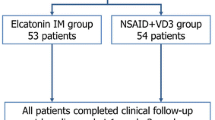
All 228 candidates for participation who registered with the administrative office from July 2008 to May 2010 were suitable as participants and consented to randomization (114 allocated to each of the elcatonin and NSAIDs groups). Figure 1 shows the flowchart of the clinical trial. Table 1 show the baseline features of the patients included in the trial. No statistical differences were seen between the elcatonin and NSAIDs groups with regard to age and baseline JQ22, RDQ, and VAS measures with Student’s t test or the Mann–Whitney U test (Table 1).
We obtained the spinal X-rays of 177 patients (83.5 %) from their attending physicians, and confirmed the number and level of vertebral fractures in each case. Because radiological findings consisted of fresh and old vertebral fractures and did not cover the whole cases, we statistically analyzed pain and fresh vertebral fractures by a two-stage method.
Nonparametric comparisons with the JQ22 for all possible pairs of spinal vertebrae from the two groups were conducted with the Steel–Dwass method. Extraction of the level with value 0 (no cases present) was not conducted. No difference on the JQ22 was seen between every available pair from the two groups at the baseline. Available X-rays and MRI images of vertebral fractures at the baseline were checked for fresh fractures. The distribution of the levels of fresh fractures (N = 145), which were confirmed by clinical findings such as tapping pain on the fractured spine, ranged from T5 to L5, mainly between T11 and L3 (Table 2).
According to Cochran–Mantel–Haenszel statistics, the p value for general association was 0.383. The results showed the fracture level did not depend on the groups.
Outcome measures
Progress of clinical manifestation was assessed by a linear mixed effects model. This linear mixed effects model is preferable when the number of participants is sufficiently large and the proportion of missing data is small enough. The results are shown as effect size (Cohen’s d); d > 0.2, small; d > 0.5 medium; d > 0.8, large).
There were statistically significant differences between the elcatonin and NSAIDs groups in the change in the JQ22, RDQ, and VAS scores (Table 3). Pain recovery showed an obvious effect size at week 4, but functional condition was slightly delayed until week 6.
The necessary data were obtained even after some dropout cases during the 4 and 6 weeks since the baseline, at which time there were 109 patients in the elcatonin group and 105 patients in the NSAIDs group. The mean differences between the elcatonin and NSAIDs groups for all three outcome measures are shown with the 95 % confidence intervals in Table 4. The mean differences between the elcatonin group and the NSAIDs group for each measure at weeks 4 and 6 were −4.8 and −8.3 for the JQ22, −1.3 and −2.6 for the RDQ, and −11.3 and −11.5 for the VAS (Fig. 2; the RDQ score was converted from a maximum of 24 to 100 to make it uniform with the other scores).
Adverse or undesirable events
No dropouts associated with adverse events were seen in the elcatonin group. Drug administration was discontinued in one patient because of an upper gastrointestinal tract disorder and in two patients because of drug eruption in the NSAIDs group.
Discussion
Background to elcatonin injection
Elcatonin (once weekly injection of 20 units) has a 30-year history of use in Japan as an osteoporosis treatment drug, and is widely used in clinical settings. In contrast, nasally administered salmon calcitonin is mainly used in other countries. A systematic review of the analgesic effect of nasally administered salmon calcitonin [18] concluded that pain scores with activities of daily living significantly and continuously decreased with administration of this agent over a period from 1 to 4 weeks after initiation of treatment. The American Academy of Orthopedic Surgeons treatment guideline for osteoporotic vertebral fractures also recommends calcitonin agents in the treatment of osteoporotic vertebral compression fractures [19]. Therefore, it is reasonable to use calcitonin to treat vertebral fragility fractures.
In Japan, an injectable form of calcitonin is used in once weekly injections and is highly regarded in terms of alleviating pain attributable to osteoporosis [1]. Moreover, since there are few serious adverse effects, early alleviation of pain and improved quality of life may be expected, making it one of the drugs of choice immediately after fracture or for patients in whom postural abnormalities or other problems occur in conjunction with vertebral fractures [1].
This trial was a nationwide, multicenter, prospective, randomized clinical trial investigating the effects of elcatonin on acute lumbar pain from osteoporotic vertebral fractures. Treatment effects were evaluated with the JQ22, the RDQ, and a VAS, with a focus on functional impairments or social participation status, and pain management effects of patients with new fragility vertebral fractures.
