Abstract
Ca-depleted photosystem II membranes obtained by treatment with acidic buffer do not contain Ca2+ in the Mn4CaO5 cluster but contain all extrinsic proteins protecting this cluster (PSII(-Ca/low pH)). However, unlike native photosystem II, Mn cluster in PSII(-Ca/low pH) samples is available for small-sized reductants. Using this property, we investigated the substitution possibility of Mn cation(s) with Fe cation(s) to obtain a chimeric cluster in PSII(-Ca/low pH) samples containing extrinsic proteins. We found that Fe(II) cation replaces Mn cation at pH 6.5, however, PSII(-Ca/low pH) membranes with the 3Mn1Fe chimeric cluster in the oxygen-evolving complex evolve O2 with high intensity in the presence of exogenous Ca2+. The O2 evolution rate is about 80% of the same rate in PSII(-Ca/low pH) membranes.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photosystem II (PSII) in oxygenic photosynthetic organisms has a catalytic complex that oxidizes water under illumination and synthesizes the molecular oxygen. The main component of the oxygen-evolving complex (OEC) is a cluster Mn4CaO5 formed by 4 manganese cations and one calcium cation interconnected by oxygen bridges [1]. Mn/Ca cluster can be extracted from PSII using various methods, including its treatment by NH2OH, Tris free base, lipophilic chelator, N,N,N′,N′-tetrapropionato-1,3-bis(aminomethyl)benzene (TPDBA) [2]. Mn-depleted PSII samples (PSII(-Mn)) have the high-affinity (HA) Mn-binding site [3]. Possibly there is a second Mn-binding site in such preparations [4].
HA site in the PSII(-Mn) samples binds with high-affinity Mn(II) cation, then bound Mn(II) cation is oxidized by YZ· [5]. This reaction is a primary step in the photoactivation process [6]. During the study of the possibility of interaction with the HA site the cations of other metals, we found highly effective binding of iron cations to this site [7]. Binding is observed at very low concentration of Fe(II) cations comparable to the binding Mn(II) concentration – 2 to 4 µM [7, 8]. Such a large similarity in binding to the HA site between Mn(II) and Fe(II) cations can be due to at least two factors: (1) the ionic radii are very close: 0.80/0.66 Å and 0.74/0.64 Å for Mn(II)/Mn(III) and Fe(II)/Fe(III), respectively; (2) Mn and Fe cations have the possibility of redox transformation at physiological pH utilized in many enzymes. In subsequent studies [8], we found that Fe cations irreversibly bind to the HA site (the bound cation cannot be removed by washing with buffer but can be extracted by citrate treatment at the acidic pH [9]). Irreversible binding needs illumination by weak light (like Mn(II) binding during photoactivation process) providing the oxidation of Fe(II) cation on the HA site [8]. Fe(III) cation bound to the HA site prevent the binding of Mn(II) cation to this site and its oxidation. In fact, Fe(III) cation blocks the HA site. According to kinetic measurements, blocking of the HA site is determined not by one iron cation, but by two or more [7, 8]. Measurement of the Fe(II) concentration in the medium during blocking showed that oxidation of 5 Fe(II) cations is necessary for this effect [10]. As in the case of photoactivation a dark interval is needed when blocking occurs under pulsed light [11]. The carboxyl-containing amino acid involved in binding the Mn(II) cation at the HA site is involved in binding the blocking iron cation [9]. High similarity in the interaction of Mn(II) and Fe(II) with the donor side of Mn-depleted PSII membranes is supported by the fact that Fe(II) cation binding is accompanied by reconstruction of the low barrier hydrogen bond between YZ and D1-His190 [12]. However, despite the great similarity between Mn(II) and Fe(II) cations in the mechanism of their interaction with PSII(-Mn) membranes, neither oxygen release nor electron transport was found in the PSII(-Mn) preparations containing iron cations.
The high binding efficiency of Fe(III) cation to the Mn-binding site and its significantly low redox potential (about 0 V for Fe(II)/Fe(OH)3 at pH 7.0 [13]), characterizing the Fe(II) cation as a strong reducing agent, made it possible to obtain preparations of PSII with chimeric clusters in the OEC consisting of manganese and iron cations. To carry out such substitution, we incubated Ca-depleted PSII membranes (PSII(-Ca)) without extrinsic proteins PsbP and PsbQ with Fe(II) cations (to get access for cations to the manganese cluster) in the dark in buffer with pH 6.5. We found that Fe(II) cations substitute two of the four Mn ions in PSII(-Ca) [14]. No O2 evolution is observed, but the heteronuclear metal cluster in PSII(2Mn,2Fe) samples is still able to supply electrons for reduction of the exogenous electron acceptor by photooxidizing water and producing H2O2 [14]. The decrease in the pH of the incubation buffer up to pH 5.7 is accompanied by an increase in the resistance of one of the Mn cations in the cluster to the action of reducing agents (Fe(II), hydroquinone) [15]. Incubation of the PSII(-Ca) membranes obtained by the NaCl treatment (PSII loose PsbP and PsbQ proteins after such treatment) preparation with Fe(II) cations at pH 5.7 is accompanied by substitution not 2 Mn cations, but only one [16]. NaCl-treated PSII(-Ca) membranes with chimeric cluster 3Mn1Fe are able to reduce the exogenic electron acceptors oxidizing water to H2O2. However, these preparations are able also to produce O2 in the presence of exogenous Ca2+ [16]. The rate of O2 generation is about 27% in relation to native membranes (PSII).