The results suggested that elcatonin treatment had greater efficacy than NSAID treatment in alleviating pain and improving quality of life when used early following vertebral fractures. The trial suggests an adequate analgesic effect with weekly administration of injectable elcatonin for osteoporosis patients, even though blinding was not possible.
These findings are similar to those of various reports on the analgesic effects of nasally administered salmon calcitonin for treatment of pain caused by vertebral fractures [20].
Economical considerations of elcatonin injection
The drug prices of the NSAIDs for 1-week use are as follows: ¥367.5 for loxoprofen sodium (60 mg), ¥275.1 for diclofenac sodium (25 mg), ¥386.4 for etodolac (200 mg), ¥541.8 for lornoxicam (4 mg), and ¥403.25 for zaltoprofen (80 mg). Compared with the price of elcatonin, the prices of the NSAIDs are less than half on a weekly basis. Prescription of elcatonin is restricted to a maximum of 6 months.
Types of osteoporotic pain
In general, osteoporotic pain includes both acute pain occurring immediately after a vertebral fracture and chronic pain accompanying spinal deformity and reduced bone mineral density. Pain that occurs immediately after a fracture corresponds to the site of the fractured vertebra and is characterized by greater pain during movement, such as turning over in bed or getting up from bed. Acute lumbar pain associated with vertebral fracture is thought to be due to causes such as local tissue strain with a fracture, inflammation, increased acid (H+) with higher bone resorption, and nerve damage [21].
In contrast, whether the decreased bone mass associated with osteoporosis is a direct cause of pain has not been sufficiently elucidated. This may be due to the difficulty in establishing clinical relationships given the diverse causes of osteoporotic pain. Experimentally, it has been reported that the pain threshold decreased and there was lasting hyperalgesia in ovariectomized mice (a model of postmenopausal osteoporosis) [22], and it has been proposed that excessive bone resorption possibly induces pain [23].
Recently the effect of combining elcatonin treatment with bisphosphonate (risedronate) treatment for patients with chronic back pain was reported, and the combination resulted in alleviation of pain and improvement of function [24].
Working mechanism of elcatonin injection
Since calcitonin inhibits osteoclastic bone resorption, there is a possibility that pain can be mitigated with this action. Most osteoporosis-associated nociceptive stimuli are exacerbated by C-fiber dysfunction, and calcitonin is thought to express its analgesic effect by inhibiting this action [25]. Calcitonin is also reported to improve blood flow, and is thus thought also to have an effect in alleviating pain associated with blood circulation disorders. Calcitonin is thought to express its analgesic effect for osteoporotic pain through this diverse-acting mechanism [26].
Future fracture prevention is the most important component of osteoporosis treatment. Risk factors for osteoporotic fractures include old age, low bone density, and preexisting fractures. Among these risk factors, preexisting vertebral fractures are known to increase fourfold the risk of further vertebral fractures and to double the risk of all fractures [27]. Once a fracture occurs (where there is a preexisting fracture), the risk of subsequent fracture and hip fracture increases and there is a strong need for active osteoporosis treatment immediately after the initial fracture. The occurrence of a vertebral fracture is a unique opportunity to start osteoporosis treatment, and when a vertebral compression fracture occurs, drug treatment is effective for analgesia and the prevention of osteoporotic progression. General bone metabolism is upregulated in the healing phase of fragility vertebral fractures [28–30], but the use of bone resorption inhibitors from an early stage is a reasonable treatment to inhibit that component of upregulation. The occurrence of pain due to fracture can promote decreased muscle strength and bone mass resulting from immobility, such as occurs when people keep themselves indoors, which may lead to increased risk of falls and progression of bone fragility.
Calcitonin preparations have a recognized effect in inhibiting osteoclastic bone resorption and preventing decreased bone mass after fractures [31, 32], have an effect equal to or better than NSAIDs in inhibiting lumbar pain due to osteoporotic vertebral fracture, and are also beneficial in improving physical functions and quality of life. Therefore, early administration of calcitonin after an osteoporotic bone fracture may be considered one of the most highly recommended treatments.
Adverse effect of calcitonin preparations
Products containing salmon calcitonin have been used globally for more than 30 years, primarily as treatment for osteoporosis. However, the safety of elcatonin should be discussed in the light of the withdrawal on safety grounds of nasally administered salmon calcitonin for use in osteoporosis by the European Medicines Agency.