It should be noted that we did not use native PSII membranes for substitution, but Ca-depleted PSII membranes that did not contain two extrinsic proteins – PsbP and PsbQ and Ca2+ cation in the OEC. In this regard, in the proposed work, we investigated the possibility of preparing a PSII samples with a chimeric cluster in OEC but containing extrinsic proteins PsbP and PsbQ. To this end, we used PSII preparations obtained by treating of PSII membranes by citrates in acid buffer [17]. Such PSII membranes do not contain a Ca2+ in the OEC but do contain all extrinsic proteins. But, crucially, unlike the native PSII membranes in citrate-treated PSII (-Ca) membranes, the Mn cluster is available for small-sized reducing agents [18, 19]. Therefore, we used such citrate-treated PSII(–Ca) preparations to replace the manganese cation with iron cations and found that at pH 6.5, one Mn cation in OEC is substituted, but the preparations with the chimeric cluster 3Mn1Fe in the presence of exogenic Ca2+ release oxygen at a high rate, which is about 80% of the control (PSII(-Ca/low pH)).
Materials and methods
Preparation of PSII-enriched membranes
Membranes (BBY type) were prepared from market spinach according to Ghanotakis and Babcock [20]. Samples were stored at – 80 °C in buffer A (50 mM 2-(N-morpholino)-ethanesulfonic acid (MES) at pH 6.5, 15 mM NaCl, and 400 mM sucrose). The rates of O2 evolution (450–550 μmol O2 mg chlorophyll (Chl)−1 h−1) were measured polarographically using a Clark–type oxygen electrode under continuous, saturating white light illumination (through a water filter) at 25 °C with 0.2 mM 2,6-dichloro-1,4-benzoquinone (DCBQ) as the exogenous electron acceptor. Concentration of PSII during the measurement of oxygen evolution was 10 µg Chl/ml. Chlorophyll concentrations were determined in 80% acetone, according to the method of Porra et al. [21].
Preparation of Ca-depleted PSII membranes (PSII(-Ca))
Two types PSII(-Ca) membranes were prepared. 1. PSII(-Ca) membranes without extrinsic proteins PsbP and PsbQ (PSII(-Ca/NaCl)) were prepared according to [22]. PSII membranes (0.5 mg/ml Chl) were incubated in the buffer 2 M NaCl, 0.4 M sucrose, and 25 mM MES (pH 6.5) for 15 min at room temperature under low illumination (4–5 μE m−2 s−1, room fluorescent light). The resulting material was washed twice and resuspended in a buffer A. Besides Ca2+ cation PSII(-Ca/NaCl) membranes lack the PsbQ and PsbP extrinsic proteins, which prevent exogenous reducing agents from attacking the Mn/Ca cluster. 2. PSII(-Ca) membranes containing PsbQ and PsbP extrinsic proteins (PSII(-Ca/low pH)) were prepared according to [17]. PSII membrane preparations in a buffer A were centrifuged, and the pellets resuspended in citrate buffer (400 mM sucrose, 20 mM NaCl, 10 mM sodium citrate, pH 3.4) at 2 mg Chl ml−1. Further, the membranes were incubated for 5 min on ice in the dark. The samples were finally diluted (1:3 v/v) with buffer A (adjusting pH to 6.5) and incubated for 20 min at 4 °C in the dark. The membranes were then pelleted, washed once with buffer A, and subsequently resuspended in buffer A.
Determination of Mn content in the PSII membranes
Mn concentrations in different samples were measured as described in a previous publication [23] with minor modifications [24]. Samples (100 μg Chl) were suspended in 1 ml of buffer A and then incubated with 50 mM CaCl2 for 2 min in the dark at 5 °C. After incubation, the samples were pelleted. The pellet was resuspended in 90 μl of 0.6 N HCl to solubilize the functional Mn remaining in the pellet. Next, 0.9 ml of glass-distilled water was added to the membrane suspension, and finally, the microfuge tube was centrifuged for 3 min at 15,000 × g (22 °C). The supernatant (0.9 ml) was filtered through a 13-mm Acrodisc syringe filter containing a 0.2 μm nylon membrane (Pall Life Sciences, Ann Arbor, USA). The filtrate (in a 1-ml glass cuvette) was mixed consecutively according to Serrat [25] with 40 μl of 2 M NaOH, 40 μl of a stock solution of 3,3′,5,5′-tetramethylbenzidine (TMB) (100 mg TMB in 100 ml of 0.1 M hydrochloric acid), and 40 μl of 5.3 M phosphoric acid. The absorbance at 450 nm was used to calculate Mn(II) concentrations (extinction coefficient of 34 mM−1 cm−1) in the samples [25]. PSII reaction center (RC) concentrations were calculated in μM using 250 molecules of Chl/RC in the PSII samples [26, 27].
Substitution of Mn ions in PSII(-Ca/low pH) membranes with Fe cations
PSII(-Ca/low pH) membranes (100 μg Chl/ml) were incubated with Fe(II) for 120 min in the dark at 4 °C in buffer A (pH 6.5). After incubation, the membranes were pelleted, washed, and resuspended in buffer A.
Determination of protein profiles of protein composition in spinach PSII membranes
One-dimensional SDS-polyacrylamide gel electrophoresis was performed in polyacrylamide gel by the Laemmli method [28] using 6% concentrating gel in 1.0 M Tris–HCl (pH 6.8), 0.4% SDS and 15% separating gel in 1.0 M Tris–HCl (pH 8.8). The protein samples for electrophoresis were prepared in a fourfold buffer (250 mM Tris–HCl, pH 6.8, 8% SDS, 40% sucrose, 0.05% bromophenol blue, 5% β-mercaptoethanol) and incubated for 5 min at 95 °C. Electrophoresis was performed in Mini-Protean III cell (Bio-Rad, USA). When the bromophenol blue has run to the bottom of the gel the electrophoresis was terminated by 1-h incubation at room temperature in solution containing 25% isopropanol and 10% acetic acid. Then, proteins on the gel were stained for 1 h with Coomassie R-250 (Bio-Rad) and incubated overnight with a solution, containing 25% ethanol and 7.5% acetic acid. Then, gel was washed two times in the distilled water.
Results and discussion
Exposure of PSII to high ionic strengths (≥ 1 M NaCl) is used often to extract Ca2+ from the OEC [22, 29, 30]. Addition of Ca2+ to such preparation reactivate the oxygen-evolving function, however, such membranes don’t contain extrinsic PsbP and PsbQ polypeptides. Ono and Inoue [17] suggested another method of Ca2+ extraction from the OEC. They used citrate treatment of PSII membranes in acidic pH buffer followed by pH adjustment (using MES buffer) to normal pH value (6.5). This procedure provides the reconstruction of all extrinsic proteins, i.e., the preparation of Ca-depleted PSII membranes containing all extrinsic proteins. Rebinding of the PsbP and PsbQ polypeptides to PSII in the absence of added Ca2+ does not restore activity, whereas addition of exogenous Ca2+ recover upon long-term incubation the O2 evolution reaction [17] which indicates the possibility of metal cation access to the Mn cluster through the protective layer of extrinsic proteins. Indeed, Yocum with coworkers [18, 19] found that Mn cluster in the OEC of the low pH-treated PSII preparation is available for small type reductant like NH2OH but not too large like hydroquinone (H2Q). The reason for this may be the channel between the solvent and the Mn cluster, that is normally occluded by a bound Ca2+ cation [18].
Availability of Mn cluster for H2Q in the low pH-treated PSII(-Ca) membranes
We used this method to prepare PSII membranes that do not contain Ca2 + but contain all extrinsic proteins: PSII membranes were incubated for 5 min in the dark in citrate buffer (pH 3.4) then the suspension was diluted with buffer A (MES buffer), adjusting the pH to 6.5. After dilution, the buffer contained 25% citrate buffer and 75% buffer A. However, before incubation of the obtained PSII(-Ca) preparations with Fe(II) cations in order to replace Mn cation(s) in the OEC, it was necessary to remove citrate from the buffer, since citrate can bind iron cations and first stability constants (log K1) of Fe(II) and Fe(III) cations chelates with citric acid is equal to 3.2 and 11.85, respectively [31]. Therefore, we removed citrate from the medium after dilution by transferring/centrifugation of the sample using buffer A. As we mentioned before, extrinsic proteins do not pass the large reductants like hydroquinone to the manganese cluster in low pH-treated PSII samples [18]. This is an important feature of low pH-treated PSII membranes and therefore, we tested the presence of such property in our preparations. Results of experiments are given in Table 1. Obtained data clearly show that H2Q cannot extract Mn cations from the low pH-treated PSII(-Ca) membranes, while Mn cluster is available for H2Q in the NaCl-treated PSII(-Ca) samples. These data are consistent with the results obtained by Vander Meulen et al. [18] and indicate that the Mn cluster in the low pH-treated PSII(-Ca) membranes is protected by extrinsic proteins.