In 2012, the European Medicines Agency withdrew calcitonin nasal spray from the market and limited the duration of other calcitonin products because of a putative association with (nonspecific) cancer [33]. This apparent association was first noticed for prostate cancer in a study of an investigational orally administered recombinant salmon calcitonin product.
Elcatonin is different from salmon calcitonin in its structure. There are also almost no reports of cancer in postmarketing surveillance since 1993 in Japan.
Limitations
Elcatonin is not a novel treatment for patients with osteoporotic lumbar pain. However, there is little scientific evidence regarding the effect of elcatonin for Japanese patients. It is important to have a sufficient number of studies that use the minimal clinically important difference of outcome measures in assessing elcatonin’s effect in lumbar pain patients.
This clinical trial provides strong evidence to support elcatonin treatment for osteoporotic fragility fracture in Japanese women but the study did not cover lifestyle factors (physical activity levels, alcohol consumption, smoking, years since menopause, etc.). Although random allocation could control for the two groups’ backgrounds, we should investigate the influence of these factors in the future.
Conclusion
We conducted an open-label randomized controlled trial comparing elcatonin injections and oral administration of NSAIDs. The results showed that elcatonin was superior to each of the five NSAIDs in this trial. Elcatonin injections are a safe and effective short-term treatment method.
Intramuscular injection of calcitonin was more effective than NSAIDs in alleviating acute lumbar pain and maintaining related activities of daily living in Japanese women with osteoporotic vertebral fracture.
References
The prevention and treatment guidelines making committee of the osteoporosis, the prevention and treatment guidelines on osteoporosis (version 2015). Life Science, Tokyo
Yoshimura N, Muraki S, Oka H, Kawaguchi H, Nakamura K, Akune T (2010) Cohort profile: research on osteoarthritis/osteoporosis against disability study. Int J Epidemiol 39:988–995
Copp DH, Cameron EC (1961) Demonstration of a hypocalcemic factor (calcitonin) in commercial parathyroid extract. Science 134:2038
Hori M, Takahashi H, Konno T, Inoue J, Haba T, Sakurada T, Noda T, Fujimoto K (1984) (Effect of elcatonin on experimental osteoporosis induced by ovariectomy and low calcium diet in beagles). Nihon Yakurigaku Zasshi 84:91–98
Hayashi T, Yamamuro T, Okumura H, Kasai R, Tada K (1989) Effect of (Asu1,7)-eel calcitonin on the prevention of osteoporosis induced by combination of immobilization and ovariectomy in the rat. Bone 10:25–28
Shibata K, Takeda M, Ito A, Takeda M, Sagai H (1998) Ovariectomy-induced hyperalgesia and antinociceptive effect of elcatonin, a synthetic eel calcitonin. Pharmacol Biochem Behav 60:371–376
Ito A, Kumamoto E, Takeda M, Shibata K, Sagai H, Yoshimura M (2000) Mechanisms for ovariectomy-induced hyperalgesia and its relief by calcitonin: participation of 5-HT1A-like receptor on C-afferent terminals in substantia gelatinosa of the rat spinal cord. J Neurosci 20:6302–6308
Orimo H, Morii H, Inoue T, Yamamoto K, Minaguchi H, Ishii Y, Murota K, Fujimaki E, Watanabe R, Harata S, Honjo H, Fujita T (1996) Effect of elcatonin on involutional osteoporosis. J Bone Miner Metab 14:73–78
Yoh K, Tanaka K, Ishikawa A, Ishibashi T, Uchino Y, Sato Y, Tobinaga M, Hasegawa N, Kamae S, Yoshizawa M (2005) Health-related quality of life (HRQOL) in Japanese osteoporotic patients and its improvement by elcatonin treatment. J Bone Miner Metab 23:167–173