Effect of Fe(II) cations on the Mn content in low pH-treated PSII(-Ca) membranes and on their oxygen-evolving activity
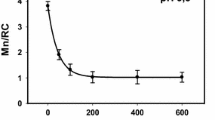
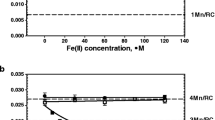
Unlike large reductant H2Q small reductant Fe(II) cations can reach Mn cluster in PSII(-Ca/low pH) samples and interact with Mn cations. The incubation of the low pH-treated PSII(-Ca) membranes with Fe(II) is accompanied by a decrease in Mn cation content in the OEC per reaction center with time incubation (Fig. 1). The degree of Mn extraction reaches a saturation value 1Mn/RC after about 80 min incubation in the dark at 4 °C and Fe(II) concentration 80 µM. The kinetics of the Mn cation displacement (presence of saturation) by the iron cation indicate that the pool of Mn cations available for reduction is exhausted and, in the area binding the Mn cations, only cations unavailable for reduction remain. According to results presented in Fig. 1 the number of remaining Mn cations per RC is 3, i.e., it is not a mixture of RC with different Mn content giving an average of 3. Similar (in nature) effect occurs during extraction of Mn cations by hydroquinone [15, 32] and by Fe(II). It was found that Fe(II) extract 2Mn cations at pH 6.5 [14] and 1 Mn/RC at pH 5.7 [16]. We suggest that it takes place the formation of a cluster consisting of 3 Mn cations and possibly 1 Fe (III) cation. In favor of the latter suggestion, it was demonstrated very high efficiency of the Fe cation binding to the free Mn-binding site [7, 8]. The vacant Mn-binding site appears as result of the Mn cation reduction by Fe(II) cation and release of Mn(II) cation from binding site. This is evidenced by the fact that (a) binding takes place only in samples where Mn is available for Fe(II) cations (there is no binding in intact PSII) [14, 16]; (b) binding is accompanied by removal of Mn cation from OEC. The presence of the Fe(III) cation in the mixed Mn/Fe cluster is indicated by an increase in the resistance of the Mn cations in such clusters to the action of H2Q [16]. Figure 2 shows concentration dependence of substitution of Mn cation in the low pH-treated PSII(-Ca) membranes with Fe(II). An important circumstance should be noted here. Fe(II) cation replaces one manganese cation in the low pH-treated PSII(-Ca) membranes (all extrinsic proteins are present in the membrane) whereas it replaces 2 Mn cations in the NaCl-treated PSII(-Ca) preparation (PsbP and PsbQ are absent) although the pH of the incubation buffer was the same—6.5. This fact indicates a significant effect of PsbP and PsbQ extrinsic proteins on the properties of the Mn cluster (possibly on the redox potential value of the Mn cation(s) in the OEC).
Confirmation of this comment is the effect of Fe cation on the oxygen evolution in the low pH-treated PSII(-Ca) with the chimeric cluster 3Mn1Fe (PSII[-Ca/low pH/3Mn1Fe]) (Table 2). In the process of the O2 evolution rate measuring in the PSII(-Ca/low pH/3Mn1Fe) membranes we discovered that such samples release oxygen at a high rate (Table 2). If we take the oxygen-evolving activity of PSII(-Ca) membranes (obtained by low pH treatment) as 100% in the presence of 30 mM exogenous Ca2+ (this value is equal to 248 µmol O2/mg Chl h and corresponds to the level of reactivation of similar preparations measured by Vander Meulen et al. [18]), then after replacing one of the Mn cations with an iron cation, the activity of the preparation (in the presence of exogenous Ca2+) decreases, but slightly—by about 20% (Table 2). That is, PSII(-Ca) membranes containing in the catalytic center not 4 Mn cations, but 3 together with 1 Fe cation oxidize water to molecular oxygen with rather high activity.
Effect of extrinsic proteins on the oxygen evolution in PSII(-Ca/low pH/3Mn1Fe) membranes
A feature of the PSII(-Ca/low pH/3Mn1Fe) membranes is the presence of all extrinsic proteins. And it can be assumed that this may be the reason for the high catalytic activity of the chimeric cluster. To test this assumption, we used the standard method of extrinsic proteins PsbP and PsbQ extraction by high concentration NaCl. To this end, PSII(-Ca/low pH/3Mn1Fe) membranes were pelleted, then the pellet was resuspended in buffer containing 2 M NaCl, 25 mM MES, 400 mM sucrose (pH 6.5), 0.5 mg Chl/ml. Membranes were incubated for 15 min at room temperature and illumination. Then membranes were washed twice by buffer A. Obtained results showed that washing of membranes PSII(-Ca/low pH/3Mn1Fe) with NaCl solution removes only 1 extrinsic protein (PsbQ), whereas washing intact PSII membranes removes 2 extrinsic proteins (PsbP and PsbQ) (Fig. 3, lanes 5 and 2, respectively). Washing of Ca-depleted membranes obtained by treatment with citrate buffer as in our work also extracts PsbP and PsbQ proteins [17]. The lack of 2 M NaCl effect on extrinsic protein PsbP is unexpected. This effect is evidence of stronger protein binding to the reaction center in membranes with chimeric cluster than in native membranes. Perhaps, stronger binding of this protein is carried out due to the participation of the amino acid residue of the PsbP protein in the coordination of the iron cation. In this regard, it should be noted that the PsbP protein has 2 Mn-binding sites and 1 Zn-binding site [see for review 33].
Polyacrylamide gel electrophoretogram of PSII membranes fragments. Lane 1, intact PSII membranes; lane 2, NaCl-washed PSII(-Ca) membranes without PsbP and PsbQ extrinsic proteins; lane 3, low pH-treated PSII(-Ca) membranes; lane 4, low pH-treated PSII(-Ca) membranes after incubation with FeSO4; lane 5, low pH-treated PSII(-Ca) membranes with chimeric cluster 3Mn1Fe in the OEC after 2 M NaCl treatment; lane 6, protein standards. The positions of the PSII extrinsic proteins (PsbO, PsbP, and PsbQ) are indicated by arrows on the left
At the same time 2 M NaCl washing does not affect the activity of preparations: activity of PSII(-Ca/low pH/3Mn1Fe) membranes in the presence of exogenic Ca2+ (30 mM) without NaCl treatment was 189 µmol O2/mg Chl (Mn/RC = 3.1) and after treatment activity was 202 µmol O2/mg Chl (Mn/RC = 3.0). It should be noted that even in native PSII membranes, from which extrinsic proteins PsbP and PsbQ are extracted by a medium with high ionic strength (Ca2+ is simultaneously removed from OEC) high concentrations of calcium can support reasonably high rates of oxygen evolution even in the absence of the PsbP and PsbQ components [34]. These proteins are not involved in the binding of Mn and Ca2+ cations forming the catalytic center of OEC but serve to stabilize the Mn4CaO5 cluster and optimize oxygen evolution at physiological calcium and chloride concentrations and shield the catalytic center [33]. Therefore, the absence of NaCl effect on the rate of oxygen evolution in PSII(-Ca/low pH/3Mn1Fe) preparation is not surprising, since the oxygen evolution activity was measured in the presence of a large concentration of Ca2+. Moreover, the main extrinsic protein PsbP involved in stabilization of Ca2+ and Cl− is not extracted (Fig. 3, lane 5) while the extracted protein PsbQ is much less effective in maintaining the function of OEC [33].
The question remains—why the substitution of Mn cation by Fe cation in the presence of PsbP and PsbQ proteins leads to significantly greater oxygen-evolving activity of the chimeric cluster 3Mn1Fe than substitution in their absence? It is not yet clear. We can suggest that the PsbP changes the conformation of the protein around the Mn cluster, which makes it more optimal for coordination of Fe or/and Mn. For example, FTIR studies demonstrated that the binding of PsbP to PS II induced protein conformational changes in the vicinity of the Mn4CaO5 cluster [35]. Another hypothetical option is that a PsbP protein is involved in the coordination of the bound Fe cation, which increases the efficiency of the catalytic center.
Effect of citrate on PSII(-Ca/low pH/3Mn1Fe) membranes
We have previously shown that Fe(III) cation(s) blocking the HA Mn-binding site in the Mn-depleted PSII membranes can be extracted with citrate both at an acidic pH 3.0 and at a neutral pH 6.5 [9]. We used the same technique to extract the cation Fe(III) from the chimeric cluster (3Mn1Fe) in the NaCl-treated PSII(-Ca) membranes after substitution of 1Mn cation with iron cation at pH 5.7 [16]. Indirect results suggest that the Fe cation is extracted [16]. However, in the experiment conducted in the present work, we did not find extraction of iron cation by citrate from the chimeric cluster in the low pH-treated PSII(-Ca) membranes. This assumption is based on the fact that oxygen activity did not change and the amount of Mn cations in the cluster did not change.
Extrinsic proteins in the low pH-treated PSII(-Ca) membranes before and after substitution of manganese cation in the OEC with iron cation
The obtained results demonstrate that chimeric (3Mn1Fe) OEC in the PSII(-Ca) preparations with (present work) or without [16] PsbP and PsbQ extrinsic proteins are very different in its efficiency to release oxygen. Therefore, we examined the content of extrinsic proteins in the PSII(-Ca) preparations after various treatments (Fig. 3). To this end, we used polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate–urea. As controls, the electrophoretograms for intact PSII (Fig. 3, lane 1) and NaCl-treated PSII(-Ca) membranes (Fig. 3, lane 2) have been done. Control sample PSII lane (Fig. 3, lane 1) demonstrate the presence of all extrinsic proteins whereas NaCl-treated PSII(-Ca) membranes contain only one extrinsic protein—PsbO (Fig. 3, lane 2). Electrophoretogram of low pH-treated PSII(-Ca) membranes (Fig. 3, lane 3) has shown the presence all extrinsic proteins that is in line with results of [17]. After incubation of this sample with Fe(II) cations for substitution we did not observe the changes in the composition of extrinsic proteins (Fig. 3, lane 4). It is interesting that washing of PSII(-Ca/low pH/3Mn1Fe) membranes with NaCl solution (standard procedure for removal of PsbP and PsbQ extrinsic proteins) does not affect the oxygen-evolving activity (Table 2) and remove only one extrinsic protein – PsbQ (Fig. 3, lane 5). This result indicates that extrinsic proteins PsbP and PsbQ have significant effect on the substitution of Mn cation with Fe cation and the properties of chimeric catalytic center in the OEC.