Takakuwa M, Iwamoto J (2012) Elcatonin in combination with risedronate is more effective than risedronate alone for relieving back pain in postmenopausal women with osteoporosis. Biol Pharm Bull 35:1159–1165
Fujita T, Ohue M, Nakajima M, Fujii Y, Miyauchi A, Takagi Y (2011) Comparison of the effects of elcatonin and risedronate on back and knee pain by electroalgometry using fall of skin impedance and quality of life assessment using SF-36. J Bone Miner Metab 29:588–597
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H (2001) Diagnostic criteria for primary osteoporosis: year 2000 revision. Osteoporosis Diagnostic Criteria Review Committee: Japanese Society for Bone and Mineral Research. J Bone Miner Metab 19:331–337
Roland M, Morris R (1983) A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 8:141–144
Suzukamo Y, Fukuhara S, Kikuchi S, Konno S, Roland M, Iwamoto Y, Nakamura T (2003) Validation of the Japanese version of the Roland-Morris Disability Questionnaire. J Orthop Sci 8:543–548
Doi T, Akai M, Endo N, Fujino K, Iwaya T (2013) Dynamic change and influence of osteoporotic back pain with vertebral fracture on related activities and social participation: evaluating reliability and validity of a newly developed outcome measure. J Bone Miner Metab 31:663–673
Chow S-C, Shao J, Wang H (2007) Nonparametrics. In: Sample size calculations in clinical research, 2nd edn. Chapman and Hall, Boca Raton, pp 355–371. doi:10.1201/9781584889830.ch14
Altman D, Machin D, Bryant T, Gardner M (2000) Statistics with confidence, 2nd edn. BMJ Books, Bristol
Knopp JA, Diner BM, Blitz M, Lyritis GP, Rowe BH (2005) Calcitonin for treating acute pain of osteoporotic vertebral compression fractures: a systematic review of randomized, controlled trials. Osteoporos Int 16:1281–1290
McGuire RA Jr (2010) Treating spinal compression fractures. AAOSNow. http://www.aaos.org/news/aaosnow/oct10/cover1.asp
Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW (2013) Cancer-induced bone pain: mechanisms and models. Neurosci Lett 557:52–59
Forman LJ, Tingle V, Estilow S, Cater J (1989) The response to analgesia testing is affected by gonadal steroids in the rat. Life Sci 45:447–454
Iba K, Yamashita T (2012) (Pain due to the osteoporosis; pain measures and management). MB Orthop 25:117–122
Ito A, Takeda M, Yoshimura T, Komatsu T, Ohno T, Kuriyama H, Matsuda A, Yoshimura M (2012) Anti-hyperalgesic effects of calcitonin on neuropathic pain interacting with its peripheral receptors. Mol Pain 8:42
Hongo M, Miyakoshi N, Kasukawa Y, Ishikawa Y, Shimada Y (2015) Additive effect of elcatonin to risedronate for chronic back pain and quality of life in postmenopausal women with osteoporosis; a randomized controlled trial. J Bone Miner Metab 33:432–439
Yoshimura T, Ito A, Saito SY, Takeda M, Kuriyama H, Ishikawa T (2012) Calcitonin ameliorates enhanced arterial contractility after chronic constriction injury of the sciatic nerve in rats. Fundam Clin Pharmacol 26:315–321
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739
Ivaska KK, Gerdhem P, Akesson K, Garnero P, Obrant KJ (2007) Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res 22:1155–1164
Hedstrom M, Sjoberg K, Svensson J, Brosjo E, Dalen N (2001) Changes in biochemical markers of bone metabolism and BMD during the first year after a hip fracture. Acta Orthop Scand 72:248–251
Ohishi T, Takahashi M, Kushida K, Hoshino H, Tsuchikawa T, Naitoh K, Inoue T (1998) Changes of biochemical markers during fracture healing. Arch Orthop Trauma Surg 118:126–130
Hoesel LM, Wehr U, Rambeck WA, Schnettler R, Heiss C (2005) Biochemical bone markers are useful to monitor fracture repair. Clin Orthop Relat Res 440:226–232
Karachalios T, Lyritis GP, Kaloudis J, Roidis N, Katsiri M (2004) The effects of calcitonin on acute bone loss after pertrochanteric fractures. A prospective, randomised trial. J Bone Jt Surg Br 86:350–358