Conclusion
The most interesting result in our opinion is the high oxygen-evolving activity of the low pH-treated PSII(-Ca/3Mn1Fe) preparations since chimeric cluster has only 3 Mn cations in catalytic center. We have previously shown that in the NaCl-treated PSII(-Ca/2Mn2Fe) membranes one of Fe cations bound to the HA Mn-binding site [14]. The HA site in the native PSII is occupied by cation Mn4 (numbering according to work [1]), connected to an irregular cubane of 3 manganese cations and Ca2+ cation. We can suggest that Fe cation also associated with the HA Mn-binding site in the chimeric clusters 3Mn1Fe, i.e., the Fe cation possibly replaces the Mn4. It is assumed that Mn4 participates in the initiation and organization of the photoactivation process [3, 36, 37]. Mn4 has 2 water molecules W1 and W2 as ligands [1] and this can be important if one (or both) of these molecules is substrate water but currently there is no convincing evidence of this. In this regard, it should be noted that Mn4 is connected to the oxygen bridge O5 [1]. Currently, O5 (slowly exchanging water Ws binds as an OH− in the S0 state being deprotonating during the oxidation to S1) is considered as the most likely oxygen atom involved in the synthesis O2 [38]. Other ligands coordinating Mn4 are carboxyl-containing amino acid residues D1-Asp170 and D1-Glu333 [1]. Thus, the binding site Mn4 contains ligands typical of the Fe(III) coordination sphere and if the Fe(III) cation is bound to this site, then it can participate in the oxidation of one of the substrate molecules of water. A second substrate water may be the water molecule bound to the Mn1 [38, 39]. This substrate water enters the reaction as OH− (O6—fast exchanging water Wf) [38]. Ca2+-bound water molecules (W3 and W4) are also considered as sources of oxygen atoms [38, 39].
It should be noted that the Fe cation is in a trivalent state in the chimeric OEC, since substitution of the Mn cation is carried out by its reduction with an iron cation. The physiological valence states of the Fe cation 2 and 3, i.e., Fe(III) cation is not able to oxidize water molecules bound to cation.
However, 4-stroke Kok cycle (water oxidation and O2 evolution) can be carried out by a chimeric cluster according to the low valent scheme (manganese cluster is in valent states II/III/III/III (S0 state) [40]. Perhaps the iron cation bound in the OEC plays a stabilizing role. In this regard, it should be noted that the cations Mn in the OEC are more resistant to the action of H2Q if the chimeric cluster contains 1 or 2 bonded iron cations [16]. Thus, we have shown that a chimeric cluster based on manganese and iron cations can oxidize water, releasing molecular oxygen. In this regard, it is interesting to note, that Dismukes with coworkers [41] recently synthesized OEC in apo PSII using Co2+ cations instead of Mn(II) cations during photoactivation. Catalytic center consisting of 4 cations of Co2+ and Ca2+ cation can oxidize water to O2 although with much less efficiency than manganese OEC (∼25% of the activity with Mn(II)). The summary of these results may be of interest for studies of the mechanism of water oxidation, the evolutionary origin of OEC and the development of artificial water splitting systems [42].
Data availability
The data that support the findings of this study are available from the corresponding author, [BS], upon reasonable request.
Abbreviations
- Chl:
-
Chlorophyll
- DCBQ:
-
2,6-Dichloro-1,4-benzoquinone
- HA:
-
High-affinity Mn-binding site
- H2Q:
-
Hydroquinone
- MES:
-
2-(N-morpholino)-ethanesulfonic acid
- OEC:
-
Oxygen-evolving complex
- PSII:
-
Photosystem II
- PSII(-Ca):
-
Ca-depleted PSII
- PSII(-Ca/low pH):
-
Ca-depleted PSII membranes using low pH treatment
- PSII(-Ca/NaCl):
-
Ca-depleted PSII membranes using NaCl treatment
- PSII(-Ca/low pH/3Mn1Fe):
-
Low pH-treated PSII(-Ca) membranes with chimeric cluster 3Mn1Fe
- PSII(-Mn):
-
Mn-depleted PSII membranes
- RC:
-
Reaction center
- TMB:
-
3,3′,5,5′-Tetramethylbenzidine
References
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60. https://doi.org/10.1038/nature09913
Ananyev GM, Dismukes GC (1996) Assembly of the tetra-Mn site of photosynthetic water oxidation by photoactivation: Mn stoichiometry and detection of a new intermediate. Biochemistry 35:4102–4109. https://doi.org/10.1021/bi952667h
Ono T-A, Mino H (1999) Unique binding site for Mn2+ ion responsible for reducing an oxidized YZ tyrosine in manganese-depleted photosystem II membranes. Biochemistry 38:8778–8785. https://doi.org/10.1021/bi982949s
Mino H, Asada M (2021) Location of two Mn2+ affinity sites in photosystem II detected by pulsed electron–electron double resonance. Photosynth Res. https://doi.org/10.1007/s11120-021-00885-5
Hoganson CW, Ghanotakis DF, Babcock GT, Yocum CF (1989) Mn2+ reduces Y+ in manganese-depleted photosystem II preparations. Photosynth Res 22:285–293. https://doi.org/10.1007/BF00048306
Becker K, Cormann KU, Nowaczyk MM (2011) Assembly of the water-oxidizing complex in photosystem II. J Photochem Photobiol B 104:204–211. https://doi.org/10.1016/j.jphotobiol.2011.02.005
Semin BK, Ivanov II, Rubin AB, Parak F (1995) High-specific binding of Fe(II) at the Mn-binding site in Mn-depleted PSII membranes from spinach. FEBS Lett 375:223–226. https://doi.org/10.1016/0014-5793(95)01215-Z
Semin BK, Ghirardi ML, Seibert M (2002) Blocking of electron donation by Mn(II) to YZ • following incubation of Mn-depleted photosystem II membranes with Fe(II) in the light. Biochemistry 41:5854–5864. https://doi.org/10.1021/bi0200054
Semin BK, Seibert M (2006) A carboxylic residue at the high-affinity, Mn-binding site participates in the binding of iron cations that block the site. Biochim Biophys Acta 1757:189–197. https://doi.org/10.1016/j.bbabio.2006.02.001
Semin BK, Seibert M (2004) Iron bound to the high-affinity Mn binding site of the oxygen-evolving complex shifts the pK of a component controlling electron transport via Y(Z). Biochemistry 43:6772–6782. https://doi.org/10.1021/bi036047p
Semin BK, Seibert M (2006) Flash-induced blocking of the high-affinity, Mn-binding site by iron cations: dependence on the dark interval between flashes and binary oscillations of fluorescence yield. J Phys Chem B 110:25532–25542. https://doi.org/10.1021/jp0652796
Semin BK, Lovyagina ER, Timofeev KN, Ivanov II, Rubin AB, Seibert M (2005) Iron-blocking the high-affinity Mn-binding site in photosystem II facilitates identification of the type of hydrogen bond participating in proton-coupled electron transport via YZ •. Biochemistry 44:9746–9757. https://doi.org/10.1021/bi047618w
Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B (1993) Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature 362:834–836. https://doi.org/10.1038/362834a0
Semin BK, Seibert M (2016) Substituting Fe for two of the four Mn ions in photosystem II: effects on water-oxidation. J Bioenerg Biomembr 48:227–240. https://doi.org/10.1007/s10863-016-9651-2
Semin BK, Davletshina LN, Rubin AB (2015) Correlation between pH dependence of O2 evolution and sensitivity of Mn cations in the oxygen-evolving complex to exogenous reductants. Photosynth Res 125:95–103. https://doi.org/10.1007/s11120-015-0155-4
Semin BK, Davletshina LN, Seibert M, Rubin AB (2018) Creation of a 3Mn/1Fe cluster in the oxygen-evolving complex of photosystem II and investigation of its functional activity. J Photochem Photobiol B 178:192–200. https://doi.org/10.1016/j.jphotobiol.2017.11.016
Ono T, Inoue Y (1988) Discrete extraction of the Ca atom functional for O2 evolution in higher plant photosystem II by a simple low pH treatment. FEBS Lett 227:147–152. https://doi.org/10.1016/0014-5793(88)80886-X
Vander Meulen KA, Hobson A, Yocum CF (2002) Calcium depletion modifies the structure of the photosystem II O2-evolving complex. Biochemistry 41:958–966. https://doi.org/10.1021/bi0109414
Vander Meulen KA, Hobson A, Yocum CF (2004) Reconstitution of the photosystem II Ca2+ binding site. Biochim Biophys Acta 1655:179–183. https://doi.org/10.1016/j.bbabio.2003.08.012
Ghanotakis DF, Babcock GT (1983) Hydroxylamine as an inhibitor between Z and P680 in photosystem II. FEBS Lett 153:231–234. https://doi.org/10.1016/0014-5793(83)80154-9