Katae Y, Tanaka S, Sakai A, Nagashima M, Hirasawa H, Nakamura T (2009) Elcatonin injections suppress systemic bone resorption without affecting cortical bone regeneration after drill-hole injuries in mice. J Orthop Res 27:1652–1658
European Medicines Agency (2013) Questions and answers on the review of calcitonin-containing medicines. Outcome of a procedure under Article 31 of Directive 2001/83/EC. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Calcitonin_31/WC500146171.pdf. Accessed 5 Sep 2015
Acknowledgments
We thank all the orthopedic surgeons, their staff, and their patients who willingly participated in this study. The participation of the following hospitals and clinics is also greatly appreciated: Ishigaki Orthopaedic Clinic, Kaji Orthopaedic Clinic, Kanjo-dori Higashi Orthopaedics, Miyamoto Orthopaedics, Miyanosawa Orthopaedic Clinic, Okuizumi Orthopaedics, Sapporo Hospital, Sapporo Kiyota Orthopaedic Hospital, Sapporo Maruyama Orthopaedic Hospital, Sapporo Orthopaedics, Shinoda Orthopaedics, Toshima Orthopaedic Clinic, Yamanote-dori Yagi Hospital, Hakodate Central General Hospital, Yoshida Orthopaedics, Namiki Orthopaedic Clinic, Sasaki Orthopaedic and Anesthetic Clinic, Shiobara Spa Hospital, Hirano Orthopaedic Clinic, Fujino Orthopaedic Clinic, Kakegawa Municipal General Hospital, Kaname Orthopaedics, Kato Orthopaedic Clinic, Okada Orthopaedics, Saito Orthopaedic Clinic, Seirei Hamamatsu General Hospital, Shiramatsu Orthopaedics, Izu-Nirayama Spa Hospital, Kamoto Orthopaedic Clinic, Matsuura Orthopaedics, Miyake Orthopaedic Clinic, Ohya Orthopaedic Clinic, Sugiyama Orthopaedic and Rehabilitation Clinic, Tanebe Orthopaedic Clinic, Togawa Orthopaedics, Morita Orthopaedic Clinic, Murai Orthopaedic Clinic, Nakamura Orthopaedic Clinic, Niigata Rinko Hospital, Ohura Orthopaedic Clinic, Watanabe Orthopaedics, Iwai Orthopaedic Clinic, Takada Orthopaedic Clinic, Takaoka-Seisikai Hospital, Wago Orthopaedic Clinic, Kamata Orthopaedic Clinic, Maki Orthopaedic Clinic, Suzu Orthopaedics, Kishimoto Orthopaedics, Maenaka Orthopaedic Clinic, Takada Iin (Orthopaedics and Internal Medicine), Ukon Orthopaedic Clinic, Yamamoto Orthopaedics, Kira Orthopaedic Clinic, Ryuso Orthopaedic Hospital, Sakata Orthopaedic Clinic, Tamura Orthopaedics, Hiramatsu Orthopaedic Hospital, Hrada Rehabilitation and Orthopaedic Clinic, Okuda Orthopaedics and Dermatology Clinic, Shintani Orthopaedic Clinic, Nezu Orthopaedic Clinic, Takita Orthopaedic Clinic, Yamagata Orthopaedic Clinic, Aibara Orthopaedics, Hirose Orthopaedic and Rehabilitation Clinic, Masuda Orthopaedics, Nishimoto Orthopaedics, Seike Orthopaedics, Tanaka Clinic, Shinseikai Hospital, Okazaki Orthopaedic Clinic, Sakaue Orthopaedics, Tomonaga Orthopaedic Clinic, Kataoka Orthopaedic Clinic, Naruo Orthopaedic Hospital, Nishi Orthopaedic Clinic, Hashiguchi Orthopaedics, Kuwabata Orthopaedic Clinic, Niimura Orthopedics, Nakasone Orthopaedics, Nobinobi Orthopaedics, Takeuchi Orthopaedics, and Uezato Orthopaedics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Naoto Endo, Keiji Fujino, Tokuhide Doi, Masami Akai, Yuichi Hoshino, Tetsuo Nakano, and Tsutomu Iwaya declare that they have no conflict of interest.
Additional information
On behalf of The Japanese Society for Musculoskeletal Medicine and the Japanese Clinical Orthopaedic Association.
About this article
Cite this article
Endo, N., Fujino, K., Doi, T. et al. Effect of elcatonin versus nonsteroidal anti-inflammatory medications for acute back pain in patients with osteoporotic vertebral fracture: a multiclinic randomized controlled trial. J Bone Miner Metab 35, 375–384 (2017). https://doi.org/10.1007/s00774-016-0765-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-016-0765-8