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll a and chlorophyll b extracted with 4 different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394. https://doi.org/10.1016/S0005-2728(89)80347-0
Ono T, Inoue Y (1990) Abnormal redox reactions in photosynthetic O2- evolving centers in NaCl/EDTA-washed PS II. A dark-stable EPR multiline signal and an unknown positive charge accumulator. Biochim Biophys Acta 1020:269–277. https://doi.org/10.1016/0005-2728(90)90157-Y
Semin BK, Seibert M (2009) A simple colorimetric determination of the manganese content in photosynthetic membranes. Photosynth Res 100:45–48. https://doi.org/10.1007/s11120-009-9421-7
Semin BK, Davletshina LN, Timofeev KN, Ivanov II, Rubin AB, Seibert M (2013) Production of reactive oxygen species in decoupled, Ca2+-depleted PSII and their use in assigning a function to chloride on both sides of PSII. Photosynth Res 117:385–399. https://doi.org/10.1007/s11120-013-9870-x
Serrat FB (1998) 3,3′,5,5′-Tetramethylbenzidme for the colorimetric determination of manganese in water. Microchim Acta 129:77–80. https://doi.org/10.1007/BF01246852
Ghanotakis DF, Babcock GT, Yocum CF (1984) Structural and catalytic properties of the oxygen-evolving complex. Correlation of polypeptide and manganese release with the behavior of Z+ in chloroplasts and a highly resolved preparation of the PSII complex. Biochim Biophys Acta 765:388–398. https://doi.org/10.1016/0005-2728(84)90180-4
Xu Q, Bricker TM (1992) Structural organization of proteins on the oxidizing side of photosystem I. Two molecules of the 33-kDa manganese-stabilizing proteins per reaction center. J Biol Chem 267:25816–25821. https://doi.org/10.1016/S0021-9258(18)35683-7
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Ghanotakis DF, Babcock GT, Yocum CF (1984) Calcium reconstitutes high rates of oxygen evolution in polypeptide depleted photosystem II preparations. FEBS Lett 167:127–130. https://doi.org/10.1016/0014-5793(84)80846-7
Miyao M, Murata N (1984) Calcium ions can be substituted for the 24-kDa polypeptide in photosynthetic oxygen evolution. FEBS Lett 168:118–120. https://doi.org/10.1016/0014-5793(84)80218-5
Furia TE (1973) CRC handbook of food additives (Vol. 1), CRC press
Kuntzleman T, Yocum CF (2005) Reduction-induced inhibition and Mn(II) release from the photosystem II oxygen-evolving complex by hydroquinone or NH2OH are consistent with a Mn(III)/Mn(III)/Mn(IV)/Mn(IV) oxidation state for the dark-adapted enzyme. Biochemistry 44:2129–2142. https://doi.org/10.1021/bi048460i
Roose JL, Frankel LK, Mummadisetti MP, Bricker TM (2016) The extrinsic proteins of photosystem II: update. Planta 243:889–908. https://doi.org/10.1007/s00425-015-2462-6
Nakatani HY (1984) Photosynthetic oxygen evolution does not require the participation of polypeptides of 16 and 24 kilodaltons. Biochem Biophys Res Commun 120:299–304. https://doi.org/10.1016/0006-291X(84)91448-7
Tomita M, Ifuku K, Sato F, Noguchi T (2009) FTIR evidence that the PsbP extrinsic protein induces protein conformational changes around the oxygen-evolving Mn cluster in photosystem II. Biochemistry 48:6318–6325. https://doi.org/10.1021/bi9006308
Zabret J, Bohn S, Schuller SK, Arnolds O, Möller M, Meier-Credo J et al (2021) Structural insights into photosystem II assembly. Nature plants 7:524–538. https://doi.org/10.1038/s41477-021-00895-0
Avramov AP, Hwang HJ, Burnap RL (2020) The role of Ca2+ and protein scaffolding in the formation of nature’s water oxidizing complex. Proc Natl Acad Sci USA 117:28036–28045. https://doi.org/10.1073/pnas.2011315117
Lubitz W, Cox CM, N, (2019) Water oxidation in photosystem II. Photosynth Res 142:105–125. https://doi.org/10.1007/s11120-019-00648-3
Shen JR (2015) The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48. https://doi.org/10.1146/annurev-arplant-050312-120129
Vinyard DJ, Ananyev GM, Dismukes GC (2013) Photosystem II: the reaction center of oxygenic photosynthesis. Annu Rev Biochem 82:577–606. https://doi.org/10.1146/annurev-biochem-070511-100425
Gates C, Ananyev G, Roy-Chowdhury S, Cullinane B, Miller M, Fromme P, Dismukes GC (2022) Why did nature choose manganese over cobalt to make oxygen photosynthetically on the earth? J Phys Chem B 126:3257–3268. https://doi.org/10.1021/acs.jpcb.2c00749
Du P, Eisenberg R (2012) Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: Recent progress and future challenges. Energy Environ Sci 5:6012–6021. https://doi.org/10.1039/c2ee03250c
Acknowledgements
The research was carried out as part of the Scientific Project of the State Order of the Government of Russian Federation to Lomonosov Moscow State University No. 121032500058-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Semin, B.К., Davletshina, L.N. High-efficiency oxygen evolution by photosystem II oxygen-evolving complex containing 3Mn per reaction center. J Biol Inorg Chem 28, 393–401 (2023). https://doi.org/10.1007/s00775-023-01987-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-023-01987-2